Abstract
Objective
To examine the healthcare utilization and costs associated with colorectal cancer (CRC) screening by colonoscopy, including costs associated with post-endoscopy events, among average-risk adults covered by Medicaid insurance.
Methods
This cohort study evaluated a population of adults (ages 50–75 years) with CRC screening between 1/1/2014 and 12/31/2018 (index = earliest test) from the IBM MarketScan Multi-State Medicaid database. Individuals at above-average risk for CRC or with prior CRC screening were excluded. CRC screening was reported by screening type: colonoscopy, fecal immunochemical test [FIT], fecal occult blood test [FOBT], multi-target stool DNA [mt-sDNA]. Frequency and costs of events potentially related to colonoscopy (defined as occurring within 30 days post-endoscopy) were reported overall, by event type, and by individual event.
Results
We identified a total of 13,134 average-risk adults covered by Medicaid insurance who received screening by colonoscopy; 63.6% (8350) had Medicare dual-eligibility while 36.4% (4785) did not have Medicare dual-eligibility. The mean (SD) cost of a colonoscopy procedure was $684 ($907) and mean (SD) out-of-pocket costs were $6 ($132). Serious gastrointestinal (GI) events (perforation and bleeding) were observed in 4.6% of individuals with colonoscopy, 4.3% had other GI events, and 3.0% had an incident cardiovascular/cerebrovascular event. Mean (SD) event-related costs were $1233 ($5784) among individuals with a serious GI event, $747 ($1961) among individuals with other GI events, and $4398 ($19,369) among individuals with a cardiovascular/cerebrovascular event.
Conclusions
This large, claims-based cohort study reports average (SD) out-of-pocket costs for Medicaid beneficiaries at $6 ($132), which could be one factor contributing to the accessibility of CRC screening by colonoscopy. The incidence of events potentially associated with colonoscopy (i.e. within 30 days after the screening) was 3–4%, and the event-related costs were considerable.
Introduction
While the incidence and mortality of colorectal cancer (CRC) have been slowly decreasingCitation1, CRC is the second-most common cause of cancer-related death in the United States (US), accounting for an estimated 53,200 deaths in 2020Citation2,Citation3. In the US, the lifetime risk of CRC is 4.4% among men and 4.1% among womenCitation3. The median age at diagnosis is 67, and older age is the main risk factor for the development of CRCCitation4.
For average-risk, asymptomatic individuals during the time period when this study was conducted, the 2016 United States Preventive Services Task Force (USPSTF) guidelines recommend CRC screening beginning at age 50 years and continuing until age 75 yearsCitation5. While several modes of CRC screening are available in the US, including fecal immunochemical testing (FIT) and fecal occult blood testing (FOBT), colonoscopy is the most commonly used modality, particularly among adults with public insurance such as Medicaid or MedicareCitation6,Citation7. Screening with colonoscopy allows for visual detection and removal of adenomatous polyps contributing to reduced overall mortality from CRCCitation8. However, it is associated with potentially serious gastrointestinal (GI) events and an unpleasant bowel preparation, which, if not performed correctly, can compromise the screening efficacy and result in repeat screeningCitation9–12.
While the overall screening rate remains below the National Colorectal Cancer Roundtable goal of 80%Citation13–15, the rate is particularly low among the uninsured due to the cost and access barriersCitation16,Citation17. Medicaid expansions from the Affordable Care Act have resulted in a significant increase in insurance access and CRC screening among low-income individualsCitation18,Citation19. However, the costs associated with CRC screening by colonoscopy in individuals covered by Medicaid insurance are not reported in the literature. The primary objective of this study was to provide the healthcare utilization and costs associated with colonoscopy with and without polypectomy and describe the frequency and costs of serious GI and other GI events following colonoscopy among a population of adults covered by Medicaid insurance. A secondary analysis examined the frequency and costs of cardiovascular events following a colonoscopy.
Methods
Study design and data source
This cohort study used administrative claims from the IBM MarketScan Multi-State Medicaid Database from January 1, 2004 to December 31, 2018. The Medicaid database contains the pooled healthcare experience of Medicaid enrollees from multiple, geographically dispersed states. The Medicaid files include records of inpatient services, inpatient admission, outpatient services, and outpatient prescription drug claims. All database records are statistically de-identified and certified to be fully compliant with US patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from Institutional Review Board approval. All variables used to define study outcomes were obtained using medical codes such as the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM), the Current Procedural Terminology fourth edition (CPT-4), and the Healthcare Common Procedure Coding System (HCPCS).
