Abstract
Objective
Network meta-analysis was used to derive estimates of the relative efficacy of inclisiran, evolocumab, alirocumab, bempedoic acid, and ezetimibe in patients with hypercholesterolemia and/or at increased cardiovascular risk due to elevated low-density lipoprotein cholesterol taking maximum tolerated dose statins.
Methods
Clinical trials published through February 2021 comparing percent change from baseline in low-density lipoprotein cholesterol were identified via a systematic review. Bayesian network meta-analyses were performed for patients with atherosclerotic cardiovascular disease and/or high cardiovascular risk on maximally tolerated statins in the base case, which included 23 trials.
Results
Results from the base-case analyses demonstrated that inclisiran, evolocumab, and alirocumab provide superior efficacy over placebo, bempedoic acid, and ezetimibe in terms of reduction in low-density lipoprotein cholesterol. Inclisiran was also comparable to alirocumab (mean difference: 0.78% [95% CrI: −8.35, 9.88]) and evolocumab (8.16%, [95% CrI: −1.82, 18.49]). Findings of a scenario which also included trials conducted in patients with heterozygous familial hypercholesterolemia were consistent with the base case. There was evidence of statistical heterogeneity across the included trials, roughly equivalent to variation of 5–10% change in low-density lipoprotein cholesterol, suggesting that any differences between treatments that were greater than 5–10% are generalizable.
Conclusions
This study provides insight regarding the comparative efficacy of drugs for which no head-to-head trials exist and suggests that inclisiran, alirocumab, and evolocumab are expected to provide similar clinically meaningful improvements in low-density lipoprotein cholesterol in patients with hypercholesterolemia on maximally tolerated statins who are at increased cardiovascular risk.
Introduction
Hypercholesterolemia and mixed dyslipidemia are disorders of lipid metabolism characterized by high levels of low-density lipoprotein cholesterol (LDL-C) and, for the latter, lower levels of high-density lipoprotein cholesterol (HDL-C)Citation1–3. Elevated levels of LDL-C are both causal and cumulative for the development of cardiovascular disease (CVD), in particular atherosclerotic cardiovascular disease (ASCVD). CVD is the leading cause of mortality worldwide with a substantial contribution from ASCVD related deathsCitation4,Citation5. The World Health Organization (WHO) reports a high global prevalence of elevated cholesterol, with the highest rates reported in Europe (54%) and America (48%)Citation3. Heterozygous familial hypercholesterolemia (HeFH) is an inherited disorder of LDL-C metabolism. HeFH is estimated to have a prevalence ranging between 1:311 to 1:250 in the general population, and 1:17 in those with ASCVDCitation6,Citation7, with rates as high as 8.3%Citation8.
Global clinical guidelinesCitation9,Citation10 recommend statins as the mainstay of treatment for patients with hypercholesterolemia, including those with HeFH. However, of the majority of high-risk patients with ASCVD do not achieve clinically meaningful reductions in LDL-C with statins aloneCitation11,Citation12. For these patients, and those unable to tolerate statins, ezetimibe is recommended as a monotherapy or in combination with statins, but has been shown to provide only modest (approximately 20%) reductions in LDL-CCitation13. Monoclonal antibodies (mAbs) alirocumab and evolocumab, which target protease proprotein convertase subtilisin/kexin type 9 (PCSK9), provide much greater efficacy (reductions of between 55% and 60%) in patients with ASCVD and/or HeFH requiring additional reduction in LDL-CCitation14. Unfortunately, the impact of these promising therapies has been limited by pricing barriers to access encountered by clinicians and patients.
Inclisiran is the first small interfering RNA (siRNA) that selectively targets the liver and supresses the translation of PCSK9, thereby increasing LDL receptor recycling, increasing LDL-C uptake and decreasing LDL-C levels in the bloodstreamCitation15,Citation16. In the ORION clinical trials, inclisiran has demonstrated superiority over placebo, on a background of maximally tolerated statin therapy, in lowering LDL-C among patients with HeFH, ASCVD, or ASCVD risk equivalentsCitation17,Citation18.
