Abstract
Objective
Lusutrombopag is a thrombopoietin receptor agonist approved to treat thrombocytopenia in patients with chronic liver disease (CLD). This post hoc analysis of the Japanese L-PLUS 1 and global L-PLUS 2 trials aimed to clarify factors related to platelet count increase after lusutrombopag treatment.
Methods
In L-PLUS 1, Pearson’s correlation coefficients were used to evaluate correlations between platelet count and spleen index, thrombopoietin concentration, white blood cell (WBC) counts, and red blood cell counts (intent-to-treat [ITT] population). Associations between platelet count increase after lusutrombopag treatment and each parameter were assessed by regression analysis and mixed-effect model for repeated measures (MMRM). Associations between time-dependent changes in platelet count increase and each parameter were also examined in the L-PLUS 2 lusutrombopag ITT population by MMRM.
Results
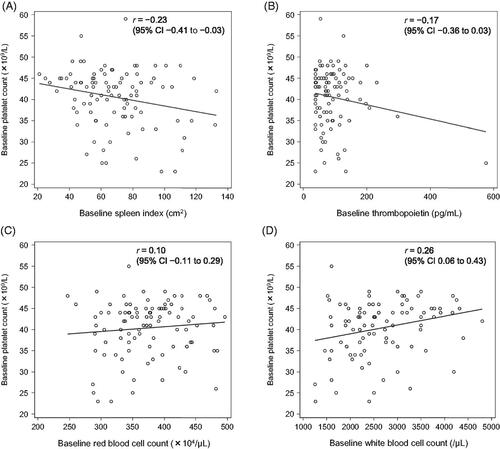
In L-PLUS 1, the baseline platelet count was correlated with pretreatment spleen index (r = −0.23, 95% confidence interval [CI] −0.41 to −0.03) and WBC count (r = 0.26, 95% CI 0.06 to 0.43). No selected parameters were associated with the maximum platelet count increase from baseline. Patients with WBC counts below the normal range showed smaller platelet count increases after lusutrombopag treatment than patients with WBC counts within the normal range (p = .0028). In L-PLUS 2 (p = .0533), findings were similar and confirmed by larger pooled data of L-PLUS 1/L-PLUS 2 (p = .0021).
Conclusions
This post hoc analysis revealed a possible association between baseline WBC count and platelet count increase after lusutrombopag treatment. WBC count could be a relevant factor for lusutrombopag efficacy.
Introduction
Thrombocytopenia is a common hematologic complication in chronic liver disease (CLD)Citation1. Patients with CLD and severe thrombocytopenia are at increased risk of bleeding, which can interfere with the performance of invasive diagnostic or therapeutic proceduresCitation2. Hence, guidelines indicate the need to raise the platelet count above certain thresholds prior to procedures, typically 50 × 109/L. Platelet transfusions can be used to improve thrombocytopenia preoperativelyCitation1; however, their effectiveness has been questioned as they may be ineffective for achieving the desired preoperative platelet countCitation3 and may be associated with complications and risk of transfusion reactions and infectionsCitation4,Citation5.
Thrombopoietin is a key cytokine in the regulation of megakaryocyte and platelet productionCitation1. Lusutrombopag (Shionogi & Co., Ltd., Osaka, Japan), a small-molecule thrombopoietin receptor agonist, is approved in the US and Japan for the treatment of thrombocytopenia, and in Europe for the treatment of severe thrombocytopenia in adults with CLD scheduled to undergo an invasive procedureCitation6,Citation7. Three double-blind, placebo-controlled trials (Japanese phase 2 b, and phase 3 [L-PLUS 1], and global phase 3 [L-PLUS 2]) have shown that lusutrombopag is efficacious and safe for patients with CLD and severe thrombocytopeniaCitation8–10. In these three trials, significantly lower proportions of patients with CLD receiving lusutrombopag (p < .01) required platelet transfusion before invasive procedures compared with placebo. Recent post-marketing surveillance in Japan reported that among 300 patients treated with lusutrombopag who underwent invasive procedures, 94% did not require a preoperative platelet transfusionCitation11. Nonetheless, thrombocytopenia is unresponsive to lusutrombopag in some patients with CLD in clinical settings. Thus far, factors that can predict an appropriate response to lusutrombopag have not been identified.