Cohort selection
A population of adults with evidence of CRC screening (colonoscopy, CT colonography, double-contrast barium enema, flexible sigmoidoscopy, FIT, FOBT, or multi-target stool DNA assay) between January 1, 2014 and December 31, 2018 were identified from the Marketscan Multi-State Medicaid Database. The date of their earliest claim for CRC screening during the selection window was defined as the index date.
Individuals were required to be 45–75 years old on the index date and have continuous enrollment with medical and pharmacy benefits for ten years prior to the index date (pre-index period). This pre-index period was used to exclude individuals who were not due for screening along with those who were at above average risk of CRC. Individuals with evidence of not being due for screening included those with any claims for colonoscopy (screening or diagnostic) during the 10 years prior to the index date, any claims for flexible sigmoidoscopy, double-contrast barium enema, or CT colonography in the five years prior to the index date, any claims for multi-target stool DNA assay during the three years prior to the index date, or any claims for FIT or FOBT during the year prior to the index date. Evidence of being at above average risk for CRC included any claims indicating a family history of GI cancer or non-diagnostic claims with a diagnosis of colorectal polyps, colorectal neoplasm (benign or malignant), or inflammatory bowel disease (ulcerative colitis or Crohn's disease). In addition, to further refine the population to individuals receiving routine CRC screening rather than diagnostic procedures, patients with at least one non-diagnostic claim with a diagnosis of blood in the stool in the three months preceding the index date were excluded. Diagnostic claims are claims for services often used in ruling out conditions and are not reliable for identifying individuals with a specific diagnosis. All medical codes used in this patient selection algorithm are reported in Supplementary Table 1.
The final study population was restricted to individuals screened by colonoscopy on the index date, who were at least 50 years of age on the index date and had at least 30 days of continuous enrollment, inclusive of the index date, in the Marketscan Multi-State Medicaid Database following the index date. Individuals with more than one screening test on the index date were excluded.
Outcomes
Demographic characteristics were abstracted from the index date and included age, sex, race, insurance plan type, dual eligibility with Medicare, urban residency, and index year. The Deyo-Charlson Comorbidity Index was measured using data from the 1 year immediately preceding the index dateCitation20. The proportion of individuals with each condition used in calculating the Deyo-Charlson Comorbidity Index during this 1-year period was also reported.
Costs associated with a screening colonoscopy, including colonoscopy, pathology, anesthesia, and prescription bowel preparation costs, were examined in the 30-days prior to and following the index date. The generic names of medications used to identify anesthesia and bowel preparation claims are listed in Supplementary Table 2. The procedures codes used to identify colonoscopy with and without polypectomy, pathology, and anesthesia are listed in Supplementary Table 1. The costs of colonoscopy were stratified by those with and without dual eligibility with Medicare and by those with and without polyp removal. Costs were reported as total, health plan paid, and out-of-pocket costs. A subgroup analysis was done on the portion of individuals with claims for pathology, anesthesia, and prescription bowel preparation, as this was hypothesized to be the highest cost subgroupCitation21.
GI and cardiovascular events that may have been associated with colonoscopy were measured during the 30 days following the initial colonoscopy (inclusive of the index date). A diagnosis was only included in the analysis if the individual did not have the same diagnosis during the 12 months preceding the index date. The primary analysis examined serious GI events (perforation and bleeding) and other GI events (paralytic ileus, nausea/vomiting/dehydration, and abdominal pain). A secondary analysis examined cardiovascular/cerebrovascular events (myocardial infarction/angina, arrhythmia, heart failure, cardiac/respiratory arrest, syncope/hypotension/shock, stroke, and transient ischemic attack).
For each event, two cost measures were calculated in the 30 days including and following the index colonoscopy screening employing methods consistent with prior studiesCitation10,Citation12. First, event-related costs captured only claims with a diagnosis of the event of interest or on pharmacy claims for the therapeutic class related to the event of interest. The generic names of medications used to identify pharmacy claims are listed in Supplementary Table 2. Second, all-cause costs were comprehensive of all claims during this period, including the colonoscopy procedure. Events and their associated costs were reported overall, by event type, and by individual event. The costs of events following colonoscopy were stratified by those with and without dual eligibility with Medicare and by those with and without polyp removal.
