Abstract
Background
Many biomedical journals follow the International Committee of Medical Journal Editors (ICMJE) recommendations and criteria for authorship. ICMJE criterion 1 provides the basis for selecting authors according to their substantial contributions to the work reported in the publication. Identifying substantial contributions and their application for author selection can be challenging, especially for multicenter studies with large numbers of investigators and contributors. Contributions are not frequently documented during study conduct and authorship decisions may lack transparency, objectivity, and context.
Methods
The International Society for Medical Publication Professionals (ISMPP) Authorship Task Force surveyed members on authorship practices, reviewed the literature defining substantial contributions to ICMJE criterion 1, and assessed existing tools or algorithms for determining authorship in industry-sponsored research. Contributions were categorized under the four sub-categories of ICMJE criterion 1: study concept and design, acquisition of data, data analysis, and data interpretation.
Results
Survey findings and literature review confirmed the need for clear and consistent interpretation, application, and documentation of ICMJE criterion 1 for transparent decisions about authorship. The Task Force reached consensus on definitions of substantial contributions to be considered when selecting authors of industry-sponsored research. The subsequent recommendations were grouped according to the sub-categories of ICMJE criterion 1. In addition, the Task Force developed recommendations regarding contributions that do not merit authorship designation.
Conclusions
The Task Force recommendations for objective and consistent interpretation of ICMJE criterion 1 will facilitate an author selection process grounded in the core principles of substantial intellectual contribution to the work’s conception or design, or to the acquisition, analysis, or interpretation of data. While these recommendations are focused on author selection practices for industry-sponsored research, they may be applicable to publications in other areas of scientific and biomedical research.
Introduction
Publication of biomedical research in peer-reviewed journals is a cornerstone of the scientific process. The International Committee of Medical Journal Editors (ICMJE) Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical JournalsCitation1 is widely adopted for reporting biomedical research in a transparent, objective, and ethical manner. The ICMJE recommendations provide a framework to ensure best practice and ethical standards in the conduct and reporting of research and other material published in medical journals. The ICMJE recommendations were formerly known as the Uniform Requirements for Manuscripts Submitted to Biomedical Journals (URMs). The URM was first published in 1978 as a way of standardizing manuscript format and preparation across journals. Over the years, the recommendations were updated to address issues in publishing that went well beyond manuscript preparation and were renamed as “Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals”Citation1.
Many peer-reviewed biomedical journals have adopted the ICMJE authorship criteria to guide authorship eligibility, and all authors are required to meet all four ICMJE criteria:
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and
Drafting the work or revising it critically for important intellectual content; and
Final approval of the version to be published; and
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ICMJE criterion 1 provides the basis for selecting authors according to their substantial contributions to the research reported in the publication. Despite the reporting of additional guidelines and resources about ICMJE criterion 1Citation2–8, there is no “user guide” to assist authoring teams with the interpretation of criterion 1. What does substantial intellectual contribution mean? How does it apply to different types of research? Which criterion is more important to qualify as an author? The answers to these questions are critical to ensure ethical authorship practices, especially as they relate to decisions for author selection. Consistent application of ICMJE criterion 1 to determine authorship is the first step to foster ethical authorship practices, trust, and overcome challengesCitation9. For example, study concept and design, acquisition of data, data analysis, and interpretation of data typically involve many individuals including investigators and study sponsors, often making attribution of contributions difficult. Furthermore, contributions to concept and design are often made before a study starts, and a long period may elapse before the publication is developed, requiring identification of contributions based on the recollections of study investigators or sponsor study teams. In addition, many, if not most, contributions that qualify for authorship are not documented, which leads to inconsistencies in authorship decisions, despite best intentions to follow ICMJE criterion 1. For example, many investigators and sponsors rely solely on the documented enrollment numbers (i.e. data acquisition) as a proxy for all “substantial contributions” which undermines other substantial contributions to concept and design, analysis, and interpretation. Overall, the definition of “substantial” contribution as it applies to ICMJE criterion 1 is ambiguous and challenging to interpret and applyCitation10,Citation11; especially in the context of large, multicenter trialsCitation6–8.
