Abstract
Objective
To compare changes in systolic and diastolic blood pressures (SBP, DBP) from baseline to following 8 weeks of treatment with a low dose combination of amlodipine 5 mg plus bisoprolol 5 mg versus up titration to the maximum daily dose of amlodipine 10 mg, in hypertensive patients uncontrolled by amlodipine 5 mg.
Methods
Individual patient data (IPD) from a randomized clinical trial (RCT) comparing the combination versus amlodipine 5 mg (EudraCT Number: 2019-000751-13) and aggregated data (AgD) from a published RCT comparing amlodipine 10 mg versus amlodipine 5 mg were utilized in an anchored simulated treatment comparison (STC). The RCT with IPD was used to create models assessing how patients might respond to the combination if they were more comparable to those patients in the RCT with AgD. A population-adjusted indirect comparison of the treatment strategies was then conducted, using amlodipine 5 mg as an anchor.
Results
In the efficacy analyses, a total of 261 patients were included in the amlodipine 10 mg arm of the RCT with AgD; and a total of 178 patients in the low-dose combination arm of the RCT with IPD. Respectively, in the Amlodipine 10 mg arm and in the low-dose combination arm, the mean age was 54.3 years-old (Standard deviation [SD] 10.6), and 57.1 years-old (13.7); 8.7% and 18.8% of patients were diabetics; and the mean baseline SBP/DBP was 149.3 (12.0)/96.5 (4.7) mmHg, and 148.8 (8.2)/90.2 (7.6) mmHg. The final model for SBP and DBP included the following variables: baseline SBP, baseline DBP, duration of hypertension, age, concomitant diabetes, sex, smoking history (final model for SBP only), and body mass index (final model for DBP only). Mean treatment differences (standard error [SE]) at 8 weeks between the combination and uptitration were −1.6 mmHg (1.9) for SBP; and −3.3 mmHg (1.3) for DBP.
Conclusion
In this indirect comparison, a more important decrease was observed in DBP with the low-dose combination as compared to the alternative therapeutic approach of up-titration from amlodipine 5 mg to amlodipine 10 mg. No meaningful difference was seen for SBP.
1. Introduction
1.1. Rationale
Antihypertensive drug monotherapy is a recommended therapeutic approach in patients with mild blood pressure (BP) elevation and low-to-moderate cardiovascular riskCitation1. The choice of antihypertensive drugs used depends on associated comorbidities. It is proposed to follow the recommendation of the international guidelines that provide treatment recommendations for different hypertensive patient groups, considering ethnicity, age, and comorbidities such as angina pectoris, chronic heart failure, post-myocardial infarction, sleep apnoea etcCitation1.
Several studies have demonstrated the efficacy of bisoprolol or amlodipine as monotherapy in management of essential hypertensionCitation2, but the ability of any agent used alone to achieve target BP values is limitedCitation3. For example, in a study in 762 patients with mild to moderate hypertension, 545 (72%) did not achieve diastolic BP (DBP) <90 mmHg after 4 weeks of monotherapy with amlodipine 5 mgCitation4.
Increasing the dose of amlodipine is likely to increase the risk of adverse effectsCitation1. In a randomized clinical trial (RCT) of patients uncontrolled on amlodipine 5 mg, 3.4% of patients who remained on amlodipine 5 mg and 11.2% of patients uptitrated to amlodipine 10 mg experienced peripheral edemaCitation4.
Except for very mild hypertension and old or frail patients, the guideline from the European Society of Cardiology and the European Society of Hypertension recommends a low-dose combination therapy rather than increasing the dosage of monotherapy to the maximum daily dose if BP is not sufficiently controlled on monotherapyCitation1. Antihypertensive combinations with distinct and complementary mechanisms of action are preferredCitation1. Amlodipine is a vascular selective calcium antagonist that lowers total peripheral vascular resistance, while bisoprolol is a selective β-1 adrenoceptor blocker that inhibits renin release and reduces sympathetic drive and, thus, cardiac output. A low-dose, fixed-dose combination may also improve patients’ adherence to therapy by lowering the pill burden and potential for polypharmacy.