Healthcare costs were based on paid amounts of adjudicated claims, including insurer and health plan payments, as well as patient cost-sharing in the form of copayment, deductible, and coinsurance. The costs for services provided under capitated arrangements were estimated using payment proxies based on paid claims at the procedure level using the MarketScan Medicaid Multi-State Database. All dollar estimates were inflated by the Medical Care Component of the Consumer Price Index for 2019Citation22.
Analysis
Mean and standard deviation (SD) were reported for continuous variables, and statistical significance was determined using Student's t-tests. Medians were also reported for cost variables. Frequencies and percentages were reported for categorical variables, and statistical significance was determined using Chi-Square tests. The alpha level for all statistical tests was 0.05; however, statistical significance should be interpreted in the context of large sample sizes. All data analyses were conducted using WPS version 4.2 (World Programming, United Kingdom).
Results
Demographics and baseline clinical characteristics
The study included a population of 13,134 adults with Medicare insurance screened by colonoscopy (), who met our inclusion/exclusion criteria. The population had a mean (SD) age of 59.3 (7.2) years, and 25.4% were over the age of 65 (). Overall, 64.3% were female, 47.9% were white, and 63.6% had dual eligibility with Medicare. The mean (SD) Deyo-Charlson Comorbidity Index was 1.9 (2.1).
Figure 1. Cohort Selection. 1CRC screening tests were mt-sDNA, colonoscopy, fecal immunochemical testing (FIT), fecal occult blood testing (FOBT), sigmoidoscopy, CT colonography and double-contrast barium enema (DCBE). 2FIT or FOBT in the year prior to the index date, no evidence of mt-sDNA test in 3 years prior to the index date, no evidence of other screening (sigmoidoscopy, CT colonography or DCBE) in 5 years prior to the index date, and no evidence of colonoscopy in 10 years prior to the index date. 3Codes and conditions for identifying above average-risk adults can be found in Supplementary Table 1.
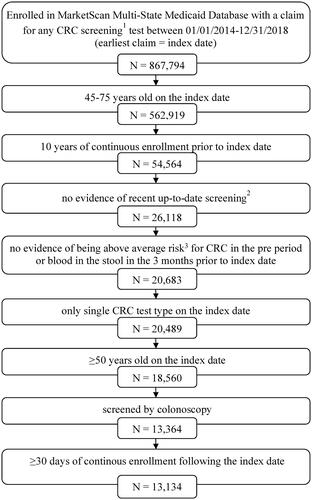
Table 1. Patient characteristics of average-risk adults with screening colonoscopy covered by Medicaid insurance.
Utilization and costs associated with colonoscopy
Among the eligible individuals with a screening colonoscopy, 32.3% had a bowel preparation prescription, 79.0% had an anesthesia claim, and 63.3% had a pathology claim within the 60 days spanning the colonoscopy (). Overall, 8448 (64.3%) had a colonoscopy with polyp removal, and 4686 (35.7%) had a colonoscopy without polyp removal.
Table 2. Utilization and costs associated with screening colonoscopy in the 30 days prior to and following colonoscopy among average-risk adults covered by Medicaid insurance.
Individuals that had dual eligibility with Medicare were less likely to have a bowel preparation prescription (18.2% vs 57.0%) and were less likely to have an anesthesiology claim (74.8% vs 86.2%) but were more likely to have a pathology claim (66.0% vs 58.6%) compared to those without dual eligibility with Medicare (). Moreover, individuals that had a colonoscopy with polyp removal were less likely to have a bowel preparation prescription (30.3%) than those without polyp removal (36.0%, p < .001) and more likely to have a pathology claim (86.9% vs. 20.7%, p < .001) (Supplementary Table 3).
The mean (SD) cost of a colonoscopy was $684 ($907) for adults covered by Medicaid (). The mean (SD) cost of colonoscopy was lower for those with dual eligibility with Medicare ($518 [$881]) compared to those without dual eligibility with Medicare ($973 [$879]). Colonoscopy-related costs were significantly higher for adults with polyp removal compared to those without ($743 [$972] vs. $578 [$765], p < .001). Mean out-of-pocket costs were under $10 ($6 for all patients, $8 with polyp removal, and $3 without polyp removal), and median out-of-pocket costs were $0, regardless of polyp removal. Among the 18.0% (N = 2367) of adults with claims for bowel preparation, anesthesia, and pathology, total colonoscopy-related costs were $897 ($1126).
