Abstract
Objective
To gather impressions from patients/caregivers/advocates with acute leukemia regarding preferences for accessing scientific information, the value of patients as authors of scientific publications, and the format of plain language summaries (PLS). In addition, use of an asynchronous virtual advisory board (VAB) was piloted with this group.
Methods
We conducted a 14-day asynchronous VAB (using SkyBoard) with six patients/caregivers/advocates. Patients/caregivers/advocates were identified in collaboration with the Acute Leukemia Advocates Network. Asynchronous VABs are an alternative to in-person advisory boards/synchronous VABs (video conferencing), enabling patients/caregivers/advocates to interact with VABs/each other in their own time. Platform features used to gather data included surveys, online forums, and content reviews of PLS.
Results
An international group of patients/caregivers/advocates were actively engaged in the VAB at convenient times for them. Privacy settings allowed for partial anonymity (personal details not shown) while enabling interactions with peers/facilitators. Key learnings included: patients/caregivers/advocates trust advocacy organizations to deliver scientific information; patients/caregivers/advocates often feel overwhelmed by the volume of scientific information available/seek a central hub for information; patients/caregivers/advocates are aware of PLS; advocates are well suited to co-author scientific publications/disseminate PLS; and PLS should include varying levels of detail to satisfy all patients/caregivers/advocates needs. Video is also the most preferred alternative format to infographic PLS.
Conclusions
Patients/caregivers/advocates welcomed the opportunity to discuss the communication of scientific information. The asynchronous VAB was an effective and efficient format for individuals to interact, assess materials and engage in virtual discussion. Feedback resulted in the development of a video abstract PLS for an oncology congress. Asynchronous VABs offer advantages to in-person advisory boards, including flexibility, convenience, and low cost.
Keywords:
Acknowledgements
The authors would like to thank the patients, caregivers, and advocates who participated in this study. The authors also thank Tyron McClain and the Acute Leukemia Advocates Network (ALAN) for their support with this initiative and System Analytic for providing technical support. This study was sponsored by Pfizer. Medical writing and editorial support were provided by Simon Stones, PhD, of Engage Scientific Solutions, and was funded by Pfizer.
Abstract
Objective
Scientific communication platforms (SCPs) support consistent and accurate communication around pharmaceutical products. As development requires significant investment of budget and time, our aim was to understand SCP perception and use.
Methods
An 18-item, online survey was developed to gather insights into company employees’ awareness and use of SCPs and emailed to >900 recipients across functions. The survey was active from 1 to 18 November 2021.
Results
Of 115 respondents, 60% were in a medical role and 52% were local employees. 46 (40%) were aware of SCPs. A higher proportion (52%) of those in a medical role were aware of SCPs. More respondents in a global role (23/53 [43%]) were aware of SCPs than those in a local role (22/60 [37%]). Of respondents aware of SCPs, most (79%) knew where to find or start looking for them, or who to ask to locate them. This was consistent for global and local employees. The majority (67%) of respondents who were aware of SCPs were also aware that they were regularly updated. Of respondents aware of SCPs, 20 (43%) reported regular or occasional use. Among 40 respondents who had used SCPs, 75% and 72.5% highly rated the usefulness and quality/accuracy of SCPs, respectively. Most frequent SCP use was in educational materials (23/40 [58%]), congress materials (14 [35%]), and publication planning/development (12 [30%]).
Conclusion
Awareness of SCPs was more common among employees in global roles and in the medical function. Our findings highlight the need for frequent communication and/or education around SCPs to cross-functional and regional colleagues to increase uptake and engagement.
Keywords:
Abstract
Objective
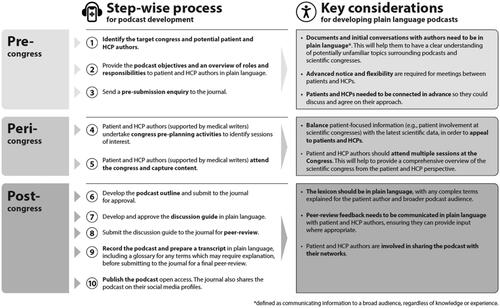
Journals are publishing peer-reviewed podcasts summarizing the latest findings presented at scientific congresses. However, they are not in plain language to appeal to broader audiences, including patients/patient advocates. Our objective was to create a process for developing plain language podcasts to increase accessibility to relevant data from congresses for patients and HCPs.
Methods
We used a collaborative process to publish a peer-reviewed, citable, plain language podcast ().
Results
The peer-reviewed plain language podcast of the European Society for Medical Oncology 2021 congress was accepted for publication in Oncology and Therapy in 2022. The podcast (and transcript/glossary) is available on PubMed, Google Podcasts, and Spotify. It focuses on topics such as patient involvement at congresses, as well as data in bladder/kidney cancer.
Conclusions
We successfully published a peer-reviewed, citeable, plain language podcast. We believe this process will facilitate the development of further podcasts, increasing the accessibility of data presented at scientific congresses, while empowering patients and HCPs to engage with research.
Keywords:
Acknowledgements
The authors thank Alex Felicvias and Tom Powles for participating in the podcast. This podcast was sponsored by Pfizer. Medical writing support was provided by Susan Tan, PhD, of Envision Pharma Group, and was funded by Pfizer.
Abstract
Objectives
Healthcare systems contribute to approximately 5% of national carbon footprints, with up to 26% of their greenhouse gas (GHG) emissions attributable to the pharmaceutical industry and its supply chainCitation1,Citation2. This study aimed to determine how leading pharmaceutical companies are engaging with environmental issues, which have made commitments to climate change targets, and what implications these have for pharmaceutical suppliers.
Methods
Content analysis was performed of the most recent (2020 or 2021) publicly available company reports of the top 20 pharmaceutical companies, by 2020 prescription revenueCitation3. Using a pre-formulated data extraction table, data on environmental reporting, climate change targets and sustainability measures were extracted. Proportions of companies that made commitments to net zero emissions, carbon neutrality and GHG emission reductions across their own operations and supply chain were determined.
Results
All companies engaged in environmental reporting. Nineteen (95%) of the companies have committed to climate change targets and reducing GHG emissions in their operations, 50% have committed to carbon neutrality and 40% to net zero emissions by a range of target years. Moreover, 90% of companies have committed to improving reporting and reducing emissions across their supply chain. Actions that companies are undertaking to reduce their supply chain emissions include engaging with suppliers to set climate change targets, including environmental sustainability in vendor selection criteria, and specific projects such as assisting suppliers to purchase renewable energy.
Conclusions
Pharmaceutical companies are engaging with environmental issues. Collaboration between companies and suppliers, including publications and communications providers, will increasingly require novel solutions for the delivery of a lower carbon, sustainable future.
Note
*Oral Presentation
Acknowledgements
We would like to thank Paul Tisdale and Jane Francis for providing English language editing and review services. We would like to thank Tom Crighton and Nathalie Reichmann for performing data checks.
References
- Pichler P, Jaccard I, Weisz U, et al. International comparison of health care carbon footprints. Environ Res Lett. 2019;14:064004.
- Eckelman MJ, Sherman JD, MacNeill AJ. Life cycle environmental emissions and health damages from the Canadian healthcare system: an economic-environmental-epidemiological analysis. PLoS Med. 2018;15:e1002623.
- Christel M. Pharm Exec top 50 companies. Pharm Exec. 2021;41:26–29.
Abstract
Objective
Over the last 2 years, one of the biggest publication trends to emerge has been the move from in-person to virtual congresses. This has given rise to the concept of virtual plenaries that run monthly between annual scientific meetings. Using ESMO and ASCO as case studies, we aimed to evaluate the impact and reach of virtual plenaries and their broader implications for publication planning.
Methods
We conducted desk research and social listening (Brandwatch, Google, congress websites) to assess abstract selection, online sentiment, and overall impact of ESMO and ASCO virtual plenaries versus annual meetings.
Results
Between February and November 2021, 10 ESMO and one ASCO virtual plenaries took place. The abstract submission process was similar between virtual plenaries and annual meetings, with options to encore, but selection criteria had a greater focus on data with scientific merit. Reach, assessed by social media listening, was broad, but lower for ESMO virtual plenaries than the annual meeting (2147 vs 49,686 total online mentions: primarily Twitter or news stories). Online sentiment for ESMO and ASCO virtual plenaries was positive, with rapid data dissemination cited as a key advantage. Shorter submission to presentation times will likely need to be factored into publication planning.