The pathogenesis of thrombocytopenia in CLD is multifactorial. The platelet count is thought to be associated with splenomegaly, portal vein hypertension, reduced thrombopoietin production, and bone marrow suppressionCitation1,Citation12. In patients with cirrhosis, the platelet count was associated with spleen size, hepatic venous pressure gradient, and other blood cell countsCitation13,Citation14. Retrospective studies conducted in Japan have analyzed the relationship between spleen volume and changes in platelet count after lusutrombopag treatmentCitation15,Citation16. Therefore, these parameters could be considered relevant to the efficacy of lusutrombopag. We previously reported the results of a post hoc analysis of covariance, in which there was no correlation between the change in platelet count from baseline by lusutrombopag treatment and either splenic volume or thrombopoietin concentration at baseline in L-PLUS 1Citation8. Herein, we conducted a post hoc analysis of both L-PLUS 1, a Japanese phase 3 trial, and L-PLUS 2, a global phase 3 trial, to explore possible associations between spleen index, thrombopoietin concentration, white blood cell (WBC) count, and red blood cell (RBC) count at baseline and platelet count increase after lusutrombopag treatment.
Methods
Study design, participants, and treatment
The detailed methodology of L-PLUS 1 (Japan; JapicCTI-132323; first patient in: 17 October 2013; last patient in: 28 March 2014; last patient last visit: 1 May 2014) and L-PLUS 2 (global; NCT02389621; first patient in: 4 July 2015; last patient in: 16 March 2017; last patient last visit: 10 May 2017) has been described in fullCitation8,Citation9. In L-PLUS 1, 97 patients were randomly assigned to treatment (lusutrombopag, n = 49; placebo, n = 48), all of whom were included in the present study. The 215 patients randomly assigned to treatment in L-PLUS 2 (lusutrombopag, n = 108; placebo, n = 107) were also evaluated.
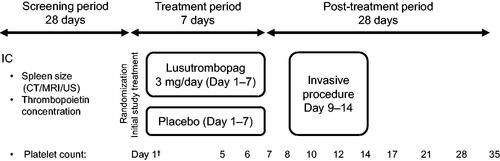
These phase 3 studies were multicenter, randomized, double-blind, parallel-group, placebo-controlled with three study periods: a screening period of up to 28 days before randomization, a treatment period of up to 7 days, and a post-treatment period of 28 days. Patients with CLD and platelet count <50 × 109/L, undergoing invasive procedures were enrolled and randomly assigned to treatment for up to 7 days (Days 1–7), after which they underwent the planned invasive procedure (Days 9–14). Spleen size and thrombopoietin concentration were measured during the screening visit or on Day 1 (baseline). Spleen size was measured by ultrasonography, computed tomography, or magnetic resonance imaging in L-PLUS 1 and was calculated by multiplying the transverse diameter by the vertical diameter. The spleen index data from L-PLUS 1 were validated by a blinded independent medical expert. The baseline values of both WBC and RBC counts were measured on Day 1 before study drug administration. The platelet count was measured on Days 1, 5–8, 10, 12, 14, 17, 21, 28, and 35 (). The primary endpoint in both clinical trials was the proportion of patients not requiring platelet transfusion before the invasive procedure. Patients received a preoperative platelet transfusion if the platelet count was <50 × 109/L.