GI and cardiovascular events and costs following colonoscopy
Serious gastrointestinal events, such as gastrointestinal bleeding, were observed in 4.6% (N = 601) of those with a colonoscopy, while 4.3% (N = 566) had other gastrointestinal events in the 30 days following colonoscopy (). Mean (SD) event-related healthcare services costs in the 30 days following and inclusive of the index date were $1233 ($5784) among individuals with a serious gastrointestinal event and $747 ($1961) among individuals with any other gastrointestinal event. All-cause healthcare costs, inclusive of the colonoscopy procedure, were $6189 ($18,195) among individuals with a serious gastrointestinal event and were $7196 ($24,949) among individuals with any other gastrointestinal event.
Table 3. Gastrointestinal (GI) and cardiovascular/cerebrovascular (CV) events and costs associated with eventsa in the 30 days following colonoscopy among average-risk adults covered by Medicaid insurance.
In adults with Medicaid insurance, a lower percentage of individuals with dual eligibility with Medicare than individuals without dual eligibility with Medicare had a serious GI event (5.9% vs 2.3%) or other GI event (4.5% vs 4.0%; ). Among those with a serious or other GI event, event-related costs were lower among individuals with dual eligibility with Medicare (). In adults with Medicaid insurance, a lower percentage of individuals with polyp removal during colonoscopy than individuals without polyp removal had a serious GI event (4.1% vs 5.5%) or other GI event (3.7% vs 5.4%; ). However, among those with a serious or other GI event, event-related costs were similar between individuals with or without polyp removal ()
We conducted a secondary analysis to describe cardiovascular/cerebrovascular events following a colonoscopy. A total of 3.0% (N = 396) had a cardiovascular or cerebrovascular event in the 30 days following and including the colonoscopy index date. Among those with a cardiovascular or cerebrovascular event, mean (SD) event-related and all-cause costs were $4398 ($19,369) and $12,750 ($30,046), respectively. A full breakdown of event-related and all-cause costs by individual event and among individuals with and without dual eligibility with Medicare and with and without polyp removal can be found in Supplementary Table 4.
Discussion
The study included 13,134 adults who received CRC screening by colonoscopy and had Medicaid insurance coverage. The mean (SD) cost of a colonoscopy was $684 ($907) among all screened individuals covered by Medicaid. The mean (SD) cost of colonoscopy was $518 ($881) among those with dual eligibility with Medicare and was $973 ($879) among those without dual eligibility with Medicare. In the 30 days following and inclusive of the colonoscopy date, 4.6% of adults with Medicaid insurance had a serious gastrointestinal event with mean event-related costs of $1233 ($5784).
Colonoscopy is the most commonly used method of colorectal cancer screening among adults with Medicaid insuranceCitation6,Citation7. Several studies have examined the CRC screening rates in this populationCitation16,Citation23, but the costs associated with CRC screening among those covered by Medicaid are not reported in the literature. We aimed to conduct a detailed analysis, to better understand the cost of CRC screening by colonoscopy as well as the costs of events following colonoscopy among individuals covered by Medicaid insurance.
In a previously published analysis of colonoscopy screening costs among average-risk adults with commercial or Medicare supplemental insurance between 2014 and June 2019, we found the mean cost of a screening colonoscopy was $2200 for adults with commercial insurance and $1486 among adults Medicare supplemental insuranceCitation24. Pyenson et al. estimated the cost of screening colonoscopies in 2010 among adults with commercial or Medicare insurance by calculating all costs on the day of the colonoscopy, excluding facility costs for inpatient staysCitation12. Using this methodology, the average cost per colonoscopy was $2146 for commercially-insured individuals and $1071 for Medicare-insured individuals. By comparison, an analysis of the Centers for Disease Control and Prevention’s (CDC) Colorectal Cancer Control Program (CRCCP), which funds CRC screening for low-income adults who are uninsured or underinsured, reported mean clinical costs of $1150 per colonoscopyCitation25. One limitation of the CDC’s reporting on the CRCCP costs is that the services included in the clinical costs are not clearly defined and likely vary between participating programs.