The objectives of this study were to use NMA to estimate the relative efficacy of inclisiran, evolocumab, alirocumab, bempedoic acid, and ezetimibe in patients with hypercholesterolemia, including ASCVD, HeFH, and/or high-very high cardiovascular (CV) risk.
Methods
Identification and selection of studies
A systematic literature review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statementCitation19. MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, and Web of Science were searched in order to identify full-text articles and conference abstracts published through February 2021 (Appendix 1). Clinical trial registries were searched to identify additional data of interest for trials in progress.
Two reviewers independently screened citations against predefined selection criteria (Appendix 1), which aimed to capture RCTs comparing the efficacy of approved doses of inclisiran, evolocumab, alirocumab, ezetimibe, and bempedoic acid therapies, compared to each other, other lipid-lowering drugs (LLDs), or placebo in adult patients with hypercholesterolemia, including ASCVD, HeFH and/or high or very high CV risk due to inadequate LDL-C control on maximally tolerated dose (MTD) statins (+/− other LLDs). High to very-high CV risk was defined according to the 2019 European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) guidelines, which acknowledged the importance of treatments to lower LDL-C for certain primary prevention patient groups (i.e. diabetes, chronic kidney disease) without ASCVDCitation20.
Data extraction and quality assessment
For each included trial, a single researcher extracted details on study design, patient characteristics, and interventions, and a second researcher traced the extracted data back to the original publication. When percent change in LDL-C data were only reported graphically (no numeric values), Engauge Digitizer was used to convert the image files into numeric values. The quality of the RCTs was assessed according to the Centre for Reviews and Dissemination instrumentCitation21.
Feasibility assessment
The feasibility of performing NMA was assessed by comparing the study design, patient characteristics, treatment, and outcome definitions across the included trials. The following factors were identified a priori as potential treatment effect modifiers based on clinical and methodological expertise:
Washout/Run-in phase
Follow-up duration
Imputation methods
CV risk and severity
Statin history (type, dose, tolerance, and background use)
Background ezetimibe use
Baseline LDL-C
Baseline % HeFH
The included studies were further categorized based on the inclusion/exclusion criteria as ASCVD and/or high CV risk or HeFH, and the following analyses were deemed feasible for the outcomes of interest:
Base case: ASCVD and/or high CV risk populations on MTD statins
“All-Comers” scenario: All hypercholesterolemia, including ASCVD and HeFH and/or high CV risk populations on MTD statins
Sensitivity analyses were performed to assess the impact of (1) timepoint selection, (2) imputation methods, and (3) statin intolerance within the ORION trials.
Statistical analysis
Bayesian NMA models were used to simultaneously synthesize the results of the included RCTs and estimate the relative efficacy of inclisiran versus comparators of interestCitation22–25. NMA has increased statistical power to detect differences in treatment effects compared to standard pair-wise meta-analysis because it combines both direct (head-to-head) and indirect evidenceCitation26. Arm-based percent change in LDL-C at 24 weeks were used as inputs for analyses, with 12-week data being used when 24-week data were not reported. Random-effects (RE) models were most appropriate given the number of studies per treatment and observed heterogeneity in patient/trial characteristics.
All analyses were based on published codesCitation27 and were implemented with OpenBuGS (version 3.2.3)Citation28. Vaguely-informative normal prior distributions with a uniform standard deviation (SD) were used, specifically U[0,1] for all binary outcomes, and U[0,X] for continuous outcomes, where X was the median within-group SD of the outcome across studies. However, sufficient data existed in the network to make results relatively insensitive to the choice of prior.
Results of the NMA are reported as the pooled posterior medians with corresponding 95% credible intervals (CrI) for each treatment versus placebo (and each other)Citation29. Heterogeneity for each comparison was primarily assessed by conducting classical pairwise meta-analysis and calculating I2, estimates of the percentage of variability due to heterogeneityCitation30. t, was also estimated for all pair-wise and network meta-analyses, as it represents a more absolute estimate of heterogeneity, and is presented in the same units as the outcome. Assuming, normally distributed population effects, these estimates represent the SD given variation in patient characteristics and methodological factors that are observed or unobserved.