A multicenter, placebo-controlled, 8-week, phase III, double-blind RCT (referred to henceforth as the BA5-A5 study) demonstrated superiority in terms of BP decrease for a combination of amlodipine 5 mg plus bisoprolol 5 mg versus amlodipine 5 mg alone, in a population with uncontrolled hypertension (systolic BP [SBP] ≥ 140 mmHg) on monotherapy with amlodipine 5 mg (EudraCT Number: 2019-000751-13)Citation5. No RCT has provided a direct comparison between uptitrating amlodipine and switching to low-dose amlodipine-bisoprolol combination therapy for patients with BP uncontrolled on amlodipine 5 mg alone. We have used data from existing RCTsCitation5,Citation6 to conduct a population-adjusted, indirect comparison of these two treatment strategies in this population.
1.2. Objective
The main objective of the study was to compare the changes in SBP and DBP from baseline to week 8 between the low-dose combination of amlodipine 5 mg plus bisoprolol 5 mg versus up-titration to the maximum daily dose of amlodipine (10 mg), in hypertensive patients uncontrolled on amlodipine 5 mg.
2. Material and methods
Individual patient data (IPD) from the BA5-A5 study (EudraCT Number: 2019-000751-13) comparing the combination versus amlodipine 5 mg was availableCitation5. Aggregated data (AgD) from published RCTs comparing amlodipine 10 mg versus amlodipine 5 mg was identified via a systematic literature review (see Section 2.1). We, then conducted a population-adjusted indirect comparison, more specifically an anchored simulated treatment comparison (STC) using the IPD and AgD described above. This allowed adjustment for potential between-trial imbalances in the distribution of the described covariates such as diabetes or baseline DBPCitation7.
2.1. Part 1 – Systematic literature review
We conducted a systematic literature review to identify RCTs reporting the difference in BP decrease at week 8 following an up-titration from amlodipine 5 mg to amlodipine 10 mg, compared to maintaining amlodipine 5 mg. The systematic literature search followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020Citation8 (further details of the systematic review, including the PRISMA checklist, are provided in an online Supplementary material to this article).
Studies to be included in the systematic literature review were: clinical trials completed or published between 2001 and 30 April 2021 that enrolled hypertensive European patients older than 18 years who were inadequately controlled after at least 4 weeks of amlodipine 5 mg monotherapy; randomized to amlodipine 10 mg monotherapy or amlodipine 5 mg ± placebo; assessed changes in mean sitting SBP or DBP following 8 weeks of therapy in both arms; enrolled a sample size consistent with a provided power calculation; and were published in English.
The following electronic databases were searched on 11 May 2021: NLM MEDLINE File (MEDLINE), Excerpta Medica (EMBASE), ClinicalTrials.gov, and the WHO ICTRP. No timelines or language restrictions were applied at the search stage. More details of the Search Strategy are available in the online Supplementary material. Duplicate references between the four sources were identified manually by one reviewer. Two independent reviewers then performed the data screening simultaneously, in parallel. A third reviewer reconciled any discrepancies.
Only one RCTCitation6,Citation9 was identified as relevant for the purpose of this analysis (NCT00558428). This study had a low risk of bias according to the revised Cochrane risk-of-bias tool for RCTsCitation10 (the evaluation was performed by one reviewer and is available in the online Supplementary material).
Briefly (see the online Supplementary material for more details), NCT00558428 was a multicenter, multinational, 8-week randomized, double-blind, parallel-arm study that evaluated the efficacy and safety of telmisartan 40 mg/amlodipine 5 mg, telmisartan 80 mg/amlodipine 5 mg, amlodipine 10 mg, and amlodipine 5 mg, in adult patients with hypertension uncontrolled on amlodipine 5 mg monotherapy. All patients underwent a 6-week run-in treatment with amlodipine 5 mg. Patients who failed to respond adequately to treatment with amlodipine monotherapy (DBP <90 mm Hg) were randomized (1:1:1:1) to one of the four treatment armsCitation6. Baseline characteristics of patients in the amlodipine 10 mg arm are shown in , and of patients in the amlodipine 5 mg arm in Supplementary material.
Table 1. Baseline characteristics and blood pressure outcomes of the studies that contributed data to the model.