Conclusions
Virtual plenaries appear to be an innovative option for real-time data presentation, allowing for accelerated data dissemination, benefitting the scientific community. For publication planning, virtual plenaries have the potential to amplify discussion around key data releases. Interest in this type of meeting is likely to increase in the future and will warrant further assessment to understand the impact for the scientific community.
Abstract
Objective
When transgender authors change their names to better match their identity, correcting their names in the published literature is a matter of privacy, safety, and ethics. Though progress has been made to develop name change policies, consensus guidelines do not exist. The objective of this analysis was to survey the prevalence and content of current name change policies.
Methods
PubsHub Journals & Congresses was used to identify active biomedical journals that allow submissions, are published in English and have an impact factor as of 2018. From these journals, a random number generator was used to select a list of 33 unique publishers, covering a range of publisher size, fields, and impact. Publisher and journal websites were searched for author name change policies.
Results
Of 33 unique publishers, 17 (52%) had publicly available name change policies. In all but two of these policies, changes could be made for any reason, including gender identity, religious conversion, or change in marital status, and the change could be made without alerting coauthors or having a notice of addendum attached to the published article. For the remaining two policies, authors were instructed to contact the publisher for more information.
Conclusions
Across the biomedical field, half of publishers have an accessible policy to facilitate name changes for transgender authors and others, setting a clear precedent and a template for other journals and publishers to follow.
Abstract
Objective
Social media use has grown exponentially over the past decade; many medical journals have active Twitter accounts. Although it is assumed that journals with high impact factors (IFs) would have greater Twitter presence, it is unknown whether journal metrics correlate with Twitter reach and engagement. We sought to examine the relationship between journal characteristics and Twitter metrics.
Methods
We selected 36 journals representing general medicine, medical specialties (neurology, oncology, infectious disease), and other topics of interest (economics/outcomes research, digital health). IFs were obtained from PubsHub Journals & Congresses to classify journals as high (IF >15), medium (>5 to ≤15), or low (≤5) impact. Twitter accounts were reviewed to determine join date, number of followers, and primary tweet topic. Meltwater was used to extract data for tweets/month and engagement.
Results
Of the selected journals, 30 (83%) had Twitter accounts. IFs ranged from 0.493 to 79.321. Twitter join dates ranged from October 2008 to June 2021 and number of followers from 52 to 868,227. Most journals (73%) primarily tweeted recently published articles. Median (range) tweets/month was 91 (2–797). IF did not correlate with tweets/month (Spearman σ, 0.041) but did correlate with followers (Spearman σ, 0.572) and engagement (Spearman σ, 0.754). Median Meltwater-assessed engagement was 37 for high-, 4 for medium-, and 1 for low-impact journals; median reach was 51,609, 4914, and 5847, respectively.
Conclusion
IF alone does not correlate with a journal’s Twitter presence. However, greater engagement was more common among journals with higher IF. Medical communications professionals may wish to consider a journal’s social media presence and identify ways to amplify engagement when recommending target journals and developing publication plans.
Keywords:
Abstract
Objective
COVID-19 has mandated remote working across many geographies. We assessed the impact on MedComms work and company culture within one agency network.
Methods
Employees completed an anonymous internet-based survey, indicating agreement/disagreement with statements that various aspects of their work were negatively affected by the previous 20 months of COVID-19-mandated remote working.
Results
Approximately 600 surveys were emailed; 194 responses were evaluable. 75% of respondents did not work remotely pre-COVID-19 and medical/scientific roles (45%) were most frequently reported. Irrespective of previous work location, COVID-19-imposed remote working negatively impacted employees’ work–life balance, although most work aspects probed showed no clear impact or were less negatively affected (e.g. career development and communication) (). Preferred future working environments were: hybrid (56%), remote (35%), office (7%), or unsure (3%).
Conclusions
This survey provides valuable insights into the impact of COVID-19-mandated remote work environments on MedComms employees. A follow-up survey is planned to gain a deeper understanding of how to best support employees through changing work circumstances.
Table 1. Negative impact of remote working during COVID-19 on MedComms industry employeesTable Footnotea
Abstract
Objective
With the evolving ecosystem of artificial intelligence (AI) in the healthcare space, we sought to examine current and anticipated applications of AI within Medical Affairs to better understand how AI strategy should be deployed by Medical Affairs functions.
Methods
Seven deep-diving interviews with four bio-pharma leaders (Eisai, Baxter, Novartis, Takeda) and three consulting agencies (Nextgen Healthcare, ZoomRx, ECT Medical), plus two consultancy-published secondary sources (The Future Today Institute, West Monroe) were conducted. Participants were identified and qualified through LinkedIn profiles, recruited through email and LinkedIn InMail, and interviewed for 60 min using Microsoft Teams during July and August 2021. The interview contained 25 open-ended questions. Participants received a complimentary copy of curated findings as an incentive. Results were analyzed qualitatively.
Results
The analysis provided insights related to Need (data are increasing in volume and complexity); Ethical transparency (logic explanation needed to prevent the “black box” effect); Outsourcing (consultancy and software partnerships increase likelihood of achieving overall strategy); Success metrics (KPIs targeting improvements in consistency, error reduction, and productivity per worker are most likely to add value); Compliance (strengthen external compliance partnerships through more regular AI-focused discussion); Staffing (introduce a layered structure overseen by a core team including practical analytical leaders); and Future opportunities (new language benchmarks, machine reading comprehension, labour efficiencies, new roles, real-time analyses and improvements in insight generation).
Conclusions
AI is already recognized as a necessary and expected means to improve insights in real-time, create labour efficiencies and help Medical Affairs functions make better decisions. Medical Affairs teams should consider the role AI plays in addressing evidence generation, information needs and communication challenges.
Abstract
Objective
An innovative, SharePoint (SP)-based tool, the Publications Query Intake Form (PQIF), was developed to streamline the publication-related queries and subsequent guidance provided by our team, and to provide metrics and a living FAQ for future reference.
Methods
The PQIF tool was launched in October 2021. In January 2022, a 7-question survey was distributed via SurveyMonkey to users (n = 28) of the PQIF to gauge the effectiveness and use of the tool.
Results
To date, 44 queries were received from 28 internal publication professionals. The top three query topics included authorship, data disclosure, and copyright. A total of 16 respondents (57%) completed the survey, all of whom indicated the PQIF is easy to use. 81% of respondents had used the PQIF as an FAQ prior to submitting their own question. Most respondents (94%) felt they received a response and resolution to their query in a timely manner and that using the PQIF resulted in a more efficient use of their time. According to 100% of respondents, the PQIF helped streamline the process of asking questions, and all indicated they would likely recommend the tool to a colleague. Qualitative data was also captured, including anecdotal positive feedback and suggestions on how to improve future updates to the tool.
Conclusions
The PQIF is an innovative, easy-to-use tool that streamlines the publication query process, reducing the number of emails sent and resulting in a more efficient use of time by publication leads and publication advisors. The tool is a resource of FAQ and provides metrics for developing targeted training and informing updates to publication procedural documents.
Abstract
Objective
To understand physician preferences for digital enhancements in scientific posters and gather information to evaluate preferred interaction methods with specialists at both virtual and hybrid scientific conferences.
Methods
One anonymous Qualtrics survey was distributed by email. The survey included 21 questions and was sent to ∼3000 physicians who utilized Medical Information services within the last year. Physicians were given one month to complete the survey with a reminder email sent at the two-week mark.
Results
Of 20 physicians who completed the survey (dermatology (n=5), cardiology (n=5), other (n=5), ophthalmology (n=2), and rheumatology (n=1), endocrinology (n=1), and neurology (n=1)), 85% responded they would be likely to attend a virtual conference in the future compared to 73% that would be likely to attend a hybrid conference in the future. The top three preferences for digital supplementary material with a poster at a virtual conference were video (40%), infographics (35%), and QR code (15%). In a hybrid conference, top three preferences included infographics (35%), videos, podcasts/audio summaries, QR code, and none (15%), and quizzes/interactive component (5%). If attending a hybrid conference in person, the preferred digital method when interacting with Medical Information experts include video call/chat function (39.3%).
Conclusions
A majority of respondents expressed a preference for utilizing digital enhancements to review data in order to optimize the understanding of research posters in both virtual and hybrid scientific conferences. In both virtual and hybrid conferences, the preferred digital supplementary material was an audio summary/podcast, while video call and chat function were the top preferred interaction methods with MI specialists.
Note
*Joint first authors
Abstract
Objective
Artificial intelligence (AI)-powered translation tools are gaining attention and acceptance to increase efficiency in medical publication development. However, these tools are not yet mature enough to be used in isolation without human review while maintaining quality and accuracyCitation1. We assessed the performance of a commercially available, AI–powered translation tool in Japanese-to-English translation of scientific and clinical documents.