Inconsistency was assessed by consideration of each individual ‘closed loop’ of direct and indirect evidence, where the result for direct evidence was compared to the indirect result from the other two links. Global statistical heterogeneity was also assessed by considering the size of the τ from the Bayesian NMA under the RE model – this parameter reflects both the amount of heterogeneity and inconsistency in the data.
Estimates for all analyses were also presented along with 95% prediction intervals, which provide a range within which future results would be expected to fall for each comparison. This approach was taken to overcome some of the limitations around the interpretation of NMA results in the presence of heterogeneity, as the width of the prediction interval incorporates the between-study heterogeneity observedCitation31,Citation32. Prediction intervals account for both imprecision in the estimate of mean effects and imprecision caused due to unexplained RE variation, i.e. true deviations from those mean effects across trials. The posterior samples were also used to estimate the rank probability of each treatment being better than each comparator.
Results
Study selection
The study selection for the systematic review and NMA is described in Appendix 2. A total of 31 trials were included in the systematic review, of which 23 trials reported populations, interventions, and outcomes of interest for the NMA and were deemed appropriate for analysis.
The majority of studies excluded from the NMA failed to report if patients were receiving MTD statins (i.e. low- or moderate-dose statins only)Citation33–36 or administered double doses of statins in the placebo arm compared to the active intervention armCitation37–40. Two studies excluded patients with ASCVDCitation41 or clinically significant CVDCitation42 and one included a control arm consisting of just ezetimibe, without background statin therapy (vs. ezetimibe + atorvastatin)Citation43.
Evidence base
Study and patient characteristics for the 23 included trials and the results of the quality assessment are provided in Appendix 2.
The majority were phase III, double-blind, global, multicenter trials; nine were conducted in North America and/or Europe and two were conducted in Asia. Most studies assessed percent change in LDL-C at 24 weeks, with the exception of five evolocumab trialsCitation44–48 which only reported results at 12 weeks, and the inclisiran trials which reported results at 150 days (21.4 weeks).
Risk of bias was low for 11 of the included trials, while the remaining trials were considered to be moderate risk, due mainly to the fact that details of the randomization process were not reported, and imbalances were observed between baseline characteristics of prognostic factors between treatment arms.
Incomplete and inconsistent reporting across a number of key characteristics with regards to established CVD, CV history, prior CV events, and CV risk factors was observed across the included trials. Most trials defined their populations based on ASCVD and/or established coronary heart disease (CHD) (Appendix 3). The proportion of patients with HeFH ranged from 1% to 17% across the trials included in the base case. The “all-comers” scenario analysis was performed to test the impact of combining the base case trials with trials conducted in 100% HeFH populations.
Network
The connected network of RCTs for the base case analyses is illustrated in Appendix 4. The thickness of the lines corresponds to the number of trials included per treatment comparison. The “all-comers” scenario analysis included an additional six trials conducted in patients with HeFH: ORION-9, ODYSSEY FH I, ODYSSEY FH II, NCT01266876, ODYSSEY HIGH FH, and RUTHERFORD-2 (Appendix 4).
NMA results
presents the NMA results for percent change from baseline (CFB), where a mean difference in CFB less than zero favors treatment over placebo. Statistically significant advantages were observed for all active treatments when compared with placebo. Similar changes were observed for inclisiran compared to alirocumab (mean between group difference [MD]: 0.78%, [95% CrI: −8.35, 9.88]) and evolocumab (MD: 8.16%, [95% CrI: −1.82, 18.49]) (). Results based on prediction intervals were consistent with the findings based on 95% CrIs (Appendix 5). Sensitivity analyses were performed to test the impact of timepoint selection, imputation methods, and statin intolerance within the ORION-10 and ORION-11 trialsCitation18 and differences in patient characteristics in the ORION-1Citation49 trial. All analyses slightly modified the magnitude, but not the direction of effect for inclisiran versus placebo (range of mean difference in CFB: −51.02 [−58.41, −43.52] when washout imputed data from the ORION trials were used, to −57.57 [-65.23, −49.89] when statin intolerant patients were excluded) (). Results from the “All-Comer” scenario analysis that added HeFH trials to the base case demonstrated results that were consistent with the base case with respect to change in LDL-C, although the magnitude of the difference in reduction in LDL-C was slightly less for all treatments versus placebo ().
