Abstract
Background
Galcanezumab, a monoclonal antibody to calcitonin gene-related peptide, was found to be safe and efficacious for the preventive treatment of chronic migraine based on the randomized, placebo-controlled double-blind period of the REGAIN study. Long-term safety and efficacy were assessed in an open-label extension.
Methods
Patients 18–65 years old with chronic migraine completing the 3-month double-blind period of REGAIN could enter a 9-month open-label extension (OLE; months 4–12). Upon entering the OLE, patients received a 240-mg galcanezumab loading dose, then 120 mg at the next month, with flexible dosing thereafter (120 or 240 mg/month). The primary efficacy measure was the mean change in the number of monthly migraine headache days from double-blind baseline to month 12. Other endpoints included response rates (based on percent reduction in monthly migraine headache days from double-blind baseline to month 12), safety and tolerability.
Results
Of patients who completed double-blind treatment, 1022 (99%) entered the OLE, with 81% completing month 12. From a baseline of 19.4 monthly migraine headache days at the beginning of the double-blind period, patients at month 12 in the previous placebo, 120-mg, and 240-mg galcanezumab groups had a mean change of −8.5, −9.0, and −8.0, respectively (SE = 0.43 to 0.55, within-group p’s < .001). At month 12, the percentage of patients with ≥50% response was 57%, 57%, and 53%, respectively. Percentage with ≥75% response was 32%, 31%, and 30%, respectively. Percentage with 100% response was 8%, 6%, and 6%, respectively. There were no significant new safety findings during the open-label period. The incidence of discontinuation from the OLE due to adverse events was 5%.
Conclusion
Galcanezumab was effective, safe, and well-tolerated, with high adherence, for up to 12 months of treatment in patients with chronic migraine.
Trial Registration
Clinicaltrials.gov identifier: NCT02614261; www.clinicaltrials.gov/ct2/show/NCT02614261
Introduction
Chronic migraine (CM) is a disabling neurological disorder characterized by 15 or more headache days per month, of which at least 8 are migraineCitation1. CM has a prevalence of approximately 1–2% and carries a significantly higher disease burden marked by greater severity of illness, more missed time from work, greater impact to the quality of life, and greater healthcare resource utilization compared with migraine that occurs with episodic frequencyCitation2,Citation3. Because patients with CM typically require long-term pharmacologic preventive treatments to manage their illnessCitation4–6, such treatments need to be not only effective and safe but also well-tolerated and easy to adhere to. However, adherence to previous standard-of-care treatments, most of which were not developed specially for the treatment of migraine, has typically been poor. Patients with CM who receive prescription migraine preventive medication have been found to have adherence rates of 29% at 6 months and 20% at 12 months in the USCitation7. Although reasons for nonadherence are frequently unknown, particularly in claims database studies, inadequate efficacy and/or tolerability likely play a roleCitation7–10. Thus, longer-term studies are necessary for evaluating the true utility of preventive medication for CM. Because of the high burden of illness associated with CM, it can be difficult to conduct placebo-controlled trials of any considerable length. Therefore, open-label extension (OLE) data become critical to the understanding of the long-term effectiveness, safety, and tolerability of migraine preventive medications in this population.
Calcitonin gene-related peptide (CGRP) has been implicated in the pathophysiology of migraineCitation11,Citation12, and several monoclonal antibodies (mAbs) that target the CGRP pathway have been developed and approved for the prevention of migraine in adultsCitation13–19, including galcanezumabCitation20–22. These CGRP mAbs selectively bind to either the CGRP ligand or receptor and are large molecules that must be injectedCitation23. Treatment with CGRP mAbs has demonstrated safety and tolerability in year-long studiesCitation24–26.
Galcanezumab is a humanized monoclonal antibody that binds to the CGRP ligand, which prevents its binding to the receptorCitation27. Phase 3 studies of 3- to 6-month durations have demonstrated the efficacy and safety of monthly subcutaneous injections of galcanezumab for preventive treatment of episodic migraineCitation20,Citation21 and CMCitation22. The REGAIN study demonstrated that galcanezumab at doses of 120 mg or 240 mg/month was superior to placebo in the reduction of monthly migraine headache days during a 3-month, randomized, double-blind treatment period in patients with CMCitation22. We now present the results of the REGAIN study through month 12, with a focus on the 9-month OLE that followed the 3-month double-blind treatment period. The objective was to assess the long-term effectiveness and safety of galcanezumab for up to a total of 12 months in adult patients with CM.