Figure 1. Timing of acquisition of selected parameters in the phase 3 study design. IC, informed consent; CT, computed tomography; MRI, magnetic resonance imaging; US, ultrasonography. †Red blood cell count and white blood cell count
Lusutrombopag treatment discontinuation criteria were set to avoid an excessive increase in platelet count and consisted of discontinuation of the 3-mg once-daily lusutrombopag dose on Days 5–7 if the platelet count reached ≥50 × 109/L with an increase of ≥20 × 109/L from baseline. Ten patients (lusutrombopag, n = 8; placebo, n = 2) in L-PLUS 1 met the criteria for treatment discontinuation, while in L-PLUS 2, 36 patients (lusutrombopag, n = 30; placebo, n = 6) met the criteria for treatment discontinuation. There was one patient in each of the lusutrombopag groups in L-PLUS 1 and L-PLUS 2 (and one in the placebo group in L-PLUS 1) who had baseline platelet count >50 × 109/L.
Assessments and statistical analysis
Using the intent-to-treat (ITT) population of L-PLUS 1, we assessed the correlations between baseline platelet count and the selected parameters (spleen index and thrombopoietin concentration during the screening period, and counts of WBC and RBC on Day 1 before treatment administration) using Pearson’s correlation coefficients (r). The ITT population included patients who were randomized to either lusutrombopag or placebo treatment in L-PLUS 1.
Next, we explored the association between the maximum increase in platelet count by treatment and the selected parameters by regression analysis. This model includes the treatment group as a fixed effect, and the selected parameter at baseline and the interaction between the treatment group and the selected parameter at baseline as covariates. This interaction effect shows the difference of slope of the selected parameter at baseline between treatment groups, which indicates the effect of the selected parameter of lusutrombopag treatment on the maximum increase in platelet count.
Finally, we investigated the difference in the time course of changes in the platelet count from baseline between selected parameters in patients who received lusutrombopag in L-PLUS 1, using a mixed-effect model for repeated measures (MMRM). The MMRM analysis included the groups based on the target parameter, time point, and interaction effect (group and time point) as a fixed effect and baseline platelet count as a covariate. Type 3 tests were performed to explore the difference between the groups; when patients were stratified by covariates (e.g. WBC low or normal), the p value was based on the null hypothesis that the mean platelet count between groups at each time point was equalCitation17. The platelet count after a platelet transfusion was excluded from the MMRM.
Regarding the groups based on the WBC and RBC counts in the MMRM, patients were classified as having normal or low (i.e. <normal) count; the clinically normal range defined by each laboratory was used for classification. For the spleen index and the thrombopoietin concentration, patients were classified according to the median value (<median or ≥median). The median was used because there are no standard reference values for these measures. If the obtained concentration of thrombopoietin was below the lower limit of quantification (LLQ), the LLQ was imputed for the analysis.
To confirm the reproducibility of the results from lusutrombopag-treated patients in L-PLUS 1, we analyzed the data from lusutrombopag-treated patients (ITT population) in the similarly designed study, L-PLUS 2. Additionally, we conducted a pooled analysis using data from the ITT populations of both studies.
Statistical significance was set at 0.05. SAS software was used for the statistical analyses (version 9.4 or higher; SAS Institute Inc., Cary, NC, USA).
Results
Correlation between the baseline platelet count and selected parameters in L-PLUS 1
In this post hoc analysis, we analyzed the data from 97 patients in the ITT population of L-PLUS 1 (), because spleen index data were not collected in L-PLUS 2. This included one patient who did not receive the study drug.
Table 1. Patient baseline characteristics of the intent-to-treat population (n = 97) in L-PLUS 1Citation8.
Assessment of the correlations between the baseline platelet count and the following selected parameters in these 97 patients produced the following results: spleen index, r = −0.23 (95% confidence interval [CI] −0.41 to −0.03); thrombopoietin concentration, r = −0.17 (95% CI −0.36 to 0.03); RBC count, r = 0.10 (95% CI −0.11 to 0.29); and WBC count, r = 0.26 (95% CI 0.06 to 0.43) (). There was a negative correlation between platelet count and spleen index and a positive correlation with WBC count, but not with thrombopoietin and RBC levels before treatment with lusutrombopag or placebo.