Methods
After application of pre-translation measures (e.g. file conversion and human translation of embedded images), 23 documents written in Japanese (e.g. clinical study materials, slides, and client comments) were translated into English via an AI-powered translation tool (T-400 [Rozetta Corp., Tokyo, Japan]) and reviewed and manually edited by a bilingual expert. We compared the translation processing speed and review time with this process versus those with pure human translation. Quality of “AI-powered” translation was assessed using a 5-point scale (1 = very poor to 5 = very good).
Results
Following the pre-translation treatment, the processing speed increased by >200-fold with the AI tool versus human translation, which was within expected parameters. The speed for quality review and editing after AI translation increased by 67% compared with internal baseline for previous human-translated projects. The quality of AI–translated content was generally acceptable (4 [good] on the 5-point scale), but human review and editing were required for all materials before their further use in medical publications.
Conclusions
Use of AI-powered translation tools combined with pre-translation measures and human editing can increase translation speed compared with pure human translation in medical publication development with no compromise on translation quality.
Acknowledgements
We thank Shama Buch of Cactus Life Sciences, Cactus Communications, Mumbai, India, for assisting the initial stage of this project and Madhuri Sankaranarayanan, Vanshika Sahni and Vaibhavi Pardeshi of Cactus Life Sciences, Cactus Communications, Mumbai, India, for their administrative support.
Reference
- Heer J. Agency plus automation: designing artificial intelligence into interactive systems. Proc Natl Acad Sci U S A. 2019;116(6):1844–1850.
Abstract
Objective
Podcast articles that undergo a formal peer review and are indexed in literature databases are a novel method to marry the rigor of traditional publications with the convenience of novel delivery systems. We determined best practices for efficient and compliant development of impactful peer-reviewed podcast articles.
Methods
We partnered early in the development of a podcast articleCitation1 to ensure a scientifically rigorous and transparent peer review process. First, the journal editors reviewed a concept document sharing author names, proposed title, and objectives. Then, a detailed discussion guide outlining the authors’ intended conversation was submitted for peer review. Peer review comments were incorporated by the authors at podcast recording. The podcast recording and transcript were then submitted for a second peer review. We examined usage metrics and Altmetrics to determine how the podcast article was received by the community.
Results
The process took 1 month from transcript/audio submission to publication. As of 4 months post-publication, the podcast article has received ∼2600 article accesses and an Altmetric score of 15. It was independently shared through 26 tweets and 24 Mendeley reads. The podcast scored higher than 87% of contemporaries. Users accessed the article mostly via TrendMD, PubMed, Google, direct link and LinkedIn. The podcast platform saw 37 unique user downloads, and 45 downloads from Figshare.
Conclusions
This collaborative process led to the timely publication of a podcast article with measurable impact in the healthcare community. As the publication community looks for new ways to publish peer-reviewed, scientific research in response to HCPs current needs, peer-reviewed podcast articles are a novel option in publications.
Reference
- Shin RK, Rammohan KW, Williams MJ. Expert perspectives on COVID-19 vaccination for people living with multiple sclerosis. Neurol Ther. 2021;10(2):415–425.
Abstract
Objective
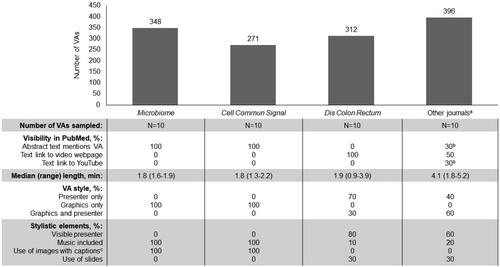
This analysis evaluated visibility and content of video abstracts (VAs) in PubMed and select journals.
Methods
VAs in PubMed were identified via search string, quantified by journal, and randomly selected for content analysis (n = 40). Journals with dermatologists, gastroenterologists, or infectious disease specialists in the top three audiences of the Journal Selector and Conference Authority database (Anju Software) were analyzed for video visibility within journal sites.
Results
Between 1 January 2020, and 1 December 2021, 1327 VAs were identified from PubMed, with 70% identified from three journals and the remaining 30% from 87 journals; median VA length was ∼2–4 min, with varying audio and visual content styles (). Among 200 journals targeting key audiences, video submissions were generally permitted, with videos primarily published in supplemental content.
Conclusions
Video format, such as visible presenter and/or graphical element use, varied across journals. Opportunities to enhance visibility include referencing VAs in abstracts and embedding VAs in main article pages. Video metrics could further inform VA importance in enhancing publications.
Figure 1. VAs identified in PubMed analyzed by journal for visibility and visual and audio content style. Abbreviation. VA: Video abstract. aOther journals included Bioessays, Current Biology, Nuclear Medicine Communications, Journal of Neurologic Physical Therapy, Advances in Neonatal Care, Epidemiology, BMC Pregnancy Childbirth, Developmental Science, and Pediatric Physical Therapy. bOne VA had a mention in the abstract text and a text link to YouTube. cImages included figures, photography, video vignettes, and illustrations.
Abstract
Objectives
India has been evolving steadily into a global hub for Medical Communications (MedComms) activities. Insufficient data exists to show the paradigm shift in MedComms which prompted the MedComms India PROfessional NetwoRking GRoup (MIRORR) to investigate the current landscape through survey responses of MedComms professionals in India.
Methods
We conducted an online questionnaire-based survey to understand the experiences, challenges, and expectations of MedComms professionals in India. The survey was administered via social media platforms and targeted emails to various biopharma and MedComms agencies. Responses were collected anonymously over six weeks between 2 December 2021–10 January 2022 and analysed via a mixed-methods approach. Analyses presented are purely descriptive.
Results
Of the 195 responses received, most were associated with MedComms agencies (60.5%), followed by biopharma (34.0%). Over 41.5% and 13% of respondents had >5 and >10 years of MedComms experience. Primarily, the roles spanned publication writing, reviewing/editing (41.5%), and non-publication content development (25.1%). Employer-driven (50%) and online on-the-job trainings (26.6%) were common training opportunities; 38.9% felt this was insufficient. Membership of any professional societies (AMWA, EMWA, ISCR, ISMPP) stood at 12.8%; of these, 60% were members of ISMPP. About 59% acknowledged the importance of professional certifications in furthering their careers. Of these, 42% aspired for CMPP certification; however, 5.6% had reservations about the usefulness of the certificate for professional growth. Analysis of free-text responses revealed engagement with global colleagues (53%) and strategic project(s) involvement (18%) among the vital future expectations.
Conclusions
MedComms professionals in India are seeking strategic partnership opportunities and resources for education and professional growth for meaningful contributions to this ever-evolving space.
Abstract
Objective
Understanding the health care provider (HCP) audience journey of consuming scientific information is critical for optimizing publication reach; a survey was conducted in 2019–2020 to understand how HCPs identify, review, and share publications of interestCitation1,Citation2. The COVID-19-pandemic, however, has impacted multiple facets of data dissemination. For this reason, a follow-up survey was conducted to assess how HCP behavior may have evolved over the past 2 years.
Methods
Expanding on the survey completed in March 2020Citation1, a 24-question, online survey was conducted via email list, social media, and personal outreach to practicing US-based HCPs.
Results
Of the 33 HCPs who responded, 67% were community practitioners; 45% have been practicing for >20 years and 30% for <5 years. Medical media channels (preferred by 73%) are now the most common means of identifying publications of interest (vs targeted PubMed/Embase searches in early 2020). Sixty-seven percent of HCPs found supplemental digital information moderately/very useful for understanding article content vs 56% in the 2020 survey. When asked about pandemic-related behavior changes, HCPs reported increases in social media use (55%), medical media use (52%), direct reading of research articles (45%), accessing supplemental digital information (39%), and sharing/recommending articles to colleagues (33%). Of note, 67% of HCPs reported that their trust in peer-reviewed publications has not changed, with 42% agreeing that publications should become more accessible to the general public.
Conclusions
Results from this small survey demonstrate that how HCPs interact with publication content is evolving, and that these changes appear accelerated by the COVID-19 pandemic. This evolution is important to understand, and be accounted for, in the framework of omnichannel publication planning.
Keywords:
References
- Ruth A, Subramanian R, Suchy J, et al. Consumption of publication content—mapping the audience journey to inform omnichannel planning in an open world. Curr Med Res Opin. 2020;36(suppl 1):7–8.
- Fazzone W, Bessler J, Darby C, et al. Trends in the use of omnichannel approaches to transform publication planning: results of a cross-sectional survey of medical publications professionals. Curr Med Res Opin. 2021;37(suppl 1):18–19.