Methods
Study design, treatment, and patients
REGAIN (ClinicalTrials.gov: NCT02614261) was a phase 3, double-blind, randomized, placebo-controlled trial comprised of 5 periods: (1) screening and washout; (2) a 1-month prospective baseline period; (3) a 3-month double-blind treatment period; (4) a 9-month OLE; and (5) a 4-month post-treatment follow-up period (). The trial was conducted at 116 sites in 12 countries. Detailed procedures have been previously reportedCitation22. This trial is registered with ClinicalTrials.gov (NCT02614261) and was conducted according to Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by the ethical/institutional review boards at all participating sites. Patients provided written informed consent prior to all study procedures and treatments.
Figure 1. Study diagram. Note: the GMB 120 mg dose group depicted in Period 3 had a 240-mg loading dose. Abbreviations: GMB galcanezumab.
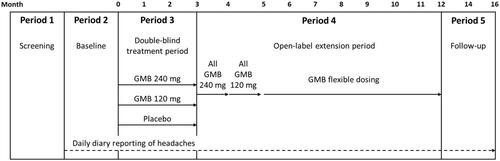
After screening/washout and completion of the prospective baseline period, eligible patients were randomly assigned (2:1:1) to receive subcutaneous injections of placebo, 120 mg/month galcanezumab (with a 240-mg loading dose), or 240 mg/month galcanezumab in the double-blind treatment phaseCitation22. Patients who completed the double-blind treatment period at month 3 could then enter the OLE. However, study sites and patients remained blinded throughout the study to the patients’ previous double-blind treatment assignment.
The dosing regimen for the OLE started all patients on a 240-mg galcanezumab loading dose (2 injections of 120 mg each) after completing double-blind treatment at month 3. At month 4, all patients then received a maintenance dose of 120 mg galcanezumab (single injection) in order to encourage the use of the lowest possible monthly maintenance dose. However, starting at month 5, dosing was flexible such that patients could receive 1 injection (120 mg) or 2 injections (a total of 240 mg) per month at the investigators’ clinical discretion ().
Detailed inclusion and exclusion criteria of REGAIN were previously describedCitation22. Patients were 18 to 65 years of age with an International Classification of Headache Disorders (ICHD)-3 betaCitation28 diagnosis of CM. During the prospective baseline period, patients must have demonstrated at least 15 headache days per month, of which at least 8 had the features of migraine headache, with at least 1 headache-free day and at least 80% compliance with the use of a daily electronic diary (eDiary) for collection of headache information. Key exclusion criteria included persistent daily headache, cluster headache, or atypical migraine subtypes, and patients with previous efficacy failure to 3 or more adequately dosed standard-of-care migraine preventives from different classes (defined as at least 2 months of treatment at the maximum tolerated dose of medications from 3 or more different classes of migraine preventives with Level-A or Level-B evidence per the American Academy of Neurology guidelinesCitation29 or botulinum toxin a or b)Citation22.
Study procedures
Patients used a handheld eDiary to record daily headache information. Acute headache medication could be used as needed, but opioid- or barbiturate-containing medications could not be used more than 3 days per month, and patients could receive no more than 1 corticosteroid injection for acute migraine treatment during the trial, and only in an emergency setting. Up to one-third of patients could continue migraine prophylactic treatment with either topiramate or propranolol if the patient was on a stable dose for at least 2 months prior to the prospective baseline period. Otherwise, patients discontinued all migraine preventives at least 30 days prior to entering the prospective baseline periodCitation20. As reported previously, only 15% of patients elected to remain on topiramate or propranolol during the studyCitation22.