Association between maximum increase in platelet count from baseline and selected parameters in L-PLUS 1
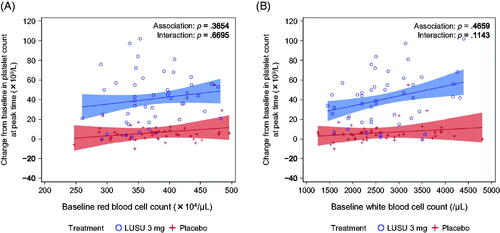
The relationships between the maximum change in platelet count after the administration of lusutrombopag or placebo and baseline spleen index, thrombopoietin, RBC, and WBC were investigated. The p values for the associations between the maximum increase in platelet count and the baseline parameters were p = .3654 for RBC count and p = .4659 for WBC count. The p values for the interaction between the baseline parameters and treatment on the maximum increase in platelet count were p = .6695 for RBC count and p = .1143 for WBC count (). Although the p value of interactions with WBC count was greater than 0.05, the difference of slope between the lusutrombopag 3 mg and placebo groups was larger compared with other parameters, and the p value for the interaction between the baseline WBC count and treatment was relatively small (). There was no relationship between the maximum change in platelet count and spleen index or thrombopoietin concentration.
Figure 3. Association between the maximum change in platelet count from baseline and selected parameters in L-PLUS 1. (A) red blood cell count and (B) white blood cell count. In the regression analysis, the fixed effect was the treatment group and the covariates were the baseline platelet count, the baseline parameters, and the interaction between baseline parameters and treatment. Filled areas indicate 95% confidence intervals for predicted outcome: blue for lusutrombopag 3 mg and red for placebo. LUSU, lusutrombopag.
Time course of the change from baseline in platelet count by selected parameters in L-PLUS 1
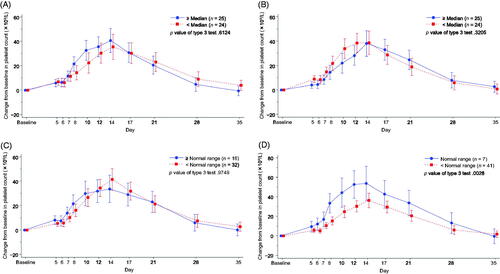
The time course of changes in platelet count was investigated. Data were classified by median value for spleen index and thrombopoietin and by normal range for RBC and WBC (). Normal RBC and WBC counts were defined differently at each study site. At the respective sites, the lower limit of normal values ranged from 2920/μL to 4500/μL for WBC count. For RBC count, the lower limit of normal values ranged from 334 × 104/μL to 443 × 104/μL, and the upper limit of normal values ranged from 460 × 104/μL to 580 × 104/μL. After lusutrombopag treatment, no notable difference in platelet count was observed in any of the groups classified by spleen index (p = .6124), thrombopoietin concentration (p = .3205), and RBC count (p = .9749), respectively; however, patients with WBC counts below the normal range had smaller increases in platelet count after lusutrombopag treatment compared with patients with WBC counts within the normal range (p = .0028) ().
Figure 4. Time-dependent change from baseline in the platelet count by selected parameters in L-PLUS 1. (A) spleen index, (B) thrombopoietin concentration, (C) red blood cell count, and (D) white blood cell count. In the mixed-effect model for repeated measures analysis, the fixed effect was the group based on the target parameter, time point and the interaction effect (group and time point), and the covariate was the baseline platelet count, with unstructured covariance. Error bars indicate a 95% confidence interval of the predicted outcome.