The International Society for Medical Publication Professionals (ISMPP; www.ismpp.org) is a professional member association formed in 2005 with the aim of enhancing integrity and transparency in medical publications, improving standards and best practices and collaborative outreach in education and advocacy. ISMPP’s membership includes more than 1800 stakeholders involved in the publication of medical research, with representation from pharmaceutical, biotechnology, and device companies, medical publication and communication agencies, and medical journal publishers. ISMPP convened a Task Force of expert medical publication professionals from multiple pharmaceutical companies (Appendix) to develop recommendations that address authorship challenges related to industry-sponsored biomedical research. The authors and all members of the Task Force are active members of ISMPP and have served in leadership roles within their organizations, including development of publication policy, education, and ethics initiatives. Importantly, they have experienced, on a routine basis, challenges with author selection in the absence of specific guidance with regards to ICMJE criterion 1.
The goals of the ISMPP Authorship Task Force were to define “substantial contributions” for authorship in accordance with ICMJE criterion 1, to optimize documentation of contributions during the research or study for subsequent contribution-based author selection, and to increase education and transparency about authorship decisions. The Task Force focused on four key areas: defining substantial contributions; documentation of contributions; education and transparency; and external communication. In this paper, we describe the principles and key concepts to define substantial contributions to ICMJE criterion 1 and present recommendations to aid in author selection. The scope of the recommendations is focused on interpretation of ICMJE criterion 1 for all types of industry-sponsored research.
Methods
The Task Force used the approaches described below to confirm industry-wide challenges and identify solutions to define “substantial contributions” for authorship.
ISMPP Task Force member survey
A survey of the ISMPP Task Force members was conducted to understand current authorship practices and challenges with a focus on interpretation of ICMJE criterion 1 for author selection. An anonymous 12-question survey () was developed by one Task Force member and sent electronically to all Task Force members (n = 34) using SurveyMonkey (Palo Alto, CA), a web-based survey software tool. The survey was conducted from July 31 to August 12, 2019. Respondents were invited to share authorship tools, including algorithms, or any guidance documents in use at their organizations to identify research contributions, as well as to provide any additional information in open-field comments. Survey data were kept strictly confidential and anonymized. Descriptive statistics were used to analyze the response rates, and the responses were described qualitatively.
Table 1. Survey of ISMPP Authorship Task Force members and results.
Literature review and other available resources
The literature review focused on identifying definitions of “substantial contributions” to ICMJE criterion 1, principles for application of ICMJE criteria, and guidance on the use of these contributions for author selection. A search in the ProQuest (https://www.proquest.com/index) collection of bibliographic databases (including Biosis, Current Contents, Embase, and Medline) of sources published 2014 through 2019 was conducted using the following search terms: (authorship* or coauthorship* or authorial*) and (contribut* or *ethic* or transparen* or number or order or select* or misus* or practic*). The search terms were chosen to identify already existing approaches to provide clarity with interpretation of ICMJE criterion 1; the Task Force’s assessment revealed that relevant peer-reviewed articles describing requirements for authorship would be identified when searching authorship*. To identify additional key references, the same search terms were used in the Scopus (https://www.elsevier.com/solutions/scopus) abstract and citation database. The 100 most cited articles were scanned by an analytical expert, and a subset was manually selected for inclusion. To identify additional articles and websites that were not captured in other searches, reference lists supplied by task force members were reviewed, and a broad search of Google using the search terms “authorship”, “ICMJE”, and “scorecard” was also conducted. This approach used a level of rigor consistent with the framework described for scoping studiesCitation12. The search was comprehensive to establish the framework for the Task Force’s recommendations. The search results were reviewed to identify publications eligible for full-text assessment based on relevance to one of the following categories: very relevant (includes an authorship scorecard or grid or focuses on contributions); authorship order (focuses on issues associated with author positions on the byline); background (focuses primarily on ethical and general issues related to authorship); and not relevant. The relevant articles were further categorized according to ICMJE criterion 1 definitions of contributions, author order, author versus contributor, and unethical versus ethical practices. Articles deemed relevant to authorship were assessed for key authorship-related themes.