Abstract
Objective
The clarity and transparency in conducting and reporting systematic review (SR)/network meta-analysis (NMA)/meta-analysis (MA) are sub-optimal. Poor reporting has been leading to diminishing value to clinicians, researchers and policy makers. In this regard, we evaluated the characteristics, trend, and quality of SR/NMA and MA across all pharmacotherapies.
Methods
Between January 2017 to December 2021, we performed a PubMed search to identify SLR/NMA/MA publications. We extracted information on journal name, impact factor, therapeutic area, country, authors, registration of study design and fulfilment of reporting guidelines.
Results
Our search yielded 1564 relevant articles; 85.3% of which included MA and NMA, published nearly 5 times more than SRs. Oncology (30%) followed by cardiology (15%) were the most commonly studied topic. China (22%), USA (15.7%), UK (14.1%), Italy (5.9%) and Canada (5.6%) largely generated such publications. Overall, 21% of the studies were retrospectively registered and 38.4% complied with reporting guidelines. Methodological quality assessment of the included studies was poor and largely inconsistent. About 81.6% authors provided a rationale for conducting the review, and less than 12% provided protocol information. Only 14% of the reviews were published in journals with IF, 9–10. Most cited articles published in IF >5 (99%) journals were found compliant to PRISMA.
Conclusion
Despite growing awareness, there is a need for improvement in conducting/reporting SR/NMAs/MAs globally; to enhance transparency and reduce risk of outcome reporting bias.
Keywords:
Abstract
Objective
Enhanced publication content (EPC) is increasingly used in peer-reviewed journals. This analysis aimed to better understand journal/publisher views and policies on EPC.
Methods
An online survey was sent to editorial staff at oncology, urology, and general medicine journals or affiliated publishers and posted on the ISMPP Connect forum (Nov-Dec 2021). The 15 questions included answer choice selection, rating scales, and free-text responses.
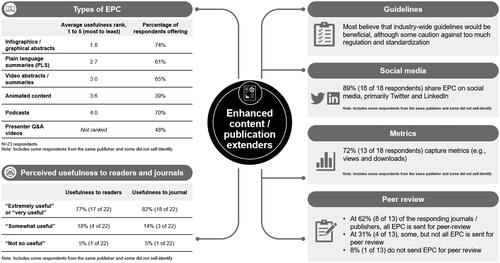
Results
The survey was completed by 23 respondents from six journals and nine publishers. Most consider EPC useful and offer several types (). Respondents stated that the advantages are increased visibility, reach (e.g. wider and more varied audiences), and impact (engagement, usage, and content retention). The most frequently noted challenges were resources (time and/or cost), author awareness, and hosting/accessibility.
Conclusions
Journal editors/publishers find that EPC is of value to readers and journals and serves to increase the visibility of and engagement with published content. Approaches to peer review were inconsistent and most respondents felt that industry-wide guidelines would be appropriate.
Abstract
Objective
We assessed the use of publication extenders in clinical trial manuscripts before and during the COVID-19 pandemic.
Methods
Using SCImago Journal Rank, the top three journals publishing clinical trial results were selected in three research fields (infectious diseases, pulmonology, oncology). Trial results with publication dates of 18 months prepandemic (01 January 2019–30 June 2020) and peripandemic (01 July 2020–31 December 2021) were identified via PubMed. Extender types (podcast, graphic/video abstract, summary, or plain language summary [PLS]) per publication were recorded. Descriptive data are presented.
Results
Of 1716 manuscripts, extenders were seen in 226 (13.2%). Extender inclusion somewhat decreased for pulmonology journals peripandemic; was unchanged in infectious diseases; and was specifically increased in oncology for JAMA Oncol (). Overall, the most frequent extender types (>1 journal) were podcasts (n = 77 [4.5%]) and visual abstracts (n = 58 [3.4%]). PLS inclusion was 0.2% (n = 4).
Conclusions
Except for JAMA Oncol, extender inclusion was generally similar before and during the COVID-19 pandemic. Podcasts and visual abstracts were most common, and utilization of PLS was surprisingly low.
Table 1. Publication extenders for clinical trials in prepandemic and peripandemic time periods across 4 research fields
Acknowledgments
Editorial support was provided by Sherri Damlo, ELS, OPEN Health, Parsippany, NJ, USA.
Abstract
Objective
Patient organizations are an emerging partner in drug research and development. We therefore analyzed perceptions of patient organization representatives regarding Plain Language Summaries (PLS) of medical publications.
Methods
Patient organization representatives across therapeutic areas commented on PLS relevance, understandability, and development process via a web-based survey.
Results
Of the 22 respondents (5 from Europe, 17 from North America), 73% were aware of PLS; all considered PLS “essential/valuable” to patients. The majority of respondents believed that independent sources, such as researchers, educational organisations, patient organizations, medical journals, or medical communication agencies, are best suited for developing PLS, while few believed that of pharmaceutical companies. Plain text and infographics were considered “helpful/very helpful” in making PLS understandable for patients, and most considered the involvement of patient organizations as “important/very important” in the development and review of PLS. Respondents (91–100%) “agreed/strongly agreed” that the most important PLS elements were “short summary of results” and “implications for patients.” However, these elements were reported to be included “very often” in only 32% and 5% of PLS, respectively. The top four characteristics diminishing the value of PLS were “too much jargon,” “conclusions/clinical relevance unclear,” “patronising towards patients,” and “badly written.” Medical journals were generally considered a “trustworthy/very trustworthy” information source, whereas 45% of respondents considered social media “not at all trustworthy.”
Conclusions
PLS are highly valuable to patients and should therefore become a standard addendum to medical publications. Survey findings suggest that PLS should ideally be developed by an independent source, reviewed by relevant patient organizations, and distributed via trustworthy channels, e.g. medical journals.
Abstract
Objective
To understand the implications of variations in first-in-class product descriptions within a first-commercial biotech company’s publications and its impact on the adoption of a standardized lexicon at launch.
Research design and methods
An initial research stage evaluated how the number of publications for first-in-class products from biotechnology companies variedCitation1. In this second analysis, a PubMed search was conducted for company-affiliated publications for these products before and after approval. In each, product descriptions were compared for novel terms or phrases, and variations tracked over time.
Results
Six companies met the criteria for stage 2 analysis and had published a sufficient number of articles to evaluate any change in product description over time and to assess the impact of variations on the adoption of the lexicon into routine use. Inconsistencies in descriptions were observed across publications for all six compounds; for two of the compounds, descriptions were not only inconsistent, but also lengthy and complex. Variations seen did not appear to solely reflect an evolution of narrative over time, or the targeting of publications to different audiences.
Conclusions
These data reveal marked variations in how companies described their innovative compounds and, in some cases, an absence of a simple description of the mechanism or profile. No gradual changes were observed, suggesting differences were possibly due to a lack of standardized lexicon being applied rather than a conscious evolution in narrative. Such variations have the potential to not only hamper understanding of the potential value of a new therapy, but also allow others to shape and own the lexicon for a new class.
Reference
- Lewis M, Falsetti S, Hatfield R, et al. Is there a blueprint for biotech pre-launch peer-review publication planning? Original abstracts from the 2021 European Meeting of ISMPP. Curr Med Res Opin. 2021;37:(sup1):21–39.
Abstract
Objective
The goal for medical communication professionals is to maximize comprehension and minimize ambiguity of evidence-based content. The meaning of content can be influenced by framing it in different linguistic structures. We aim to evidence the selection of linguistic frameworks in medical communications to maximize clarity and understanding.
Methods
A questionnaire was developed on the online platform Gorilla Experiment Builder (www.gorilla.sc). Participants were presented with 55 statement-pairs taken from a schizophrenia scientific communications platform. The statement pairs were categorized as follows: Complex vs concise, positive vs negative, active vs passive, and percentage vs fraction vs number. Participants were instructed to choose which statement they prefer based on “clarity, impact and effectiveness.” Participants had 45 seconds to choose between the two statements to ensure completion.
Results
The questionnaire had 155 respondents, including Healthcare professionals (9%), clients (23.3%) and internal employees (67.8%). A review of standard scientific content illustrated that statements tend to be complex, negative and written in the passive voice. Student’s T-tests showed that participants preferred scientific content to be concise, positive, and active, and for data to be presented as percentages (as opposed to fractions or numbers); all preferences were significant (p < .001). The only non-significant comparisons were for expressing data as fractions vs numbers.