Time course of change from baseline in platelet count by selected parameters in L-PLUS 2 and pooled analysis
The relationship between the baseline WBC count and the change in platelet count over time after lusutrombopag treatment was further investigated using global data from L-PLUS 2 (n = 108). Demographics are shown in Supplementary Table S1. As spleen size data were not available in L-PLUS 2, thrombopoietin, RBC, and WBC levels were used as selected parameters. Normal values were defined at the dedicated central laboratory for L-PLUS 2; the WBC count lower limit of normal was 4100/μL. Normal RBC values differed depending on sex and other factors; the lower limit ranged from 380 × 104/μL to 410 × 104/μL, and the upper limit ranged from 540 × 104/μL to 590 × 104/μL. The mean baseline WBC count in L-PLUS 2 was higher than that in L-PLUS 1 ( and Supplementary Table S1), and the highest recorded WBC count in L-PLUS 2 was 9100/μL. The increment of the platelet count tended to be smaller in patients with a WBC count below the normal range compared with those with a WBC count within the normal range. However, the p value of the difference was greater than 0.05 (p = .0533) (). No notable differences in platelet count were observed in the two groups classified either by the thrombopoietin concentration or the RBC count (Supplementary Figures S1 and S2).
Figure 5. Time-dependent change from baseline in the platelet count by the white blood cell count in (A) L-PLUS 2 and (B) L-PLUS 1 plus L-PLUS 2. In the mixed-effect model for repeated measures analysis, the fixed effect was the group based on the target parameter, time point and the interaction effect (group and time point), and the covariate was the baseline platelet count, with unstructured covariance. Error bars indicate 95% confidence intervals of the predicted outcome.
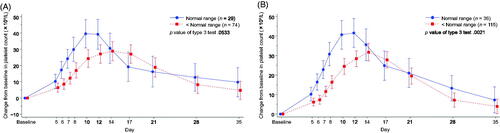
Pooled analysis by WBC count was performed using combined data from L-PLUS 1 (n = 48) and L-PLUS 2 (n = 103) to evaluate the association in a larger population. For the classification of patients by WBC, the normal range applied in each trial was used. Patients with a WBC count lower than the normal range showed smaller platelet count increments than those with a WBC count within the normal range (p = .0021) ().
Discussion
The thrombopoietin receptor agonist lusutrombopag eliminates the need for platelet transfusions prior to invasive procedures by increasing the platelet count in patients with CLD with severe thrombocytopeniaCitation8–10. This post hoc analysis aimed to clarify factors related to platelet count increase after lusutrombopag treatment, using data from two pivotal phase 3 clinical trials conducted in Japan (L-PLUS 1Citation8) and in 23 countries other than Japan (L-PLUS 2Citation9). We found that the increase in platelet count after lusutrombopag treatment was lower in patients with a WBC count below the normal range at baseline compared with patients with a WBC count within the normal range in the Japanese study data (L-PLUS 1). A similar tendency was observed for the global study data (L-PLUS 2), which was further confirmed by the larger pooled data of L-PLUS 1 and L-PLUS 2. Altogether, these findings suggested an association between WBC count before treatment and the response to lusutrombopag over time, regardless of the geographic region in which the study was conducted.
Identifying factors that affect changes in platelet count over time after lusutrombopag treatment may be useful during lusutrombopag administration in clinical settings. In both phase 3 studies, lusutrombopag was to be administered for 7 days, in principle, and an invasive procedure was scheduled to be performed 2–7 days after the final dose. Consequently, the increase in platelet count resulting from lusutrombopag treatment must be maintained until the invasive procedures. In the present analysis, a mean increase in platelet count >20 × 109/L was observed from Day 8 of treatment in patients with WBC counts within the normal range. In patients with WBC counts below the normal range, a mean increase in platelet count >20 × 109/L was observed later, that is, on Day 10 of treatment. This increase persisted until Day 17. Notably, platelet counts in both groups were reversed on Day 17 of treatment according to the analysis of L-PLUS 2 and the pooled analysis, but this was considered unlikely to have a meaningful clinical impact given that the temporal occurrence was after the invasive procedure. These data suggest that patients with WBC counts within the normal range respond better to lusutrombopag treatment, and they are more likely to be able to undergo an invasive procedure without needing a blood transfusion. Given that the WBC count can be determined as part of routine clinical practice, WBC count may serve as a convenient index that can predict the response to lusutrombopag.