Figure 1. Forest plot of NMA results for percent change from baseline in LDL-C for base case population. Abbreviations. CrI, credible interval; NMA, network meta-analysis.
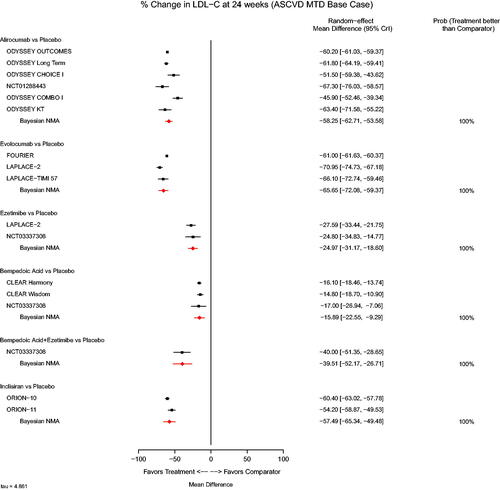
Table 1. League table NMA results for percent change in LDL-C for base case population.
Table 2. Results of sensitivity analyses based on varied data inputs for inclisiran for percent change in LDL-C for base case population.
Table 3. League table of NMA results for percent change in LDL-C for “all-comers” population.
Exploration of heterogeneity and inconsistency
Heterogeneity
Results from each pairwise meta-analysis possible are reported in Appendix 6 along with measures of statistical heterogeneity, which were found to be high in four of the six meta-analyses performed (p < 0.05). Values of t, which are presented in units of percentage CFB in LDL, suggest that for pair-wise estimates, the % change in LDL-C would vary across 95% of trials with a range from 3.9 (for inclisiran) to 6.9 (for alirocumab).
The prediction intervals indicated that both inclisiran and the PCSK9 inhibitor mAbs remained distinctly better than bempedoic acid, ezetimibe, and placebo, with the overlap in effects between those three drugs being apparent (Appendix 6).
Inconsistency
Four closed loops exist with the network: (1) placebo/evolocumab/ezetimibe, (2) placebo/alirocumab/ezetimibe, (3) placebo/bempedoic acid/ezetimibe, and (4) placebo/bempedoic acid + ezetimibe/ezetimibe. No evidence of inconsistency was identified (Appendix 7).
Discussion
Findings from the NMA demonstrate the superior efficacy of inclisiran, alirocumab, and evolocumab over placebo, bempedoic acid, bempedoic acid plus ezetimibe, and ezetimibe alone in terms of terms of their ability to lower LDL-C in patients who do not achieve clinically meaningful reductions in LDL-C with statins alone. Inclisiran, alirocumab, and evolocumab were comparable when added to current standard of care in terms of percent change in LDL-C, in particular taking into consideration the observed heterogeneity (difference for alirocumab vs. inclisiran: 0.78%, [95% CrI: −8.35, 9.88] and evolocumab vs. inclisiran: 8.16%, [95% CrI: −1.82, 18.49]).
Statistical heterogeneity was generally significant across the included trials (p < 0.05), with values of heterogeneity relative to total variation (I2) classically understood to be highCitation30. However, as I2 represents the proportion of total heterogeneity that cannot be explained due to sampling error, a high value of I2 does not necessarily represent clinically substantive heterogeneity; many of the included studies had large sample sizes and therefore study level sampling error was relatively small. A more absolute estimate of heterogeneity is the estimate of t, which, assuming normally distributed population effects, represents the SD around the observed relative effects given variation in patient characteristics and methodological factors that are observed or unobserved. For example, consider the comparison of evolocumab versus placebo: given the NMA estimate of t of ∼5, if the average effect is −65.77%, the true effects (i.e. independent of any sampling error) will vary across 95% of trials with a range of roughly −56% to −76%. This variation is reflected in the prediction intervals which account for both imprecision due to true differences in effects across the included trialsCitation50, and imprecision in the individual trial estimates, leading to an interval for evocolumab and placebo that is slightly wider: −53% to −78% (Appendix 6). These values suggest that differences in percent change in LDL-C estimated in the NMA would likely be generalizable for any observed, significance differences greater than 5%-10%.