Assessments
A migraine headache day was a calendar day with a headache lasting at least 30 min and meeting ICHD-3 beta criteria for migraine or probable migraineCitation22,Citation28. A headache could also qualify as a migraine if the patient believed the headache was a migraine at onset and was relieved by a triptan or ergotCitation22. A headache day was a calendar day with any headache lasting at least 30 min. The primary efficacy measure was the mean change from the double-blind baseline in the number of monthly migraine headache days. Other outcomes measures included the percentage of patients with ≥30%, ≥50%, ≥75%, and 100% reduction from double-blind baseline in monthly migraine headache days (response rates) and mean change from double-blind baseline in other headache parameters (such as headache days and headache hours) and the Patient Global Impression of Severity of Illness (PGI-S). Scores on the Patient Global Impression of Improvement (PGI-I) are also reported. The PGI-S and PGI-I are single-item patient-rated scales based on the clinician-rated versionsCitation30; in the present study, patients were asked to rate the level of severity and improvement of their migraine illness. Safety assessments included the collection of adverse events and vital signs at all visits, weight and laboratory analytes every 3 months, electrocardiograms at baseline, month 3, month 6, and month 12, and immunogenicity (anti-drug antibodies) at all visits through month 6 plus months 9 and 12. Note that patients also self-reported on the Migraine-Specific Quality of Life Questionnaire (MSQ)Citation31 and Migraine Disability Assessment (MIDAS)Citation32, and those results are available in a separate publicationCitation33.
Statistical analyses
For repeated efficacy measures, data across both the double-blind and OLE periods were analyzed using the total randomized population that had received at least 1 dose of investigational product in the double-blind period (hereafter referred to as the total population). Only patients with both a baseline and at least one postbaseline assessment were included in analyses. Diary data were considered missing for any month with 50% or fewer entries. Safety analyses were based on the open-label treatment population, which was comprised of all patients who received at least 1 dose of galcanezumab during the OLE. Results are reported by patients’ previous double-blind treatment group.
Change from baseline in continuous efficacy variables over 12 months was analyzed using a mixed model repeated measures methodology with fixed categorical effects of treatment, country, baseline medication overuse (yes/no), concomitant migraine preventive use (yes/no), month, and treatment-by-month interaction, as well as the continuous fixed covariates of baseline and baseline-by-month interaction. Change in categorical variables with repeated measures over 12 months was analyzed by a categorical pseudo-likelihood-based repeated measures model for binary outcome employing a generalized linear mixed model with fixed terms for treatment, baseline medication overuse, concomitant preventive use, month, treatment-by-month, and baseline. All efficacy analyses used month 0 as the baseline, also referred to as the ‘double-blind baseline.’ For analysis of baseline characteristics, groups were compared using Fisher’s exact test.
The response rate at each month was defined as the percentage of patients meeting a pre-defined threshold (≥30%, ≥50%, ≥75%, or 100%) in the reduction of monthly migraine headache days from the double-blind baseline. Maintenance of response was evaluated using two different methods. First, among galcanezumab-treated patients who were ≥50% responders at month 3, we evaluated the percentage who met the criteria for ≥50% for at least 6 of the 9 months of the OLE. Second, among all OLE patients, we evaluated the percentage of patients who met ≥50% response at any time during the open-label treatment phase and subsequently maintained ≥40% response for ≥6 and ≥9 consecutive months until the patient’s OLE endpoint.
Treatment-emergent adverse events (TEAEs) were defined as reported adverse events (AEs) first occurring or worsening during the OLE compared with the open-label baseline (that is, all visits prior to entering the OLE). The coding of AEs was based on the Medical Dictionary for Regulatory Activities (MedDRA) version 21.0. Analyses were conducted using SAS Enterprise Guide 7.0 (SAS Institute, Cary, NC). All comparisons used a two-sided alpha of 0.05.
Results
Patient disposition and demographics
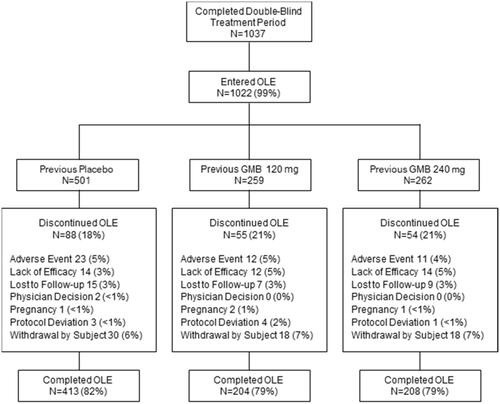
Of the 1113 patients in the total population, 1037 (93%) completed the double-blind treatment period. Of the 1037 patients who completed the double-blind period, 1022 (99%) opted to enter the OLE (501 previous placebo; 259 previous 120 mg galcanezumab; 262 previous 240 mg galcanezumab) (). A total of 197 patients (19%) discontinued during the OLE, with 5% of patients in the OLE discontinuing due to an AE and 4% due to lack of efficacy.