In the ITT population of L-PLUS 1, a positive correlation was seen between baseline platelet count and WBC count, whereas a negative correlation was observed between platelet count and spleen index. Similar results were reported by Latorre et al.Citation14 in real-world clinical practice. This suggests that the patient population in this post hoc analysis may be representative of the general patient population.
Notably, the authors of the real-world study did not find a correlation between baseline platelet count and thrombopoietinCitation14, and no correlation between baseline platelet count and thrombopoietin was observed in the current post hoc analysis (the slope was negative). Although it is known that the cytokine thrombopoietin plays a central role in platelet productionCitation1, no consensus has been reached regarding the correlation between thrombopoietin levels and platelet count. In previous reports of data from patients with cirrhosis, platelet count was high when thrombopoietin levels were high in liver transplant recipientsCitation18–20. However, some analyses of patients with CLD reported conflicting results regarding the relationship between the concentration of thrombopoietin and the platelet countCitation21–25. The negative slope and lack of correlation between platelet count and thrombopoietin levels in our study may be related to the regulation of thrombopoietin mediated by negative feedback.
Our results did not show a notable association between spleen index and the increase in platelet count after lusutrombopag treatment. We also calculated and analyzed spleen volume, but no relevant associations were found (data not shown). In previous retrospective analyses performed in Japan, it was reported that the platelet count increase after lusutrombopag treatment was lower when spleen size was >800 mLCitation15,Citation16. In L-PLUS 1, the mean (± SD) spleen size in the lusutrombopag group was 529 ± 254 mLCitation8. Therefore, one reason for the lack of association between spleen index and increase in platelet count may be that the mean spleen size was relatively small compared with that in patients previously analyzed in JapanCitation15,Citation16.
A recent study by Brown et al. reported no association between baseline platelet count and platelet count after lusutrombopag administrationCitation26. In the present study, we found that the increase in platelet count after lusutrombopag treatment was lower in patients with WBC count below the normal range than in patients with WBC count within the normal range. Patterns of hematopenia are not uniform in patients with CLDCitation27. All of the patients we evaluated had severe thrombocytopenia, but more patients were below the normal range in terms of WBC count than RBC count; as such, the pattern of hematopenia was not uniform even within this patient group. Some factors that have been reported as causes of thrombocytopenia in CLD patients and may contribute to the abnormal hematologic indices are decreased cytokine production, bone marrow suppression, and hypersplenism (splenomegaly)Citation12. These factors also affect hematocytes. Reportedly, thrombopoietin levels are decreased in patients with CLD compared with controlsCitation28. This is thought to be a result of liver damage as thrombopoietin is primarily produced in the liverCitation29. However, a relationship between thrombopoietin and platelet count was not shown in our analysis. The granulocyte-macrophage colony-stimulating factor is involved in WBC production and is not produced in the liver, but rather by a variety of nonhematopoietic cells, including fibroblasts and endothelial cellsCitation13,Citation20, and thus, may be less affected by CLD. Consequently, the possibility that cytokines affected platelet count and WBC count is thought to be low.
Although it may be possible to demonstrate the impact of bone marrow suppression by investigating the differential WBC count (neutrophils and lymphocytes), the phase 3 results analyzed herein did not include differential WBC count data. While a positive correlation was seen between baseline platelet count and WBC count, a negative correlation was found between platelet count and spleen size. Rapid platelet destruction in the spleen of patients with WBC counts below the normal range may have contributed to the smaller increase in the platelet count after lusutrombopag treatment that was observed in patients with a WBC count below the normal range.
Our study has some limitations. This study was not planned in advance; rather, it was a post hoc exploratory analysis of two clinical trialsCitation8,Citation9. The patients enrolled in both studies were not representative of real-world patients because they included only patients with severe thrombocytopenia for whom concomitant therapies that could influence platelet count were prohibited. Additionally, patients with Child-Pugh class C liver disease were excluded from L-PLUS 1. Spleen size parameters were not collected in L-PLUS 2. In terms of reference ranges for WBC and RBC, different ranges were used at different laboratory sites in L-PLUS 1. It is, therefore, possible that interpretations varied, according to the reference ranges used. Furthermore, the sample sizes were limited in number; thus, confirmation of the analyses in a wider population is desirable.