In addition to the survey and literature review, existing algorithms, both publishedCitation5,Citation7 or in use by pharmaceutical companies, were reviewed.
Development of expert framework
The information and concepts generated from the Task Force member survey and literature search were used to develop recommendations that were grouped according to the corresponding ICMJE criterion 1 categories of study concept and design, acquisition of data, data analysis, and data interpretation. Each definition of a substantial contribution required consensus by a subset of the Task Force and was revised to ensure there was no duplication, that definitions were clear and easily interpreted, and that they were applicable to different study designs, phases, and specialties (e.g. clinical, preclinical, health outcomes). The Task Force further assessed and agreed upon the different types of contributions to research to identify those that qualify for authorship and those that do not qualify for authorship but warrant acknowledgement for their contributions to the work described in the manuscript.
Results
Task Force member survey
Nineteen Task Force members (19/34, 56%) completed the survey, although some did not answer all twelve questions (). All respondents agreed that the top priority for author selection is a clear and consistent interpretation of what constitutes a “substantial contribution” per ICMJE criterion 1. Twenty to thirty percent of respondents did not use formal definitions of concept and design, data acquisition, analysis, and interpretation per ICMJE criterion 1; the other respondents all defined these terms differently. Respondents indicated that their organizations communicate qualifications for authorship with investigators at the start of each study (14%, 2/14), during publication steering committee meetings (14%, 2/14), or in response to a request or dispute (43%, 6/14). Most (57%, 8/14) described the qualifications for authorship at the start of the publication development process. Twenty-nine percent (4/14) of respondents did not share authorship criteria with investigators or collaborators.
Literature review and other available resources
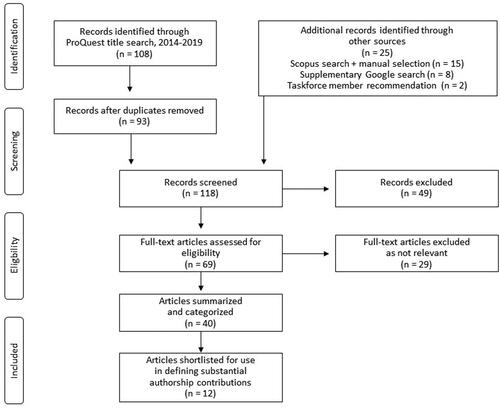
The flow diagram for the literature review is shown in . A total of 108 references, 93 of which were unique, were identified in ProQuest. An additional 25 articles were identified from Scopus, by Task Force members, or through a Google search. A total of 69 publications were deemed eligible for full-text review and assessment based on screening of titles and abstracts, of which 40 were determined to be relevant to ICMJE criterion 1. Twelve articles from the literature search or from additional publications provided by the Task Force members were found to be applicable and used by the ISMPP Task Force in defining substantial authorship contributionsCitation5–7,Citation13–21. This assessment was supplemented by five authorship algorithms provided through the authorship survey described above. Substantial contributions defined in the references were then mapped to each category of ICMJE criterion 1 (study concept and design, acquisition of data, analysis, and interpretation) and reviewed to ensure appropriateness and clarity.
Expert recommendations on defining and interpreting criterion 1
Contributions qualifying for authorship
The Task Force synthesized the information from the survey, literature review, and materials that members submitted and, through consensus, developed recommendations for defining and interpreting ICMJE criterion 1, as shown in . The definitions of contributions for authorship within each ICMJE criterion 1 category are summarized below:
Table 2. Recommendations for selection of authors of industry-sponsored research publications based on ICMJE criterion 1A.
Concept and design: Development or substantial modification of research idea, study design, methodology protocol, statistical analysis plan, or a combination of these activities; or
Data acquisition: Significant contribution of data (quality and quantity) to the final analyses; or
Data analysis: Performance of the data analysis and assurance of the integrity of the data and statistical analyses; or
Data interpretation: Derivation of conclusions, placement of results into context, or identification of knowledge gaps for future exploration.