Conclusions
People have linguistic preferences for what makes a statement clear, impactful and effective. Linguistic frameworks presenting scientific information in a concise, positive manner and using the active voice are preferred to traditional complex, passive and negative comparisons. These findings suggest ways of maximizing comprehension and minimizing ambiguity in medical and scientific content. Further studies are needed to determine the generalizability of these findings.
Abstract
Objective
We assessed the quality of patient engagement throughout the development of a unique patient-led and co-authored analysis of the lived experience of myasthenia gravis. We evaluated the self-reported experience of being involved in the qualitative data generation (patient council member) or as a patient author or non-patient author.
Methods
Following the lived experience analysis (September 2020), patient council members (n = 8) completed a self-reported experience survey based on the Patient Focused Medicines Development Patient Engagement Quality Guidance criteria for assessing patient engagementCitation1,Citation2. The survey covered 8 domains and used a Likert scale (strongly disagree to strongly agree). We calculated the overall experience score as a percentage of the maximum possible score. A similar survey was subsequently used in November 2021 to evaluate the experience of the patient (n = 1a) and non-patient authors (n = 3) following the research publicationCitation3.
Results
Patient council members rated their experience of taking part in this analysis highly: average experience score of 90% (71.6/80; n = 8). Respondents also identified approaches to enhance future collaborations, e.g. using translators during meetings. Involvement in the patient co-authored publication was also rated highly: experience score was 92% (78/85; n = 1a) for the patient author and 97% (63.3/65; n = 3) for non-patient authors.
Conclusions
Patients who took part in this patient-led analysis as patient council members or patient authors had a positive experience and provided useful insights on ways to improve patient engagement in future research and patient co-authored publications. aOne of the patient authors sadly passed away following the preparation of the manuscript and was unable to complete the survey.
Keywords:
References
- Patient Focused Medicine Development. Patient engagement quality guidance. 2018. [cited 2022 Jan 12]. Available from: https://patientfocusedmedicine.org/peqg/patient-engagement-quality-guidance.pdf.
- Woolley KL, Arnstein L, Hamoir AM et al. Development and use of 2 tools to facilitate and evaluate patient authorship. Poster presentation at the 15th Annual Meeting of the International Society for Medical Publication Professionals; 15–17 April 2019; National Harbor, MD, USA.
- Law N, Davio K, Blunck M, et al. The lived experience of myasthenia gravis: a patient-led analysis. Neurol Ther. 2021;10:1103–1125.
Abstract
Objective
Medical information (MI) on rare diseases is published in a wide variety of journals due to inherent limitations of studies in this field. With increasing emphasis on digital information sources, rare disease clinicians are challenged to keep up-to-date with MI available in both traditional and non-traditional sources. In this case study, we surveyed lysosomal disease (LD) clinicians to gain insights into preferred MI sources and consumption behavior.
Methods
We conducted a global, 30-question, qualitative online survey (July 2021) covering demographics, MI identification, consumption and sharing practices.
Results
Most of 39 respondents are from Europe (56%), work in the academic setting (92%) and practice in LDs ≥15 years (72%). They were more likely to retrieve MI through congress attendance (95%) and online searches (92%) than through social media (28%) or medical professional media channels (21%). Highly rated aspects of MI were that information is peer-reviewed (97%), published in a high-impact journal (49%) and available through open access (48%). While title (98%) and abstract (94%) are very often read, accompanying digital information (23%) and supplements (10%) were read less frequently. Infographics (31%) and plain language summaries (PLS; 20%) were the most valued digital information types. Clinicians most often share information with peers via in-person conversations (69%) and email (64%). Availability of PLS increases the likelihood of sharing information with patients, and 82% considered it very to extremely important to have MI available in the patient’s local language.
Conclusions
This study indicates that LD clinicians prefer traditional channels to receive and share medical information. Accompanying digital information can support clinicians in sharing MI with patients.
Note
¶Encored at the 18th Annual WORLDSymposium, February 7--11, 2022, San Diego, CA, USA
Abstract
Objective
Open access (OA) publishing improves the accessibility of medical research by providing free access to journal articles. This analysis evaluates the accessibility and OA status of research articles by Bristol Myers Squibb (BMS) over a one-year period.
Methods
Primary and secondary research manuscripts accepted or published between 1 June 2020 and 30 June 2021 were identified in the publication management tool, Datavision. A database of OA status of articles, Unpaywall, was used to characterize the OA status, including type of OA (e.g. Gold) and license (e.g. Creative Commons, CC), for each publication.
Results
82 research manuscripts were accepted or published during the 1-year period. 88% (72/82) of manuscript were freely and immediately accessible while 12% (10/82) were published without OA. 55% (45/82) were published in hybrid journals with OA options and 28% (23/82) were published in OA journals while 5% (4/82) were available in repositories. Among OA and free articles, 46% (33/72) were published with hybrid OA, 38% (27/72) with Gold OA, 11% (8/72) with Bronze OA, and 6% (4/72) with Green OA. Of these articles, 31% (22/72) were published with CC-BY-NC-ND license, 29% (21/72) with CC-BY, 24% (17/72) with CC-BY-NC, and 17% (12/72) without a license. The most common license among hybrid OA articles was CC-BY-NC-ND (61%, 20/33) compared to CC-BY-NC (52%, 14/27) among OA Gold articles.
Conclusions
Nearly 90% of BMS research manuscripts published or accepted between 1 June 2020 and 30 June 2021 were immediately available to readers without a paywall. Future analysis will monitor OA publishing trends for all BMS publications.
Keywords:
Abstract
Objective
ORCID iDs are unique, persistent author identifiers providing disambiguation and transparency in publications. We analysed trends in ORCID iD registration by pharma employees and use in pharma-sponsored publications.
Methods
ORCID iD registrations by employees of 14 pharma companies between 01 January 2018 and 07 January 2021 were extracted and pooled using ORCID’s application programming interface and organization email domains. Previously, ORCID iD use in publications by six of these companies was assessed using PubMed to search for and extract metadata from their sponsored publications published 01 January 2018–07 January 2019Citation1. For comparison, this analysis was re-performed for the same six companies for 01 January 2020–07 January 2021.
Results
ORCID iD registrations by employees at all 14 companies increased by 169% from 01 January 2018 to 07 January 2021. At the publication level, 1289 papers sponsored by the six companies were analysed in the current study (vs 843 previously). A larger proportion of papers had one or more associated ORCID iDs (42.6% vs 27.8% previously), and more of these papers had multiple iDs (52.6% vs 32.5% previously). Overall, more ORCID iDs were listed (1647 vs 388 previously), and a smaller proportion of these belonged to first/last authors (19.0% vs 56.4% previously). More authors who were listed with an ORCID iD and associated with multiple papers used their iDs consistently (23.8% vs 9.1% previously), but three-quarters still did not use them consistently. The proportion of ORCID iDs belonging to authors who are pharma employees decreased (11.5% vs 17.5% previously).
Conclusions
ORCID iD registration by pharma employees and use by authors in pharma-sponsored publications are increasing. However, sustained engagement efforts from pharma and publishers are needed to ensure authors of pharma-sponsored publications use their iDs consistently.
Keywords:
Acknowledgements
Editorial support was provided by Velissaria Vanna (Oxford PharmaGenesis). This study was funded by Open Pharma.
Reference
- Sabir S, Farrow P, Buys M, et al. Registration and use of ORCID by pharma. 2020 European Meeting of ISMPP; London, UK.
Abstract
Objective
The European Medicines Agency (Clinical Trial Regulation No. 536/2014) requires plain language summaries (PLS) be included in the Clinical Trials Information SystemCitation1. The United States has voluntary guidelines. This review investigated gaps in the current guidance for PLS publications.
Methods
PubMed, PubMed Central, and Google Scholar were searched (01DEC2021–06DEC2021); terms included “plain language summary,” “patient lay summary,” and combinations of terms. Duplicate and irrelevant articles were omitted. A general search for PLS-publishing journals (irrespective of article type) was also conducted. Journal guidelines were compared to regulatory guidelinesCitation2,Citation3.
Results
Five journals published clinical trial PLS as an article type and had guidelines available (all from Future Science Group). PLS guidelines were found for Cochrane Consumer Network, Taylor & Francis, and Adis Journals (Springer Healthcare Publishing). Guidelines are compared in .
Conclusions
Publication guidelines for PLS are limited. A steering committee to provide formalized guidance on PLS publication practices is recommended to align pharmaceutical, publishing, and communications industries and expand the reach of scientific research.