Prior research has indicated that post-colonoscopy event rates are higher among adults with Medicaid insurance compared to commercial or Medicare insuranceCitation10,Citation24,Citation26. In our analysis of post-colonoscopy events among average-risk adults with commercial or Medicare supplemental insurance between 2014 and June 2019, we reported a serious GI event rate of 1.3% among individuals with commercial or Medicare supplemental insuranceCitation24. Analysis of colonoscopies performed in California between 2005 and 2010 found that adults with Medicaid insurance had 47% higher adjusted odds of a serious GI event, 129% higher adjusted odds of a non-serious GI event, and 141% higher adjusted odds of a cerebrovascular event than adults with commercial insurance in the 30 days following a colonoscopyCitation10. Similarly, a study of colonoscopies in Florida performed between 1997 and 2004 found that adults with Medicaid insurance had 35% higher adjusted odds of a serious GI event than adults with Medicare insuranceCitation26. The reasons for higher serious GI event rates among Medicaid beneficiaries may be due to a higher baseline comorbidity burden or a higher likelihood of being treated by a low-volume endoscopistCitation27.
One challenge with estimating costs using administrative claims data is that not every claim related to treating a condition will include a diagnosis of the condition. Estimating costs based solely on claims with a diagnosis of the condition will underestimate its true cost as it will not capture outpatient prescriptions or claims that would have been less costly or not present had the condition not been present but which did not include the specific diagnosis in the claims record. However, estimating costs by capturing all claims within a defined window will overestimate the true cost as it includes claims for services unrelated to the condition or procedure of interest. This is particularly true for individuals with multiple comorbidities, such as those included in this study who had a mean DCI of 1.9 (which is higher when compared with other CRC studies where the DCI ranges from <1 to 1.5)Citation10,Citation26. For these reasons, we present both event-related costs along with all-cause costs for events following a colonoscopy.
Limitations
There are several limitations of this analysis. First, Medicare pays first for the Medicare-covered services that are also covered by Medicaid; therefore, costs among individuals with dual eligibility with Medicare may be underreported in this analysis. For example, the lower percentage of bowel preparation prescriptions is likely because Medicare paid for the prescription and therefore the claim was not in the Medicaid dataset. Second, this study was limited to individuals with Medicaid health coverage, and coverage of screening colonoscopy will vary based on the adoption of the Affordable Care Act Medicaid expansion in some states. This coverage variability should be considered when interpreting the generalizability of health plan paid and out-of-pocket costs. Third, there is the potential for misclassification of covariates or study outcomes may be present as individuals were identified through administrative claims data as opposed to medical records. As with any claims databases, the data within the MarketScan Research Databases are subject to data coding limitations and data entry errors.
Fourth, conditions newly occurring within 30 days of the colonoscopy procedure were assumed to be events related to the procedure, but some may be randomly occurring proximate events. Fifth, individuals who died within 30 days of the procedure were potentially excluded from the analysis. This may result in an underestimate of the frequency and costs of events with high mortality, such as GI perforation. Finally, this analysis does not capture the cost of over-the-counter medications, such as those for commonly used non-prescription bowel preparation options.
Conclusions
This study provides a detailed analysis of the costs of colonoscopy and events following colonoscopy in a Medicaid-insured population. Costs of screening with colonoscopy as well as the rate and costs of potentially related events in this low-income population with public insurance, who may have a higher comorbidity burden, and less ability to pay for any out-of-pocket costs are important. The average (SD) out-of-pocket costs for Medicaid beneficiaries were $6 ($132) which could be one factor that contributes to the accessibility of CRC screening by colonoscopy (other factors include time off work for the procedure, accessibility of a medical provider, and time/cost of bowel prep). The incidence of events potentially associated with colonoscopy (i.e. within 30 days after the screening) was 3–4%, and event-related costs were considerable. Findings from this analysis will better inform future analyses and assessments of CRC screening strategies.
Transparency
Declaration of funding
This study was funded by Exact Sciences Corporation.
Declaration of financial/other relationships
NP and KW are employed by IBM Watson Health which received funding from Exact Sciences Corporation to conduct this study. DAF is a consultant for Exact Sciences and Guardant Health. LM is an employee of Exact Sciences Corporation. PL serves as Chief Medical Officer for Screening at Exact Sciences through a contracted services agreement with Mayo Clinic. PL and Mayo Clinic have contractual rights to receive royalties through this agreement.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in study design, analysis, and interpretation of the data, drafting and revising the paper and approving the final version of the manuscript. All authors agree to be accountable for all aspects of the work.
Ethics approval and informed consent
All database records are statistically de-identified and certified to be fully compliant with US patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from Institutional Review Board approval.