In conclusion, the findings of this post hoc analysis of the L-PLUS 1 and L-PLUS 2 trials revealed a possible association between baseline WBC count and the platelet count increase after lusutrombopag treatment. The usefulness of WBC count as a relevant factor for lusutrombopag efficacy should be confirmed in future prospective studies.
Transparency
Declaration of funding
This work and manuscript development support were funded by Shionogi & Co., Ltd., Osaka, Japan. The study sponsor contributed to conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources management, supervision, validation, and writing, review, and editing of the manuscript, insofar as Takamichi Baba, Manami Yoshida, Motofumi Iguchi, Takuhiro Sonoyama, Takahiro Fukuhara, and Takeshi Kano are employees of Shionogi & Co., Ltd., Osaka, Japan.
Declaration of financial/other relationships
Katsuaki Tanaka received consultancy fees from Shionogi & Co., Ltd., outside the submitted work. Takamichi Baba, Manami Yoshida, Motofumi Iguchi, Takuhiro Sonoyama, Takahiro Fukuhara, and Takeshi Kano are employees of the study sponsor, Shionogi & Co., Ltd., Osaka, Japan.
Author contributions
Conceptualization: Katsuaki Tanaka, Takamichi Baba, Takuhiro Sonoyama, Takahiro Fukuhara, and Takeshi Kano; Data curation: Takamichi Baba; Formal analysis: Takamichi Baba; Investigation: Katsuaki Tanaka, Takamichi Baba, Manami Yoshida, Motofumi Iguchi, Takuhiro Sonoyama, Takahiro Fukuhara, and Takeshi Kano; Methodology: Takamichi Baba; Project administration: Motofumi Iguchi; Resources: Katsuaki Tanaka, Takahiro Fukuhara, and Takeshi Kano; Supervision: Katsuaki Tanaka and Takeshi Kano; Validation: Takamichi Baba; Visualization: Takamichi Baba and Motofumi Iguchi; Writing – original draft: Manami Yoshida and Motofumi Iguchi; Writing – review and editing: Katsuaki Tanaka, Takamichi Baba, Manami Yoshida, Motofumi Iguchi, Takuhiro Sonoyama, Takahiro Fukuhara, and Takeshi Kano.
Supplemental Material
Download PDF (591 KB)Acknowledgements
The authors would like to thank Keyra Martinez Dunn, MD, of Edanz, Japan, for providing medical writing assistance, which was funded by Shionogi & Co., Ltd., Osaka, Japan, through EMC K.K., Japan, in accordance with Good Publication Practice (GPP3) guidelines (https://www.ismpp.org/gpp3).
References
- Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48(6):1000–1007.
- Giannini EG, Greco A, Marenco S, et al. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol. 2010;8(10):899–902.
- Schiffer CA, Anderson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1519–1538.
- McCullough J. Current issues with platelet transfusion in patients with cancer. Semin Hematol. 2000;37(2 Suppl 4):3–10.
- Perrotta PL, Snyder EL. Non-infectious complications of transfusion therapy. Blood Rev. 2001;15(2):69–83.
- Kim ES. Lusutrombopag: first global approval. Drugs. 2016;76(1):155–158.
- Miller JB, Figueroa EJ, Haug RM, et al. Thrombocytopenia in chronic liver disease and the role of thrombopoietin agonists. Gastroenterol Hepatol. 2019;15(6):326–332.
- Hidaka H, Kurosaki M, Tanaka H, et al. Lusutrombopag reduces need for platelet transfusion in patients with thrombocytopenia undergoing invasive procedures. Clin Gastroenterol Hepatol. 2019;17(6):1192–1200.