The conventional approach to compare results from the published literature is by using standard indirect comparison methodsCitation11,Citation12. However, these methods are only based on AgD and, therefore, limited by cross-trial differences. The distribution of sex, and body mass index, in the amlodipine 5 mg arms were similar between the NCT00558428 and BA5-A5 studies, but patients in the BA5-A5 study were slightly older, had a lower baseline DBP and presented more frequently with diabetes at baseline ().
2.2. Part 2 - Population-adjusted indirect comparison: anchored simulated treatment comparison
In case of cross-trial differences, population-adjusted indirect comparisonsCitation13,Citation14 applying outcome regression methods such as STC allow for consideration of all potential effect modifiers that are imbalanced between the compared populationsCitation15. The population-adjusted indirect comparison was done via an anchored STC (the term “anchored” refers to the amlodipine 5 mg arm present in both studies that contributed to the analysis).
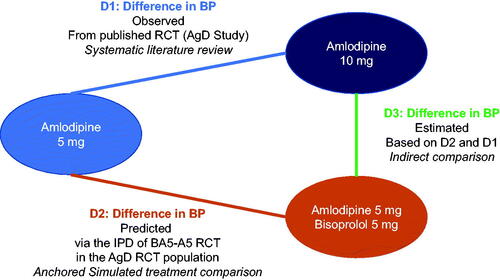
The IPD from the BA5-A5 study was used to create models assessing how patients might respond to the combination if they were more comparable to those patients in the NCT00558428 study with AgD. This comparability was assessed based on the distribution of fundamental baseline characteristics present in both RCTs. It enabled calculation of a predicted difference in BP decrease between amlodipine 5 mg and the combination (D2 in ) in a population closer to the NCT00558428 study. This predicted difference in BP decrease (D2) and the observed difference in BP decrease between amlodipine 10 mg and 5 mg in the NCT00558428 study (D1 in ) were then used to perform a population-adjusted indirect comparison. These intermediate results allowed us to estimate the difference in BP decrease between the combination and amlodipine 10 mg (D3 in ).
Figure 1. Design of the model.
Abbreviations. AgD, aggregated data; BP, blood pressure; D1, difference in BP observed; D2, difference in BP predicted; IPD, individual patient data; RCT, randomized clinical trial.
2.2.1. Selection of variables for the simulated treatment comparison: effect modifiers and prognostic variables
An effect modifier is a covariate (not necessarily a prognostic factor) that alters the effect of treatment on outcome events, according to levels of the effect modifier in different treatment groups. Anchored indirect comparisons performed via outcome regression methods such as STC should adjust for all imbalanced effect modifiers to reduce bias. Also, they may adjust for prognostic variables to improve model fit and to reduce standard error (SE)Citation16.
Only effect modifiers or prognostic variables available in both the NCT00558428 and BA5-A5 studies were considered. Thus, the following variables were integrated as effect modifiers in the outcome models: baseline systolic and diastolic BP, duration of arterial hypertension, age, and concomitant diabetes. This was consistent with the results of a literature review of potential effect modifiers (detailed in the online Supplementary material) that identified baseline BP and the presence of diabetes as principal effect modifiers of antihypertensive therapyCitation17. The following prognostic variables were considered in the models as well: sex, body mass index, and smoking.
2.2.2. Population
Our analysis was based on all randomized patients in the study BA5-A5 who took at least one dose of double-blind trial medication and for whom a baseline BP measurement and at least one post-dose trough BP measurement were available. This was the population analysed for the efficacy endpoint of the study NCT00558428.
2.2.3. Statistical methods
The BA5-A5 and NCT00558428 studies measured BP using the same approach. Efficacy outcomes were assessed after 8 weeks of treatment or at last observation during the follow-up period (i.e. last observation carried forward)Citation6.
Two different models were run, one for SBP, and one for DBP. The applied statistical concept can be described in three steps, summarized in : (1) Development of a linear regression model for the outcome “BP at week 8” based on IPD, (2) Estimation of the relative treatment effects of low-dose amlodipine/bisoprolol combination therapy and amlodipine 5 mg in the AgD, (3) Population-adjusted indirect comparison between low-dose amlodipine/bisoprolol combination therapy and amlodipine 10 mg.