Table 1. Comparison of regulatory and journal publisher guidelines for PLS
References
- European Medicines Agency (Clinical Trial Regulation No. 536/2014). Available from: https://ec.europa.eu/health/human-use/clinical-trials/regulation_en
- EU Guidance for writing clinical lay summaries. Available from: https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-10/2017_01_26_summaries_of_ct_results_for_laypersons.pdf
- Draft FDA Guidance on provision of plain language summaries. Available from: https://mrctcenter.org/blog/resources/2017-09-06-guidance-document-draft-fda-guidance-provision-plain-language-summaries/
Abstract
Objective
Individuals who identify as transgender, nonbinary, or genderqueer face obstacles to healthcare access such as discrimination and care refusalCitation1. Gender-inclusive language in peer-reviewed publications could directly promote equity in research and clinical practiceCitation2. This study examines requirements for and instances of gender-inclusive terminology in biomedical publications.
Methods
Using the proprietary software Pubstrat, the top 5 US-based journals in HIV/AIDS, reproduction, and psychiatry were identified by impact factor. Journal guidelines were examined to determine whether they addressed gender-inclusive language. Published inclusivity guidelines from leading institutions within the therapeutic areas were also identified. Finally, a targeted PubMed search spanning 10 years (2012–2022) and including select terms from institutional guidelines was conducted to identify the frequency of conventional or nonpreferred terms versus inclusive or preferred terms.
Results
Of 15 journals evaluated, only 7 had (limited) recommendations for authors. Inclusive terms of importance, particularly in the HIV field as noted in institutional guidelinesCitation2, such as “sexual orientation,” “perinatal transmission,” and “sex assigned at birth” were found to be slightly more prevalent (5132 over 4417 results) than nonpreferred terms such as “sexual preference,” “mother-to-child transmission,” and “biological sex.” The largest adopters of inclusive language were Arch Sex Behav, J Homosex, and LGBT Health. Several recommended terms such as “another sex” (vs “opposite sex”)Citation3 and “lactating parent” (vs “nursing mother” or “breastfeeding mother”)Citation4 are yet to appear in PubMed-indexed publications.
Conclusions
Many publications have begun to use more gender-inclusive language, but there remains a need for consistent guidelines at the journal level. By creating and implementing inclusive, journal-specific guidelines, medical publications professionals could further advance equity in biomedical research and ultimately promote gender-inclusive healthcare.
Note
*Oral Presentation
References
- Moseson H, Zazanis N, Goldberg E, et al. The imperative for transgender and gender nonbinary inclusion. Obstet Gynecol. 2020;135:1059–68.
- NIAID HIV Language Guide. The National Institute of Allergy and Infectious Diseases (NIAID). [cited 2022 Jan 14]. Available from: https://www.hptn.org/sites/default/files/inline-files/NIAID%20HIV%20Language%20Guide%20-%20March%202020.pdf.
- Gender. American Psychological Association. [cited 2022 Jan 14]. Available from: https://apastyle.apa.org/style-grammar-guidelines/bias-free-language/gender.
- Bartick M, Stehel EK, Calhoun SL, et al. Academy of Breastfeeding Medicine position statement and guidance: infant feeding and lactation-related language and gender. Breastfeed Med. 2021;16:587–890.
Abstract
Objective
To understand and improve the process for journal shortlisting, individual involvement, and the key factors impacting choice.
Methods
An 18-item, online survey was developed to gather individuals’ experiences of selecting journals, and circulated via LinkedIn, Open Pharma, ISMPP Forum, MAPS Connect, and personal email invitations. The survey was active from 8–25 November 2021.
Results
In total, 62 respondents completed the survey, with the majority from North America (44%) and Western Europe (39%). Three quarters of respondents were from medical communications agencies (39%) and pharmaceutical companies (37%). Perceived article quality within the journal, journal metrics, and geographical reach were ranked the most important selection criteria; cost and personal invitation were ranked the least important. Only 7 respondents reported that journal familiarity would not impact their choice. Two thirds of respondents (66%) were aware of and used journal selection tools. Most respondents reported review times (87%) and publication times (85%) impacted journal choice, with 68% reporting ≤3 months as a reasonable submission-to-acceptance time, and 73% considering ≤1–3 months as a reasonable acceptance-to-publication time. Interestingly, most respondents (74%) expected to pay publication fees of $2500–$5000 per article and would pay more for open access and rapid review. Of 59 respondents who checked for predatory journals, 81% checked the indexing status. Almost half (45%) considered journal choice at manuscript initiation and 87% reported that authors had the final choice.
Conclusions
Our findings show that individuals involved in scientific publications select journals primarily based on perceived journal merit (quality, impact factor, reach, and speed of publication), and are not dissuaded by publication fees, opting for OA and rapid publication when available.
Keywords:
Abstract
Objective
As a proxy measure of the growing importance of patient-reported outcomes (PROs) in healthcare decision-making, the annual number of studies reporting or mentioning PROs in the peer-reviewed literature over the last 25+ years was assessed.
Methods
A Medline search was conducted (1995‒2021) of the following categories of PROs and patient-specific methodologies: quality-of-life (QoL), work productivity, patient preference, treatment satisfaction, discrete choice experiment (DCE), willingness to pay (WTP), and treatment adherence or persistence. Results were reported for all diseases combined and for several major disease areas including oncology, cardiovascular disease (CVD), and depression/anxiety. Specific instruments were also searched, including the Short-Form 36 (SF-36), EuroQoL 5 Dimension (EQ5D), and Work Productivity and Activity Impairment (WPAI) questionnaire. Numbers of publications in 1995 and 2021 and the magnitude of increase over that period were reported.
Results
Numbers of publications reporting or mentioning PROs or patient-specific methodologies in 1995 and 2021 were: QoL (2806 and 38,584; 14-fold increase); work productivity (6 and 367; 61-fold); patient preference (38 and 1022; 27-fold); treatment satisfaction (8 and 444; 56-fold); DCE (1 and 387; 387-fold); WTP (17 and 1152; 68-fold); and treatment adherence or persistence (10 and 1209; 121-fold). Numbers for oncology, CVD, and depression/anxiety were smaller, but all increased by similar magnitudes. Reporting of the specific PRO instruments also increased: SF-36 (70 and 1656; 24-fold); EQ5D (3 and 943; 314-fold); and WPAI (1 and 80; 80-fold).
Conclusions
Reporting and mentioning of PROs in the peer-reviewed literature have increased substantially and will likely continue to grow since these instruments are an important measure of disease burden and treatment effect.
Keywords:
Abstract
Objective
To assess the reporting prevalence of TCTs, describe reason(s) reported for the termination (RRT), and check the availability of results from TCTs.
Methods
We retrieved interventional trials related to COVID-19 from clinicaltrials.gov as of 22 December 2021. We identified TCTs using their recruitment status and analyzed the RRT. Sub-categories for RRT analysis included “Scientific,” “Non-scientific” and “Not specified.” We also assessed the availability of results for included TCTs with or without corresponding peer-reviewed publications.
Results
Out of the total of 4050 interventional COVID-19 trials, 164 were classified terminated. Of those, only 25 TCTs (15.24%) had their results available on the clinicaltrials.gov site. The majority of RRT were attributed to issues other than scientific data compared to those related to scientific data (78.86% vs 19.43%). The scientific RRT mainly included efficacy and/or safety concerns with or without reference to the interim analysis. Other RRT (non-scientific) included administrative issues (22.46%), study conduct discrepancies (65.22%), and external information triggering the stoppage of trials (12.32%). Of 25 TCTs with the availability of results, only five published their results through peer-reviewed publications, whereas eleven TCTs published their results in peer-reviewed publications but not on the clinicaltrials.gov site.
Conclusion
Poor availability of results of TCTs indicated the scope of improvement in reporting practices of terminated trials. Additionally, well-informed, meticulous trial planning, along with publication creation efficiencies and conduct may help avoid trial terminations due to non-scientific RRT.
Keywords:
Abstract
Objective
Shared decision making, which is transforming medical practiceCitation1,Citation2, requires compliant, evidence-based medical communicationsCitation2,Citation3. Our objective was to investigate: (1) the number of shared decision making publications; (2) their availability as free full-text (ie, available to patients); and (3) relevant research from medical communicators.
Methods
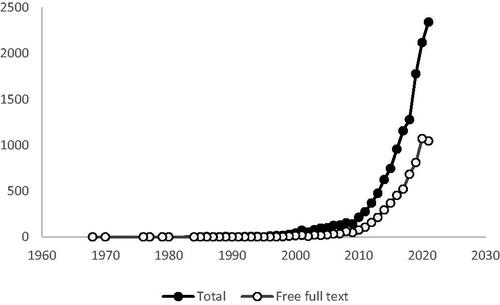
For this bibliometric study, we searched: (A) PubMed (“Shared Decision Making” OR “Shared Decision-Making”; 1 January 1968–31 December 2021), analyzing the total number of publications, the percentage of free full-text publications, and temporal changes; (B) published ISMPP abstracts (2020–2021).