The definitions of substantial authorship contributions described above and in should be used to facilitate proactive and transparent documentation of contributions required for authorship. The documented contributions should be evaluated in a systematic, objective, and transparent manner to select authors. Educating stakeholders on the application of these recommendations will be critical. We recommend discussing the criteria for author selection early in the research process or at the start of a clinical trial, and sponsors should set expectations for how contributions to the study and research data will be evaluated for author selection. Authorship in publications should not be promised or guaranteed and should reflect the contributions to the specific work reported in the publication.
Contributions not qualifying for authorship
Not all contributions to the conduct of a study are intellectual or substantial enough to warrant authorship. Some important examples of contributions that do not qualify for authorship but may be acknowledged, have been previously identified by ICMJE in their recommendationsCitation1. With respect to industry-sponsored research, we have included in relevant examples of contributions that were identified during the mapping for ICMJE criterion 1. As noted above, enrollment alone without considering quality and relative quantity does not necessarily qualify an investigator for authorship. Also, membership in Steering Committees or Advisory Boards or role as lead principal investigator should not automatically qualify an individual for authorship without their contributions to the concept or design, data acquisition, analysis, or interpretation. An individual who provides oversight or management of a group but does not actively contribute in an intellectual and substantial manner in the research project would also not qualify for authorship, but may be acknowledged.
Discussion
The ICMJE recommendations are widely accepted as the standard for author selection in biomedical publications. Their use and general adoption are broad, spanning industry, research institutions, and academia. However, the process of authorship determination based on ICMJE criterion 1 can be challenging, especially in the context of large multicenter trialsCitation6–8.
The ISMPP Task Force member survey confirmed challenges related to consistency and objectivity in applying the available guidelines including lack of clarity around interpretation of ICMJE criterion 1, inadequate documentation of contributions to the research/study overall, and use of subject enrollment as the sole criterion. Additional underlying factors may include limited transparency and communication about author selection at study initiation, during study conduct, and at the publication initiation stage, as well as author inclusion based on cultural and institutional practices (e.g. including research supervisors).
The literature review established a basis for understanding existing guidance and tools for determining authorship. Identifying articles relevant to authorship tools and other key authorship-related themes and evaluating them in depth helped to ensure objectivity and allowed the Task Force to build upon prior knowledge in identifying gaps and developing recommendations. We concluded that data available in the literature to define what constitutes a substantial contribution is scarce. In addition, we found that defining what is not a substantial contribution for authorship is just as necessary as defining what is substantial. Therefore, the work reported in this manuscript is an important contribution to the literature to interpret and apply ICMJE criterion 1 for author selection and invite scientific disclosure.
Using these focused approaches and expert consensus, we developed definitions (high-level and descriptors) of contributions to research in each of the four components of criterion 1 (concept/design, data acquisition, data analysis, and data interpretation) that merit authorship when they represent a substantial component of the work relative to the publication (). These definitions are designed to add clarity to criterion 1 and support broader understanding and adoption of the ISMPP expert recommendations by sponsors, publication professionals, and authorsCitation10. In addition, we identified examples of contributions that are not sufficient to qualify an individual for authorship (), but may be included in an acknowledgement.
Consistently adopting and incorporating these recommended definitions of substantial contributions to ICMJE criterion 1 into the organizational process, procedures, and culture is critical to improve the author selection process. The definitions for criterion 1 developed by the Task Force could also serve as a basis for ranking and scoring contributions. The ISMPP Authorship Task Force is utilizing this approach to build an Authorship Algorithm ToolCitation22 that can be used for industry-sponsored research. In addition, educational resources for internal and external stakeholders is a future planned output of the Task Force.
The recommendations described in this manuscript focus on author selection prior to the start of a study and before a publication is initiated. It is possible that additional substantial intellectual contributions (e.g. analysis, interpretation) are made after the start of a publication which may warrant revising the authorship bylineCitation23.
Limitations of this work include a small survey sample size with a focus on industry-sponsored biomedical research. Additionally, we acknowledge that a systematic literature review is the gold standard for synthesizing previous work; however, the use of a scoping methodologyCitation12 was more aligned with the purpose and scope of this project. ICMJE authorship criteria 1 was the focus of this work because industry-sponsored research is frequently submitted to journals that follow ICMJE Recommendations. Other authorship guidelines, such as journal-specific requirements, were not considered. Contributions were evaluated with publication professionals from pharmaceutical companies and not with authors, academics, journal editors, or with ICMJE members. While our work focused on author selection practices for industry-sponsored research, we believe the recommendations may be applicable to publications in other areas of scientific and biomedical researchCitation24.