Table 2. Fundamental steps in development of the statistical model.
2.3. Ethics approval and informed consent
The BA5-A5 study (EudraCT Number: 2019-000751-13) with IPD was already approved by an Independent Ethics Committee (Medical Chamber in Gdańsk) on the 26 February 2019 before any trial-related activities was carried out. The subjects’ informed consent to participate in this RCT were given before any trial-related activities were carried out.
For this indirect comparison, the study design did not involve collection of additional data or interaction with any subjects enrolled. Thus, additional approval of the protocol research or informed consent was not needed.
3. Results
Patients with at least a baseline and follow-up BP measurement were included in the assessment of the efficacy outcomes. In the study with AgD NCT00558428, this corresponded to a total of 255 (96%) randomized patients in the amlodipine 5 mg arm, and 261 (95%) in the amlodipine 10 mg arm; in the BA5-A5 study with IPD, this corresponded to a total of 179 (96%) in the amlodipine 5 mg arm, and 178 (98%) in the low-dose combination arm (). Respectively, in the amlodipine 10 mg arm and in the low-dose combination arm, the mean (SD) age was 54.3 years-old (10.6), and 57.1 years-old (13.7); 36.2% and 49.7% of patients were female; 8.7% and 18.8% presented with diabetes; and the mean baseline SBP/DBP (SD) was 149.3 (12.0)/96.5 (4.7) mmHg, and 148.8 (8.2)/90.2 (7.6) mmHg ().
Step 1 (): The final model for SBP included the following variables: baseline SBP, baseline DBP, duration of hypertension, age, concomitant diabetes, sex, and smoking history. The final model for DBP included the following variables: baseline SBP, baseline DBP, duration of hypertension, age, concomitant diabetes, sex, and body mass index. An analysis of diagnostic plots for the systolic and diastolic model indicated no violation of the model assumptions. Linearity and normal distributed error terms appeared plausible.
Step 2 (): The predicted difference in BP decreases between the combination and amlodipine 5 mg, in a population comparable to the study with AgD NCT00558428, was −6.5 mmHg (SE, 1.8) in systolic BP and −5.5 mmHg (SE, 1.2) in diastolic BP ().
Table 3. Blood pressure outcomes based on the simulated treatment comparison.
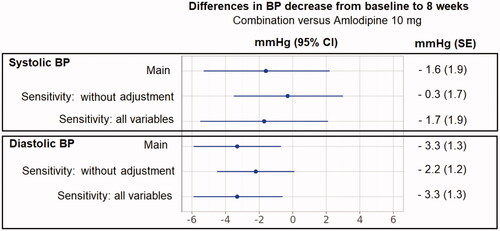
Step 3 (): Amlodipine 5 mg/bisoprolol 5 mg combination therapy reduced DBP compared with amlodipine 10 mg monotherapy (mean treatment difference −3.3 mmHg [SE, 1.3; 95% CI −5.9, −0.7]). The combination therapy and amlodipine 10 mg exerted similar effects on SBP (mean treatment difference −1.6 [SE, 1.9; 95%CI −5.3, 2.2]).
Two sensitivity analyses were conducted. The first one was a standard indirect comparison, without any population adjustment. The second one was a population-adjusted indirect comparison including all selected prognostic variables without variable selection (Step 1). These analyses provided results similar to the main analysis ().
Figure 2. Differences in BP decrease from baseline to 8 weeks for amlodipine 5 mg/bisoprolol 5 mg combination therapy versus amlodipine 10 mg monotherapy.
Abbreviations. BP, Blood pressure; CI, Confidence intervals; SE, standard error. “Main analysis”: the final model for SBP and DBP included the following variables: baseline SBP, baseline DBP, duration of hypertension, age, concomitant diabetes, sex, smoking history (final model for SBP only), and body mass index (final model for DBP only). “Sensitivity: without adjustment” refers to a standard indirect comparison, without any population adjustment. “Sensitivity: all variables” refers to an analysis which includes all selected prognostic variables, without variable selection meaning with addition of body mass index for SBP and smoking history for DBP (Step 1 in ).
Furthermore, without any population adjustment, 116 (65.2%) and 103 (39.5%) patients were observed to achieve a BP < 140/90 mmHg, in the low-dose combination arm, and the amlodipine 10 mg arm, respectivelyCitation9.