Supplemental Material
Download MS Word (58 KB)Acknowledgements
Medical Writing services were provided by Jessamine Winer-Jones, Ph.D. of IBM Watson Health. Programming services were provided by Steven Gelwicks of IBM Watson Health. Analytic services were provided by Kathryn DeYoung of IBM Watson Health at the time the analysis was conducted.
Data availability statement
The data that support the findings of this study are available from IBM Watson Health. Restrictions apply to the availability of these data, which were used under license for this study.
References
- Ward EM, Sherman RL, Henley SJ, et al. Annual report to the nation on the status of cancer, featuring cancer in men and women age 20-49 Years. J Natl Cancer Inst. 2019;111(12):1279–1297.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30.
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA A Cancer J Clin. 2020;70(3):145–164.
- SEER. Cancer Stat Facts: Colorectal Cancer. 2020. [cited 2020 August]; Available from: https://seer.cancer.gov/statfacts/html/colorect.html.
- U. S. Preventive Services Task Force. Screening for colorectal cancer: US preventive services task force recommendation statement. Jama. 2016;315(23):2564–2575.
- Klabunde CN, Cronin KA, Breen N, et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1611–1621.
- Wheeler SB, Kuo T-M, Meyer AM, et al. Multilevel predictors of colorectal cancer testing modality among publicly and privately insured people turning 50. Prev Med Rep. 2017;6:9–16.
- Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696.
- Reumkens A, Rondagh EJA, Bakker MC, et al. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol. 2016;111(8):1092–1101.
- Wang L, Mannalithara A, Singh G, et al. Low rates of gastrointestinal and non-gastrointestinal complications for screening or surveillance colonoscopies in a population-based study. Gastroenterology. 2018;154(3):540–555. e8.
- Chokshi RV, Hovis CE, Hollander T, et al. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197–1203.
- Pyenson B, Scammell C, Broulette J. Costs and repeat rates associated with colonoscopy observed in medical claims for commercial and Medicare populations. BMC Health Serv Res. 2014;14(1):92.
- Meester RGS, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121(13):2281–2285.
- Wyatt TE, Pernenkil V, Akinyemiju TF. Trends in breast and colorectal cancer screening among U.S. adults by race, healthcare coverage, and SES before, during, and after the great recession. Prev Med Rep. 2017;7:239–245.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use – United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(10):253–259.
- de Moor JS, Cohen RA, Shapiro JA, et al. Colorectal cancer screening in the United States: Trends from 2008 to 2015 and variation by health insurance coverage. Preventive Medicine. 2018; Jul112:199–206.
- Knight JR, Kanotra S, Siameh S, et al. Understanding barriers to colorectal cancer screening in Kentucky. Prev Chronic Dis. 2015;12:E95.
- Hendryx M, Luo J. Increased cancer screening for low-income adults under the affordable care act Medicaid expansion. Med Care. 2018;56(11):944–949.
- Lyu W, Wehby GL. The impacts of the ACA Medicaid expansions on cancer screening use by primary care provider supply. Med Care. 2019;57(3):202–207.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- Scheiman JM, Fendrick AM, Nuliyalu U, et al. Surprise billing for colonoscopy: the scope of the problem. Ann Intern Med. 2021;174(3):426–428.
- Consumer Price Index details report tables [cited 2020. 1 October]; Available from: https://www.bls.gov/cpi/home.htm.
- Zerhouni YA, Trinh Q-D, Lipsitz S, et al. Effect of Medicaid expansion on colorectal cancer screening rates. Dis Colon Rectum. 2019;62(1):97–103.
- Fisher DA, Princic N, Miller-Wilson LA, et al. Healthcare costs of colorectal cancer screening and events following colonoscopy among commercially insured average-risk adults in the United States. Curr Med Res Opin. 2021;17:1–71.
- Subramanian S, Tangka FKL, Hoover S, et al. Costs of colorectal cancer screening provision in CDC's colorectal cancer control program: comparisons of colonoscopy and FOBT/FIT based screening. Eval Program Plann. 2017;62:73–80.
- Chukmaitov A, Bradley CJ, Dahman B, et al. Association of polypectomy techniques, endoscopist volume, and facility type with colonoscopy complications. Gastrointest Endosc. 2013;77(3):436–446.
- Chukmaitov A, Dahman B, Bradley CJ. Outpatient facility volume, facility type, and the risk of serious colonoscopy-related adverse events in patients with comorbid conditions: a population-based study. Int J Colorectal Dis. 2019;34(7):1203–1210.