Results
Publications on shared decision making are surging. Of the 13,470 publications identified, only 6107 (45%) are available as free full-text, but this percentage is increasing (). Of the 70 published ISMPP abstracts, only 1 (1.4%) included shared decision makingCitation3.
Conclusions
The surge in publications on shared decision making reflects its growing importance in medicineCitation1,Citation2. Patients cannot easily access most of these publications, but availability is increasing. As medical communications support shared decision makingCitation2,Citation3, medical communicators should be aware of and could contribute to these publications.
Keywords:
References
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891.
- Beach MC, Sugarman J. Realizing Shared Decision-making in Practice. JAMA. 2019;322(9):811–812.
- Arnstein L, Lobban D, Ebina H, et al. Compliance-related questions and comments about plain language summaries of publications: a thematic analysis. Curr Med Res Opin. 2020;36:(sup1):7.
Abstract
Objective
There is increasing interest in the use of artificial intelligence (AI) to facilitate identification, extraction and summarization of scientific evidence. Natural language processing (NLP) tools can summarize information, which could potentially help us navigate through the ever-increasing volume of scientific data. The objective of this analysis was to compare currently available NLP tools versus medical writers (MWs) in their ability to create summaries of scientific publications, specifically looking at time-efficiency, readability and quality.
Methods
The body text (excluding abstract) of five scientific publications were inputted into two, publicly available, NLP-based summary tools. Concurrently, two MWs were also tasked to write unstructured summaries. Ten reviewers were block-assigned five batches of blinded summaries, comprising of four samples (two AI-generated, two MW-generated) and scored their readability and quality using 5-point Likert-scale questions. Descriptive statistics were used.
Results
Mean time required to produce five summaries was 9.8 h for MWs and 12 min for AI. The summaries written by MWs were rated as easier to read (MW =3.6 and AI =2.5; higher scores indicate easier readability) and rated as more “client ready” (proxy for quality) (MW =1.7 and AI =3.4; lower scores indicate more “client ready”). The reviewers felt that flow and relevance of information included in the summaries were the most distinguishing factors between the MW- and AI-generated summaries.
Conclusions
AI tools were more time-efficient, while summaries written by MWs were easier to read, had better flow and included more relevant information.
Abstract
Objective
A growing number of research-performing organizations (institutions) and funding agencies have policies that support open research practices – sharing of research data, code and softwareCitation1. We wanted to better understand if and how organizations are monitoring these practices, and why.
Methods
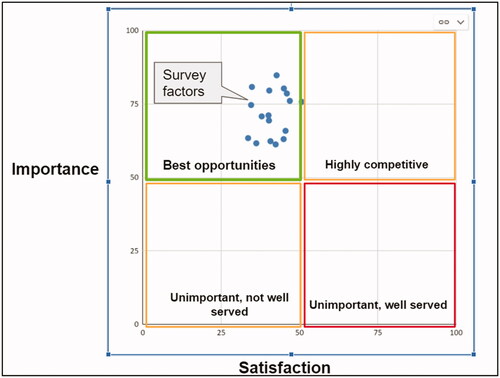
We sent a survey to funder and institutional representatives. We assessed and scored the importance of and satisfaction with 17 “factors” (tasks) associated with understanding open research practices. This includes tasks such as knowing if a research paper includes links to research data in a repository; and knowing reasons why research data are not publicly available.
Results
We received 122 completed responses. Half of respondents had tried to evaluate researchers’ open research practices in the past and 78% plan to do this in the future. The most common method used to find out if researchers are practicing open research was personal contact with researchers and the most common reason for doing it was to increase their knowledge of researchers’ sharing practices. The mean importance of all factors to respondents was 71.7, approaching the 75 threshold of “very important.” The average satisfaction of all factors was 41.3, indicating a negative level of satisfaction with ability to complete these tasksCitation2 ().
Conclusions
The growth of policies and requirements for making research data and code available does not appear to be matched with solutions for determining if these policies have been complied with. More publishers should introduce mandatory data availability statements (DAS) in research articles and visible links to research data on publications to help address this issue.
Keywords:
References
- Gaba JF, Siebert M, Dupuy A, et al. Funders’ data-sharing policies in therapeutic research: a survey of commercial and non-commercial funders. PLoS One. 2020;15:e0237464.
- Hrynaszkiewicz I, Cadwallader L. Dataset from: a survey of funders’ and institutions’ needs for understanding researchers’ open research practices. 2021. Available from: https://doi.org/https://doi.org/10.6084/m9.figshare.14994498.v1
Abstract
Objective
Medical congresses are now often hybrid events with content available live, online, and on demand. Potential digital assets to extend and enhance congress presentations include web links, QR codes, and social media channels. Here we investigated the extent to which publication extenders are being utilized.
Methods
Selected presentations of interest at ESMO 2021 covered breast cancer (BC), ovarian cancer, head and neck squamous cell carcinoma, and other solid tumors (OC/HNSCC/other). Each presentation was checked for web links and/or QR codes, which were accessed; available extenders were downloaded. Social media trends were investigated by searching Twitter mentions related to ESMO 2021.
Results
167 presentations were accessed: BC (n = 132); OC/HNSCC/other (n = 35). 43 (26%) had a QR code: BC (n = 35); OC/HNSCC/other (n = 8). Only 23 (14%) had extended content: BC (n = 18), OC/HNSCC/other (n = 5). 13 were linked to phase 3 data; 5 were linked to phase 1/2 data, and 5 were linked to real-world evidence. Extenders included patient lay summaries (PLS) (n = 12), mobile-friendly view (n = 6), supplemental data (n = 3), videos (n = 3), and trial details (n = 2). Approximately 28,000 tweets mentioned “ESMO 2021”; more than 1000 mentioned “poster,” 128 mentioned “download,” 8 mentioned PLS. Although 11 tweets related to publication extenders mentioned “breast cancer,” none of those tweets directed followers to extender content.
Conclusion
Digital assets have the potential to extend and enhance poster and oral presentation content. However, only a small percentage of ESMO presentations surveyed were accompanied by extended content or featured on social media. Publication professionals should reinforce the value of publication extenders and encourage social media engagement to enhance the reach and value of medical information.
Keywords:
Abstract
Objective
Scientific congresses rapidly adopted virtual platforms during the pandemic, necessitating process optimization for developing presentations and ancillary materials on accelerated timelines. Here we outline key considerations and process refinements through our experience.
Methods
Milestones (abstract dispositions, upload/presentation of poster/oral) from 4 major oncology/hematology congresses (ASCO, EHA, ESMO, ASH 2019–2021) were explored to assess evolution of timelines pre- and during pandemic. Virtual congress platform–driven factors for content enhancement/engagement were also considered.
Results
Across the 4 congresses evaluated, in 2019 the median development time for poster/oral (dispositions to in-person presentation) was 8 weeks (range, 6.3–10.0); in 2020–2021 it was reduced to 6 weeks (range, 5.1–6.3). Because of shorter development times, we integrated several approaches: clear, proactive, streamlined communications with authors/congresses about timelines and content; standardizing processes and templates; early content/creative development (e.g. before abstract dispositions); simultaneous author reviews on shared platforms while maintaining good publications practices; shorter review times for authors; expedited agency-pharma turnarounds; and, where appropriate, reviewing content via teleconference with real-time updates. To enhance engagement in a virtual setting, novel approaches included creating enhanced poster/oral content (e.g. visual posters, digital/HTML posters), polling, dynamic data visualization, mini oral/slides for posters, making content accessible via QR codes linked to sponsor-hosted hubs/microsites, and developing additional materials (e.g. plain language summaries, infographics, speaker videos/narrations) for non-expert readers.
Conclusions
In pivoting to virtual/hybrid, abridged timelines and a need to stand out have prompted refinement of the agency-pharma publications processes and rapid use of enhanced content. These considerations provide a solid foundation for future meetings, which are likely to continue to contain a virtual component.
Abstract
Objective
To gather opinions from pharma professionals on what patient centricity means to them and their organization, the current state of progress, and barriers encountered.
Methods
A survey of industry professionals was commissioned by Cactus Communications, driven by the pharma journal PM360, and distributed via email databases and social media. The survey aimed to assess how respondents define patient centricity, the current state of patient involvement in drug development and clinical research publication, and the barriers to greater patient involvement.