4. Discussion
To our knowledge, this is the first study to compare the low-dose combination of amlodipine 5 mg and bisoprolol 5 mg as an alternative therapeutic approach to up-titration to the maximum daily dose of amlodipine 10 mg. In this indirect comparison, no meaningful difference was found for the effects of the combination vs. amlodipine 10 mg on SBP, although a decrease in DBP was observed with the combination. The primary and sensitivity analyses led to similar conclusions.
These results can be put into perspective with the non-inferiority margin of 2 mmHg defined for DBP in the NCT00558428 study, the upper 95%CI for the difference in effect on SBP between these treatments (2.2 mmHg) was close to this non-inferiority margin.
The use of an antihypertensive regimen containing amlodipine 5 mg rather than 10 mg might contribute to limiting the frequency of dose-dependent adverse effects of amlodipine, such as edema. In the NCT00558428 study, 8.2% of patients in the amlodipine 5 mg arm experienced peripheral edema, compared with 26.8% of patients in the amlodipine 10 mg armCitation6. In the BA5-A5 study, 1.1% and 2.2% of patients experienced peripheral edema in the combination arm (which included treatment with amlodipine 5 mg) and amlodipine 5 mg monotherapy arm, respectively. Population-adjusted indirect comparisons are only meaningful if effect modifiers are accounted for in the analysis. However, to the contrary of the BP outcomes, the effect modifiers for the safety outcomes were not described in the published results of the NCT00558428 study, thus the interpretation of such analysis would be limited. Furthermore, a decrease in the frequency of peripheral edema would need to be set against the potential for appearance of side-effects due to the addition of bisoprolol.
Inability to account for race/ethnicity as an effect modifier was a limitation of our study. All patients included in the BA5-A5 study, and 77% of patients in the NCT00558428 study were Caucasian (20% were Asian, and 2% Black in NCT00558428)Citation6. Caucasian and Asian populations would be expected to present a similar BP response when up-titrated from amlodipine 5 mg to 10 mgCitation18. Black patients tend to respond to amlodipine with greater BP reductions compared to Caucasian patientsCitation19. Black race would be unlikely to act as a potential effect modifier in this analysis, as very few Black patients contributed data to the analysis as described above, although this may limit the generalisability of our results to populations of hypertensive patients with a more diverse racial/ethnic mix. We were also unable to account for effect modifiers that were not described adequately in either of our source studies (e.g. dyslipidemia, myocardial infarction, stroke, chronic renal failure, serum uric acid), although we were able to include diabetes and baseline BP, the most relevant effect modifiersCitation17. Furthermore, our systematic literature review retrieved only one RCT that met our inclusion criteria and the NCT00558428 study might not be representative of a broader population of patients with hypertension. However, another RCT was identified during our systematic literature reviewCitation4, but evaluating the BP decrease at week 6, and was therefore not eligible for our analysis. In this study, the BP decrease at 6 weeks was similar to the one at 8 weeks in the NCT00558428 (e.g. mean changes in SBP/DBP from baseline of −9.8/–8.3 mmHg and −11.1/8.0 mmHg for amlodipine 10 mg, respectively in Drummond et al.Citation4 and NCT00558428). This provides some reassurance that the study on which we based our analysis provided data typical of the effects of amlodipine on BP in patients with hypertension. Finally, this study was an indirect comparison, via the amlodipine 5 mg arm, and it might be of interest to test the robustness of the findings with a direct comparison between the combination and amlodipine 10 mg.
Due to bisoprolol intrinsic properties, further to BP, it would bring value to compare the pulse pressure, the mean arterial pressure, and the heart rate between the low-dose combination and amlodipine 10 mg. However, these were not part of the outcomes of the NCT00558428 trial, and thus the present study could not address this research question.
5. Conclusions
In this indirect comparison, conducted in patients with hypertension inadequately controlled by amlodipine 5 mg monotherapy, a more important decrease was observed in DBP with the low-dose combination as compared to the alternative therapeutic approach of up-titration from amlodipine 5 mg to amlodipine 10 mg. No meaningful difference was seen for SBP. Administration of low-dose combination therapy may be a rational therapeutic strategy for this population.