To our knowledge, this NMA is the first to combine such a broad number of treatment comparisons and also consider timepoints for change in LDL-C beyond 12 weeksCitation51–54. Previously published NMAs in hypercholesterolemia that assessed differences in LDL-C did not include more recently published evidence on inclisiran or bempedoic acid, and the one study that didCitation52 assessed results over 12 weeks. Most other published NMAs also included trials wherein the statin dose was doubled in the control arms (i.e. ODYSSEY OPTIONS I, ODYSSEY OPTIONS II) which, in turn, underestimated the relative efficacy of alirocumab. Given these differences, along with other differences in our analytic approach, direct comparison of the results of the current analysis with other published NMAs should be done with caution.
Limitations
The selection of 24 weeks as the main timepoint of interest in the base case analysis was based on the fact that it was the most commonly reported timepoint across all trials (with the exception of five evolocumab trialsCitation44–48) percent change in LDL-C typically plateaued by 24 weeks, and up-titration of alirocumab typically occurred at week 12. Although the primary efficacy endpoints in the ORION trials were percent change at day 510 and time-adjusted change in LDL-C, these longer duration timepoints, and specifically time-adjusted analyses, were not available from other trials. Sensitivity analyses that included 90-day or time-adjusted percent change in LDL-C inputs from the ORION trials (based on MMRM models) showed results that were slightly less favorable for inclisiran versus placebo. This is likely due to the dosing regimen of inclisiran, wherein patients are administered an initial dose on Day 1 and then are not dosed again until Day 90. Assessing change in LDL-C at Day 90 after a single dose may not provide sufficient time for treatment optimization.
Mixed-effects model repeated measures (MMRM) and last observation carried forward (LOCF) were the most common methods to impute missing data within the included trials, though some trials employed standard pattern mixture models (PMM) that assumed data were ‘missing at random.’ These methods were accepted as broadly comparable in the NMA, with MMRM results selected for the base case analysis when available. In the most recently published trials (including ORION, CLEAR Harmony, and CLEAR Wisdom), PMM that assumed data are ‘not missing at random’ (i.e. ‘washout-imputed’ approaches) were used for their primary endpoint analyses, making these studies outliers with regards to methodology. Patient-level data from the ORION trials were therefore used to derive results based on MMRM methods in order to provide more comparable inputs for the base case analysis. Sensitivity analyses based on washout imputed results from the ORION trials demonstrated results that were slightly less favorable with inclisiran as compared with placebo (difference of 7%). Unfortunately, MMRM results from the bempedoic acid trialsCitation32,Citation55 were not reported, and therefore, assuming a similar difference in effect based on choice of method for the ORION trials, it is possible that the reduction in LDL-C provided by bempedoic acid over placebo may be closer to 23%.
Finally, the included trials used inconsistent definitions and criteria for categorizing CV risk. These inconsistencies, coupled with poor reporting, precluded meaningful statistical control or adjustment for their impact. A recent review conducted by the United States Institute for Clinical and Economic Review (ICER) concluded that there was no significant difference in the percentage LDL-C reduction for high CV-risk patients unable to achieve disease control on MTD statins, for patients with and without established ASCVDCitation56. This was consistent in our findings of the “all-comers” scenario analysis, which included trials in ASCVD/risk equivalent patients and those with HeFH. Additional exploratory analyses not reported herein, including meta-regression for baseline LDL-C, and scenarios to exclude outliers with regard to CV risk, were also performed and resulted in findings that were consistent with the base case.