Results
The survey results of 31 pharma and associated industry professionals showed varied definitions of patient centricity: The majority (68%) defined it as “placing the patient’s well-being at the core of all initiatives”. Interestingly, only 6% of the participants defined it as “including patients as authors, peer reviewers, guest editors, and contributors to journals for clinical trial publications”.
“Determining which patients to engage with/representativeness” was identified as the strongest barrier (40% respondents) to patient/caregiver engagement as advisors in the drug R&D process. Further, the “historical precedent to only ask for clinicians’ input” was the strongest barrier (57% respondents) to patient-centricity overall.
“Disease-state education initiatives” (60% respondents) and “post-launch patient education” (52% respondents) are the most common areas where patients are currently involved.
Conclusion
Our survey results indicate that while patient centricity has been gaining momentum as an industry buzzword and a strategic priority for pharma, there exists a critical need for industry standards, guidelines, and educational initiatives to establish definitions of patient centricity and to determine when and to what extent patients should be involved in drug development, medical communication initiatives, and clinical publications.
Acknowledgements
We thank Maarten Beekman (Medical Impact+, South Holland, The Netherlands), Leigh M. Boehmer (Association of Community Cancer Center, Rockville, MD, USA), Lily Chu (International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis, Burlingame, CA, USA), and Jessica (Byam) Klein (Ipsen, Boston, MA, USA) for their inputs on the survey questionnaire.
Abstract
Objective
To understand how social media listening can inform communications strategy as illustrated by a case study in the early treatment of COVID-19.
Methods
Using artificial-intelligence-powered social listening and analytics, social and digital expressed emotions and conversations across multiple channels can be evaluated to improve understanding and uncover trends and strategic insights. The full COVID-19 conversation stream over a 10-month-period was collected. Nonrelevant news articles, and investor- and approval-related conversations were filtered out.
Results
Overall conversation volume for COVID-19 was high (44,140,096 mentions; 39,502,848 posts) with multiple spikes throughout the year, indicating a high level of engagement. Key spikes aligned with general sentiment and news reporting on the pandemic. Conversation drivers primarily focused on vaccines and COVID-19 cases. Conversation volume for long COVID was low, accounting for 0.43% of all COVID-19 conversations, with conversation drivers focused on studies and symptoms related to long COVID treatment and diagnosis. The majority of conversation was from patients versus healthcare providers (HCPs), highlighting the importance of identifying patient influencers as well as HCPs and investigators who can engage on social media. Finally, emotional distribution demonstrated that patients expressed hope, excitement, and positivity towards early treatment.
Conclusions
Social media listening can be used as an additional source of insight to inform communications planning and fine-tune tactics, particularly around patient engagement. Low conversation volume from HCPs combined with the positive treatment sentiment presented an opportunity to increase engagement and lead the conversation among the patient audience. Furthermore, by recognizing the most frequently used source types we were able to target communications to the right audience through the most appropriate channel.
Keywords:
Abstract
Objective
While diverse pharma stakeholders are now consuming scientific content digitally more than ever before, the content itself is still largely designed for print consumption and for a homogenous stakeholder groupCitation1. We aimed to understand from a range of pharma stakeholders how useful and trustworthy they find digital scientific content and their suggestions for improving trust and ease of consumption.
Methods
Qualitative interviews were conducted with two healthcare professionals (HCPs), two medical science liaisons (MSLs), three patient advocates, and one pharma executive representing payers. Interview questions focused on how the different stakeholders find scientific content, trust signals they look for, their favorite formats, and their expectations of pharma.
Results
Each pharma stakeholder group uses a different channel and medium to access content, ranging from PubMed to patient advocacy groups. It is therefore important for pharma to tailor scientific content for each stakeholder and consumption channel being used.
Patients and HCPs would like to see more scientific content developed in collaboration with trusted players like patient-advocacy groups and professional societies, respectively. HCPs prefer on-demand self-education via easy-to-find drug information, whereas patients expect HCPs to know and communicate to them all drug information, including side effects and long-term risks. MSLs on the other hand want bite-sized content with more visual elements unifying data from multiple publications.
Conclusion
Content personalization may be an effective way for Medical Affairs teams to communicate optimally and enhance trust in pharma. While limited in scope, this series of interviews sheds some light on unique stakeholder preferences and their expectations of pharma.
Keywords:
Reference
- Wolf F, Shah S, Meier C, et al. What doctors want and what this means for biopharma now. Boston Consulting Group; 2020. Available from: https://www.bcg.com/en-il/publications/2020/what-doctorswant-and-what-this-means-for-the-biopharma-industry
Abstract
Objective
In recent years, the publication of articles with digital features (DFs), such as infographics and video abstracts, has increasedCitation1. DFs are published with the aim of broadening readership and increasing engagement, however it is not yet known if certain DF types have a greater impact on article metrics than others. We investigated whether certain DF types yielded higher article accesses than others.
Methods
All articles published with DFs in Adis journals in 2020 (n = 58) were identified and categorized into one of eight DF categories (video abstract [n = 11], video [n = 11], graphical abstract [n = 8], graphical plain language summary [PLS; n = 3]), animated trial design figure [n = 9], infographic [n = 7], podcast [n = 7], slide deck [n = 2]). Only articles published with a single DF were included in the analysis. We calculated the average prorated accesses for each DF per year to account for the varying publication months.
Results
Overall, articles published with an accompanying video abstract had the highest average accesses per year (3111 accesses), followed by graphical PLS (1564 accesses). After further investigation into the different video abstract formats, we found that video abstracts consisting of a talking head along with animation/narration elements had the highest average accesses per year (6652 access).
Conclusions
This preliminary analysis suggests that articles published with video abstracts, particularly talking heads with animation/narration elements, receive, on average, a higher number of accesses compared to articles with any other DF type. However, additional studies and data are necessary to confirm these initial conclusions and to identify other factors that may impact article metrics, such as indication, study type, and author renown.
Keywords:
Reference
- Patel J, Halford C, Shepherd A. Article enhancements: a beginners guide. [cited 2022 Jan 17]. Available from: https://ismpp.memberclicks.net/social-media-and-web-metrics-resources?servId=10046?servId=10046&servId=10046?servId=10046&servId=10046?servId=10046&servId=10046.
Abstract
Objective
Recent studies suggest that authors “hype” their findings in publications observed more frequently with male authors in high impact factor (IF) (>10) journalsCitation1–5. We examined these gendered differences using a scholarly information platform.
Methods
General science and medical journal original research articles (IF >10) in 2002–2021 were assessed using Dimensions, identifying author gender (first and last author) with genderize.io, applying a 90% probability threshold. Hype wordsCitation5 were supplemented with similar, handpicked words using GloVe encodings.
Results
Across 1,668,863 publications from 311 journals, the percentage of publications with hyped words in abstracts increased over time and male first authors used the most common “hype” words more frequently than their female counterparts (). Gendered trends were also observed by first/last author in titles and abstracts.
Conclusions
This study confirms increased hyping over time in publications across a broad range of science and medical journals with high IFs, mostly by male authors. More conservative data interpretation by authors may reduce this gendered imbalance.
Figure 1. Percentage of publications with "hype" words in abstract. Inset shows number of publications with “hype” words in abstract. aThe number of publications identified in selected journals of interest were as follows: Nature (n = 51,454), Science (n = 43,588), The Lancet (n = 33,161), and The New England Journal of Medicine (n = 29,692). bP value based on unpaired proportions, using a 2-tailed t-test.
Acknowledgement
Editorial support was provided by Emily Kelly and Christy Vitale of Parexel International, Hackensack, NJ, USA
References
- Tsujimoto Y, Aoki T, Shinohara K, et al. Physician characteristics associated with proper assessment of overstated conclusions in research abstracts: a secondary analysis of a randomized controlled trial. PLoS One. 2019;14:e0211206.
- Shinohara K, Suganuma AM, Imai H, et al. Overstatements in abstract conclusions claiming effectiveness of interventions in psychiatry: a meta-epidemiological investigation. PLoS One. 2017;12:e0184786.
- Hyland K, Feng J. ‘Our striking results demonstrate…’: persuasion and the growth of academic hype. J Pragmat. 2021;182:189–202.
- Millar N, Budgell B, Salager-Meyerc F, et al. Hype in reports of clinical research: the authors’ perspectives. English Specif Purp. 2020;60:53–64.
- Lerchenmueller MJ, Sorenson O, Frank F, et al. Gender differences in how scientists present the importance of their research: observational study. BMJ. 2019;367:I6573.