Baseline demographics and disease characteristics are presented for the total population in . Patients were predominantly female (85%) and white (79%), with a mean age of 41.0 years. During the prospective baseline period, they had a mean of 19.4 migraine headache days per month, with levels of functioning (MSQ-RFR) and disability (MIDAS) consistent with a severely disabled chronic migraine population. Characteristics were generally similar across the 3 treatment groups.
Table 1. Characteristics of the total population at baseline of the double-blind period.
Double-blind baseline characteristics of the patients who continued into the OLE (N = 1022) can be found in Supplement Table 1. Their characteristics did not differ from those of the total population (N = 1113). A total of 15% of patients who entered the OLE (150/1022) continued to use an allowed ongoing concomitant prophylactic migraine medication (topiramate or propranolol). The most frequently used concomitant medications (≥5%) among all patients during the OLE were ibuprofen (29%), paracetamol (19%), sumatriptan (13%), topiramate (11%), thomapyrin N (9%), omeprazole (7%), naproxen (7%), diphenhydramine hydrochloride (7%), multivitamin (7%), acetylsalicylic acid (6%), amoxicillin (6%), salbutamol (5%), vitamin D (5%), diclofenac (5%), and loratadine (5%).
Exposure
The majority of galcanezumab exposures in the OLE were to the 240-mg dose regimen. Beginning at the first flexible dosing visit, 64% of patients received the 240-mg dose, increasing to as high as 75% at subsequent dosing visits. The reason for physician dose selection was not captured. Compliance with treatment was high, with only 5 patients missing a dose during the OLE. Adherence to treatment, calculated as the percentage of galcanezumab patients who completed a treatment period, was also high. For the 9-month OLE period, adherence was 81% (825/1022; the total number of patients who completed all 9 months of treatment in the OLE divided by the total number of patients who entered the OLE). The 12-month adherence rate was 74% (412/555; the total number of 12-month treatment completers from the randomized 120-mg and 240-mg galcanezumab groups [n = 204 + 208] divided by the total number of patients randomized to galcanezumab who received at least 1 dose of double-blind galcanezumab [n = 278 + 277]).
Efficacy
During the OLE, the previous galcanezumab 120-mg and 240-mg groups generally maintained or improved upon gains from the double-blind treatment period (). After the first open-label dose of galcanezumab 240 mg at month 3, the previous placebo group experienced a rapid mean reduction of 6.8 migraine headache days within the first month, catching up with the previous double-blind galcanezumab groups by month 4, and then maintaining that reduction over time. The reduction in migraine headache days ranged from 8.0 to 9.0 at month 12 from the double-blind baseline of 19.2 to 19.6. A similar trend was seen in headache days; by month 12 the reduction in headache days ranged from 9.1 to 9.7 days ().
Figure 3. Change from double-blind baseline in the number of migraine headache days. Abbreviations: DB double-blind, GMB galcanezumab, LS least squares, OLE open-label extension, SE standard error. p-value comparisons vs. placebo: **p < .01; ***p ≤ .001.
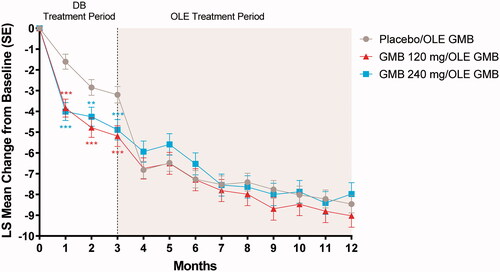
Table 2. Changes in continuous efficacy variables across the double-blind and open-label treatment periods.