Conclusions
Findings from the NMA show that the addition of inclisiran and PCSK9 inhibitor mAbs to current standard of care for patients with hypercholesterolemia on MTD statins who are at increased CV risk, results in statistically significant and clinically meaningful improvements in LDL-C. Based on the level of observed heterogeneity, conclusive differences can be generalized for any statistically significant difference in percent change in LDL-C that is greater than 5 to 10 points in size. In this regard, evidence suggests that inclisiran, alirocumab, and evolocumab are expected to provide similar improvements in LDL-C over the studied period.
Transparency
Declaration of funding
This work was funded by Novartis Pharma AG. The publication of this study was not contingent on the sponsor’s approval or censorship of the manuscript.
Declaration of financial/other relationships
HB, AC, KF, and JT are employees of Evidera, a consultancy which provides consulting and other research services to pharmaceutical, medical device, and other organizations. In their salaried positions, they work with a variety of companies and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received funding from Novartis for the involvement of their employees in this research. MD, AD, RJ, and AR are employees of Novartis. AV has conducted clinical trials funded by/received speakers' honoraria/advisory board from Astra Zeneca, Boehringer-Ingelheim, Daiicki-Sanyo, Lilly, Napp, Novartis, Novo Nordisk, Regeneron, Sanofi and Tosoh.
A reviewer on this manuscript has disclosed that they received honoraria from Sanofi Aventis and Novartis. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Inclisiran_Manuscript_Suppl_01Feb2022_v1.0.docx
Download MS Word (380.6 KB)Acknowledgements
Authors would like to thank Arushi Bamrara (Novartis), Harshul Natani (Novartis), Christine Worsley (Tolley Health Economics Ltd.) and Jo Noble-Longster (Tolley Health Economics Ltd.) for their support on the systematic literature review, and Tracy Westley (Evidera) for her assistance with the NMA. Special thanks to Sean Smith (Evidera) for his support in the preparation of this manuscript.
References
- Barbir M, Breen J, Neves E, et al. Diagnosis, management and prognosis of familial hypercholesterolaemia in a UK tertiary cardiac Centre. Clin Lipidol Metabol Disord. 2019;14(1):1–10.
- Duell PB, Gidding SS, Andersen RL, et al. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: the Cascade FH registry. Atherosclerosis. 2019;289:85–93.
- World Health Organization (WHO). WHO global health observatory data, Raised Cholesterol 2020. Available from: https://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/.
- Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472.
- Domanski MJ, Tian X, Wu CO, et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76(13):1507–1516.
- Akioyamen LE, Genest J, Shan SD, et al. Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and Meta-analysis. BMJ Open. 2017;7(9):e016461.
- Hu P, Dharmayat KI, Stevens CAT, et al. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: a systematic review and meta-analysis. Circulation. 2020;141(22):1742–1759.
- De Backer G, Besseling J, Chapman J, et al. Prevalence and management of familial hypercholesterolaemia in coronary patients: an analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosis. 2015;241(1):169–175.
- European Society of Cardiology. 2019 Guidelines on dyslipidaemias (management of); ESC clinical practice guidelines 2019. [February 2021]. Available from: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Dyslipidaemias-Management-of.
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e563–e595.
- De Backer G, Jankowski P, Kotseva K, et al. Management of dyslipidaemia in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis. 2019;285:135–146.
- Kotseva K, De Backer G, De Bacquer D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiolog. 2019;26(8):824–835.
- Ray KK, Molemans B, Schoonen WM, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. 2021;28(11):1279–1289.
- Baum SJ, Toth PP, Underberg JA, et al. PCSK9 inhibitor access barriers-issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40(4):243–254.
- Khvorova A. Oligonucleotide therapeutics - a new class of cholesterol-lowering drugs. N Engl J Med. 2017;376(1):4–7.
- Kosmas CE, Munoz Estrella A, Sourlas A, et al. Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases. 2018;6(3):63.
- Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382(16):1520–1530.
- Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–1519.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network Meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784.
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188.
- CRD’s guidance for undertaking reviews in health care. Layerthorpe (UK): Centre for Reviews and Dissemination, University of York; 2008.
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897–900.
- Jansen JP, Crawford B, Bergman G, et al. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11(5):956–964.