Transparency
Declaration of funding
This study was sponsored by Merck KGaA (CrossRef Funder ID: 10.13039/100009945).
Declaration of financial/other relationships
All authors are employed by Merck Healthcare KGaA, Darmstadt, Germany. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
CF, JF, and UH were involved in the conception and design, interpretation of the data. JF conducted the analysis. CF drafted the paper, JF, and UH revised it critically for intellectual content. All authors have approved the final version and agree to be accountable for all aspects of the work.
Supplemental.docx
Download MS Word (141.3 KB)Acknowledgements
Dr Mike Gwilt (GT Communications) edited a draft written by the authors for English and style, funded by Merck Healthcare KGaA.
The authors thank Professor Zbigniew A Gaciong (Department of Internal Medicine, Hypertension and Vascular Diseases The Medical University of Warsaw 1a Banacha Street, 02 097 Warsaw, Poland), and the Investigator of the BA5-A5 study, for sharing the individual patient level data to make this work possible.
References
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104.
- Geddes JS. Calcium antagonists and beta blockade–a useful combination. Postgrad Med J. 1983;59 (Suppl 2):62–69.
- Schrader J, Salvetti A, Calvo C, et al. The combination of amlodipine/valsartan 5/160 mg produces less peripheral oedema than amlodipine 10 mg in hypertensive patients not adequately controlled with amlodipine 5 mg. Int J Clin Pract. 2009;63(2):217–225.
- Drummond W, Munger MA, Rafique Essop M, et al. Antihypertensive efficacy of the oral direct renin inhibitor aliskiren as add-on therapy in patients not responding to amlodipine monotherapy. J Clin Hypertens Greenwich Conn. 2007;9(10):742–750.
- EudraCT Number: 2019-000751-13. EU Clinical Trials Register; [cited 2021 Nov 26]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-000751-13/PL.
- Neldam S, Lang M, Jones R. Telmisartan and amlodipine single-pill combinations vs amlodipine monotherapy for superior blood pressure lowering and improved tolerability in patients with uncontrolled hypertension: results of the TEAMSTA-5 study. J Clin Hypertens Greenwich Conn. 2011;13(7):459–466.
- Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–211.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Boehringer Ingelheim. An Eight-week Randomized, 4-arm, Double-blind Study to Compare the Efficacy and Safety of Combinations of Telmisartan 40mg + Amlodipine 5mg Versus Telmisartan 80mg + Amlodipine 5mg Versus Amlodipine 5mg Versus Amlodipine 10mg Monotherapy in Patients With Hypertension Who Fail to Respond Adequately to Treatment With Amlodipine 5mg Monotherapy. Clinical Trial Registration NCT00558428, clinicaltrials.gov; [cited 2014 Oct 17]. Available from:https://clinicaltrials.gov/ct2/show/NCT00558428.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
- Lumley T. Network Meta-analysis for indirect treatment comparisons. Stat Med. 2002;21(16):2313–2324.
- Caro JJ, Ishak KJ. No head-to-head trial? Simulate the missing arms. PharmacoEconomics. 2010;28(10):957–967.
- Ishak KJ, Proskorovsky I, Benedict A. Simulation and matching-based approaches for indirect comparison of treatments. PharmacoEconomics. 2015;33(6):537–549.
- Phillippo DM, Dias S, Ades AE, et al. Assessing the performance of population adjustment methods for anchored indirect comparisons: a simulation study. Stat Med. 2020;39(30):4885–4911.
- Phillippo D, Ades AE, Dias S, et al. NICE DSU Technical Support Document 18: Methods for population-adjusted indirect comparisons in submission to NICE. 2016. Available from: http://nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf
- Campo C, Segura J, Ruilope LM. Factors influencing the systolic blood pressure response to drug therapy. J Clin Hypertens. 2002;4(1):35–40.
- Kario K, Robbins J, Jeffers BW. Titration of amlodipine to higher doses: a comparison of Asian and Western experience. Vasc Health Risk Manag. 2013; 9:695–701.
- Lindhorst J, Alexander N, Blignaut J, et al. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr. 2007;18(4):241–247.