Conclusions
An objective and consistent interpretation of the ICMJE criterion 1 based on substantial intellectual contribution to the research is crucial for authorship determination in industry-sponsored research. The recommendations from the ISMPP Authorship Task Force are the first to provide a clarifying framework for interpreting the four components of ICMJE criterion 1 for substantial intellectual contribution (study concept or design, data acquisition, data analysis, or data interpretation) during author selection discussions before the start of a publication. These recommendations serve as a basis for the ISMPP Authorship Algorithm Tool currently in development. We believe the recommendations for interpreting ICMJE criterion 1 provide complementary guidance to the ICMJE definition of authorship that should be adopted consistently across industry-sponsored research to ensure ethical authorship practices and consistent processes for authorship decisions.
Transparency
Declaration of funding
This paper was not funded. Open Access was supported by ISMPP and the authors’ respective companies.
Declaration of financial/other relationships
MLC is an employee and stockholder of GlaxoSmithKline, SAS and SAN are employees and stockholders of Bristol Myers Squibb, EAW is an employee and stockholder of Pfizer Inc., MBH is an employee and stockholder of Merck & Co., KDM is a former employee and current stockholder of Sanofi. TS is an employee and stockholder of Novo Nordisk. MIK is an employee and stockholder of AstraZeneca.
A reviewer on this paper has disclosed that they are an active member of ISMPP, has served on ISMPP committees with some of the members of this working group and has consulted for some of the co-authors. Another reviewer disclosed that they are an active member of ISMPP but has not worked on anything related to this publication. Another reviewer has also disclosed that they are a member of ISMPP and serves on the Ethics and Standards Committee. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors were involved in the conception and design, analysis and interpretation of the data, drafting of the manuscript and revising it critically for intellectual content, approved the final version for submission, and agreed to be accountable for all aspects of the work.
Acknowledgements
The authors thank Victoria Garcia of Bristol Myers Squibb for graphical support and Jan Søvig Christensen of Novo Nordisk for analytical assistance with the literature search. We are grateful to Robert Matheis, President and CEO of ISMPP, for his enthusiastic support of the Task Force and his professional leadership.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- International Committee of Medical Journal Editors [Internet]. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals; 2021 [cited 2021 July 9]. Available from: http://www.ICMJE.org.
- Resnik DB, Master Z. Authorship policies of bioethics journals. J Med Ethics. 2011;37(7):424–428.
- Resnik DB, Tyler AM, Black JR, et al. Authorship policies of scientific journals. J Med Ethics. 2016;42(3):199–202.
- Battisti WP, Wager E, Baltzer L, et al.; International Society for Medical Publication Professionals. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464.
- Warrender JM. A simple framework for evaluating authorial contributions for scientific publications. Sci Eng Ethics. 2016;22(5):1419–1430.
- Marušić A, Hren D, Mansi B, et al. Five-step authorship framework to improve transparency in disclosing contributors to industry-sponsored clinical trial publications. BMC Med. 2014;12:197.
- Whellan DJ, Ellis SJ, Kraus WE, et al. Method for establishing authorship in a multicenter clinical trial. Ann Intern Med. 2009;151(6):414–420.
- National Research Collaborative & Association of Surgeons in Training Collaborative Consensus Group. Recognising contributions to work in research collaboratives: Guidelines for standardising reporting of authorship in collaborative research. Int J Surg. 2018;52:355–360.
- Aliukonis V, Poškutė M, Gefenas E. Perish or publish dilemma: Challenges to responsible authorship. Medicina. 2020;56(3):123.
- Malički M, Jerončić A, Marušić M, et al. Why do you think you should be the author on this manuscript? Analysis of open-ended responses of authors in a general medical journal. BMC Med Res Methodol. 2012;12(1):189.
- Helgesson G. Scientific authorship and intellectual involvement in the research: should they coincide? Med Health Care Philos. 2015;18(2):171–175.
- Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
- Camby I, Delpire V, Rouxhet L, et al. Publication practices and standards: recommendations from GSK Vaccines' author survey. Trials. 2014;15:446.
- Brand A, Allen L, Altman M, et al. Beyond authorship: attribution, contribution, collaboration, and credit. Learn Pub. 2015;28(2):151–155.
- Kim T-I. Endorsement of the Contributor Roles Taxonomy for the clarification of authorship [editorial]. J Periodontal Implant Sci. 2017;47(1):1.
- Hammer MJ, Miaskowski C. Authorship ethics in the era of team science. Oncol Nurs Forum. 2017;44(6):655–657.
- Amato AA, Baskin PK, Gross RA. Updating Neurology® authorship criteria: Ensuring inclusion of those making valuable intellectual contributions. Neurology. 2018;90(19):865–866.
- McNutt MK, Bradford M, Drazen JM, et al. Transparency in authors' contributions and responsibilities to promote integrity in scientific publication. Proc Natl Acad Sci U S A. 2018;115(11):2557–2560.
- Phillippi JC, Likis FE, Tilden EL. Authorship grids: Practical tools to facilitate collaboration and ethical publication. Res Nurs Health. 2018;41(2):195–208.
- Winston RB. Jr. A suggested procedure for determining order of authorship in research publications. J Couns Dev. 1985;63(8):515–518.
- Panter M. Credit where credit is due: best practices for authorship attribution [Internet]. AJE Best Practices Series; [cited 2019 August]. Available from: https://www.aje.com/dist/docs/Authorship_Attribution_EN.pdf.
- ISMPP authorship algorithm: standardising the application of the ICMJE authorship criteria [Internet]. Oxfordshire (UK): The Publication Plan - Authorship; 2021 August 17 [cited 2022 Feb 24]. Available from: https://thepublicationplan.com/2021/08/17/the-ismpp-authorship-algorithm-standardising-the-application-of-the-icmje-authorship-criteria/.
- Stocks A, Simcoe D, Toroser D, et al. Substantial contribution and accountability: best authorship practices for medical writers in biomedical publications. Curr Med Res Opin. 2018;34(6):1163–1168.
- Kornhaber RA, McLean LM, Baber RJ. Ongoing ethical issues concerning authorship in biomedical journals: an integrative review. Int J Nanomedicine. 2015;10:4837–4846.
Appendix
International Society for Medical Publication Professionals Authorship Task Force Members and Contributors: Heather Abourjaily (Biogen)†, Radhika Adiga (Novo Nordisk), Norbert M. W. Brunhuber (Vertex), Michelle L. Carfagno (GlaxoSmithKline), Anny Chen (Novartis), Jeffrey W. Clemens (Eli Lilly), Yaswant Dayaram (Bayer), Lisa Doherty (Takeda), Rikke Egelund Olsen (Hoffmann-La Roche), Namit Ghildyal (Janssen), Susan Hannah (Bristol Myers Squibb), Margaret B. Hodgson* (Merck Sharp and Dohme), Carolyn Hustad* (Merck Sharp and Dohme), Brittany Jordan (Allergan), Meera I. Kodukulla (AstraZeneca), Teri Michelini* (Sanofi), Karen D. Mittleman* (Consultant), Santosh Mysore (GlaxoSmithKline), Radha S. Narayan (Alexion), Susan A. Nastasee (Bristol Myers Squibb), Simon Page (Ipsen), Avishek Pal (Novartis), Abbie Pound (Ipsen), Rekha Rao (Amgen), Merry Saba (Novartis), Anja Schmidt (Bayer), Sonia A. Schweers* (Bristol Myers Squibb), Aruna Seth (Envision), Tine Sorgenfrei (Novo Nordisk), Rosanna Tedesco* (GlaxoSmithKline), Sarah Thomas (Ipsen), Yaming Wang (Alphabet Health), Carolyn Watson (Grifols), Elizabeth A. Whann (Pfizer), Fran Young (Shire)††, and Tatjana Zanki (Pfizer).
*Steering committee member
†Now at Vertex.
††Now at Takeda.