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network Meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428.
- Lu G, Ades A. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124.
- Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? it all depends on the distribution of effect modifiers. BMC Med. 2013;11:159.
- Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network Meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–617.
- Lunn D, Spiegelhalter D, Thomas A, et al. The BUGS project: evolution, critique and future directions. Stat Med. 2009;28(25):3049–3067.
- Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6(1):79.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- IntHout J, Ioannidis JP, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247.
- Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022–1032.
- Hirayama A, Honarpour N, Yoshida M, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk–primary results from the phase 2 YUKAWA study. Circ J. 2014;78(5):1073–1082.
- Kiyosue A, Honarpour N, Kurtz C, et al. A phase 3 study of evolocumab (AMG 145) in statin-treated japanese patients at high cardiovascular risk. Am J Cardiol. 2016;117(1):40–47.
- Teramoto T, Kobayashi M, Tasaki H, et al. Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins- ODYSSEY Japan randomized controlled trial. Circ J. 2016;80(9):1980–1987.
- Teramoto T, Kobayashi M, Uno K, et al. Efficacy and safety of alirocumab in Japanese subjects (phase 1 and 2 studies). Am J Cardiol. 2016;118(1):56–63.
- Farnier M, Jones P, Severance R, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–146.
- Nakamura T, Hirano M, Kitta Y, et al. A comparison of the efficacy of combined ezetimibe and statin therapy with doubling of statin dose in patients with remnant lipoproteinemia on previous statin therapy. J Cardiol. 2012;60(1):12–17.
- Roeters van Lennep HW, Liem AH, Dunselman PH, et al. The efficacy of statin monotherapy uptitration versus switching to ezetimibe/simvastatin: results of the EASEGO study. Curr Med Res Opin. 2008;24(3):685–694.
- Bays H, Gaudet D, Weiss R, et al. Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100(8):3140–3148.
- Thompson PD, MacDougall DE, Newton RS, et al. Treatment with ETC-1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. J Clin Lipidol. 2016;10(3):556–567.
- Ballantyne CM, McKenney JM, MacDougall DE, et al. Effect of ETC-1002 on serum low-density lipoprotein cholesterol in hypercholesterolemic patients receiving statin therapy. Am J Cardiol. 2016;117(12):1928–1933.
- Luo P, Wang L, Zhu H, et al. Impact of atorvastatin combined with ezetimibe for the treatment of carotid atherosclerosis in patients with coronary heart disease. Acta Cardiol Sin. 2016;32(5):578–585.
- Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate-or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870–1883.
- Giugliano RP, Desai NR, Kohli P, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007–2017.
- Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331–340.
- McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59(25):2344–2353.
- Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380(9836):29–36.
- Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376(15):1430–1440.
- Higgins JTJ, Chandler J, Cumpston M, et al. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from: www.training.cochrane.org/handbook
- Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network Meta-analysis. Eur Heart J. 2016;37(6):536–545.
- Toth PP, Bray S, Worth G. Relative efficacy of alirocumab, bempedoic acid, evolocumab, ezetimibe and inclisiran added to statins for reduction of low density lipoprotein cholesterol - a network meta-analysis of randomized clinical trials. Circulation. 2020;142(S3):A13503.
- Toth PP, Worthy G, Gandra SR, et al. Systematic review and network meta-analysis on the efficacy of evolocumab and other therapies for the management of lipid levels in hyperlipidemia. J Am Heart Assoc. 2017;6(10):e005367.
- Zhao Z, Du S, Shen S, et al. Comparative efficacy and safety of lipid-lowering agents in patients with hypercholesterolemia: a frequentist network meta-analysis. Medicine (Baltimore). 2019;98(6):e14400.
- Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA. 2019;322(18):1780–1788.
- Lin GK, Jih J, Agboola F, et al. Bempedoic acid and inclisiran for patients with heterozygous familial hypercholesterolemia and for secondary prevention of ASCVD: effectiveness and value 2021 evidence report. https://icer.org/wp-content/uploads/2021/02/ICER_High-Cholesterol_Evidence-Report_012221.pdf.