- Peck-Radosavljevic M, Simon K, Iacobellis A, et al. Lusutrombopag for the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures (L-PLUS 2). Hepatology. 2019;70(4):1336–1348.
- Tateishi R, Seike M, Kudo M, et al. A randomized controlled trial of lusutrombopag in Japanese patients with chronic liver disease undergoing radiofrequency ablation. J Gastroenterol. 2019;54(2):171–181.
- Sasaki R, Shiino C, Imawari M, et al. Safety and effectiveness of lusutrombopag in Japanese chronic liver disease patients with thrombocytopenia undergoing invasive procedures: interim results of a postmarketing surveillance. Hepatol Res. 2019;49(10):1169–1181.
- Mitchell O, Feldman DM, Diakow M, et al. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39–50.
- Qamar AA, Grace ND. Abnormal hematological indices in cirrhosis. Can J Gastroenterol. 2009;23(6):441–445.
- Latorre R, Vaquero J, Rincón D, et al. Determinants of platelet count are different in patients with compensated and decompensated cirrhosis. Liver Int. 2016;36(2):232–239.
- Hirooka M, Ochi H, Hiraoka A, et al. Role of severe thrombocytopenia in preventing platelet count recovery in thrombocytopenic patients with chronic liver disease. J Gastroenterol Hepatol. 2020;35(2):299–304.
- Uojima H, Arase Y, Itokawa N, et al. Relationship between response to lusutrombopag and splenic volume. World J Gastroenterol. 2018;24(46):5271–5279.
- Dmitrienko A, Koch GG. Analysis of clinical trials using SAS: a practical guide. 2nd ed. Cary, (NC): SAS Institute Inc.; 2017.
- Goulis J, Chau TN, Jordan S, et al. Thrombopoietin concentrations are low in patients with cirrhosis and thrombocytopenia and are restored after orthotopic liver transplantation. Gut. 1999;44(5):754–758.
- Martin TG, 3rd, Somberg KA, Meng YG, et al. Thrombopoietin levels in patients with cirrhosis before and after orthotopic liver transplantation. Ann Intern Med. 1997;127(4):285–288.
- Peck-Radosavljevic M, Zacherl J, Meng YG, et al. Is inadequate thrombopoietin production a major cause of thrombocytopenia in cirrhosis of the liver? J Hepatol. 1997;27(1):127–131.
- Aref S, Mabed M, Selim T, et al. Thrombopoietin (TPO) levels in hepatic patients with thrombocytopenia. Hematology. 2004;9(5–6):351–356.
- Schöffski P, Tacke F, Trautwein C, et al. Thrombopoietin serum levels are elevated in patients with hepatitis B/C infection compared to other causes of chronic liver disease. Liver. 2002;22(2):114–120.
- Stockelberg D, Andersson P, Björnsson E, et al. Plasma thrombopoietin levels in liver cirrhosis and kidney failure. J Intern Med. 1999;246(5):471–475.
- Vyzantiadis T, Theodoridou S, Giouleme O, et al. Serum thrombopoietin levels in thrombocytopenic patients with liver cirrhosis. Haematologica. 2002;87(8):890–891.
- Adinolfi LE, Giordano MG, Andreana A, et al. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol. 2001;113(3):590–595.
- Brown RS, Imawari M, Izumi N, et al. Assessing the periprocedural magnitude of platelet count change in response to lusutrombopag. JHEP Rep. 2021;3(2):100228.
- Lu YF, Li XQ, Han XY, et al. Peripheral blood cell variations in cirrhotic portal hypertension patients with hypersplenism. Asian Pac J Trop Med. 2013;6(8):663–666.
- Eissa LA, Gad LS, Rabie AM, et al. Thrombopoietin level in patients with chronic liver diseases. Ann Hepatol. 2008;7(3):235–244.
- Jelkmann W. The role of the liver in the production of thrombopoietin compared with erythropoietin. Eur J Gastroenterol Hepatol. 2001;13(7):791–801.