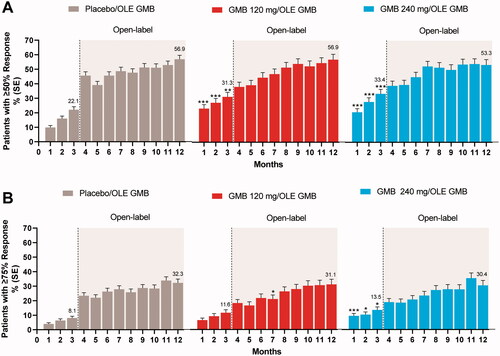
The percentages of patients meeting pre-defined response rates either increased or remained stable during the OLE. A majority of patients achieved at least a 30% reduction from their double-blind baseline for migraine headache days in the first month after beginning open-label treatment. At month 12, 67% in the previous placebo group, 75% in the previous 120-mg galcanezumab group, and 68% in the previous 240-mg galcanezumab group had ≥30% reduction from double-blind baseline for migraine headache days. The percentage of patients with ≥50% reduction from their double-blind baseline ranged from 53% to 57% at month 12 (). The percentage of patients with ≥75% reduction from their double-blind baseline ranged from 30% to 32% at month 12 (). The percentage of patients with 100% reduction from their double-blind baseline ranged from 6% to 8% at month 12.
Figure 4. Percentage of patients with ≥50% and ≥75% reduction from double-blind baseline in migraine headache days. A. ≥50% Response. B. ≥75% Response. Abbreviations: GMB galcanezumab, OLE open-label extension, PBO placebo, SE standard error. p-value comparisons vs. placebo: * p ≤ .05; ** p < .01; ***p < .001.
Among patients who had achieved ≥50% response at the end of the double-blind treatment period, the majority (88%) met that response level for at least 6 months in the OLE. Among all patients in the OLE, 40% met the ≥50% response level and subsequently maintained ≥40% response for at least 6 consecutive months until their OLE endpoint, while 30% met the ≥50% response level and subsequently maintained ≥40% response for all 9 months of the OLE.
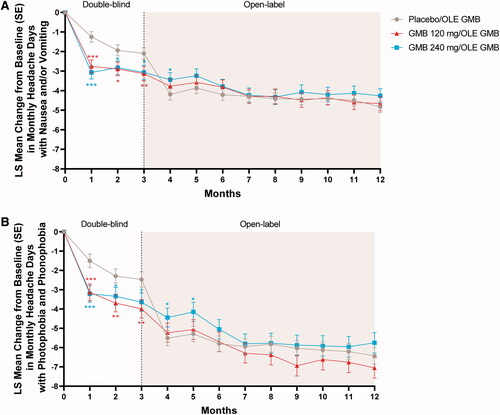
Mean changes in other secondary headache measures based on the eDiary data also showed improvement or maintenance of improvement during the OLE (). On average, patients had 50–60 fewer headache hours per month by month 12 (), 4.3–4.8 fewer migraine headache days with nausea or vomiting per month (), and 5.8–7.1 fewer migraine headache days with photophobia and phonophobia per month (). Mean changes in all secondary headache measures showed a similar pattern of rapid response in the previous placebo group after the first month of open-label treatment with galcanezumab, essentially catching up with the previous galcanezumab groups by month 4.
Figure 5. Mean change from double-blind baseline in monthly headache hours. Abbreviations: GMB galcanezumab, LS least squares, SE standard error, OLE open-label extension. p-value comparisons vs. placebo: *p < .05; ** p < .01; ***p < .001.
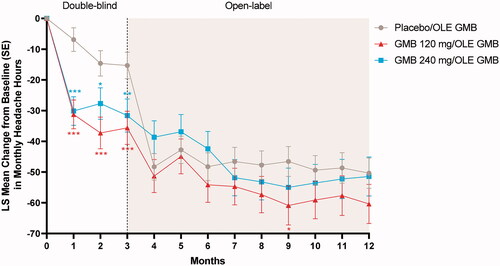
Figure 6. Mean change from double-blind baseline in migraine headache days with nausea and/or vomiting (A) and migraine headache days with photophobia and phonophobia (B). Abbreviations: GMB galcanezumab, LS least squares, OLE open-label extension, SE standard error. p-value comparisons vs. placebo: *p < .05; **p < .01; ***p < .001.
Mean changes in Patient Global Impression of Severity (PGI-S) rating are also shown in and indicated that patients went from an average severity rating of “markedly severe” to an average rating of “mild” to “moderate” severity at month 12. The average Patient Global of Impression of Improvement (PGI-I) score at month 12 indicated that patients rated their migraine illness as “much better” ().
Safety
A total of 70% of patients reported ≥1 TEAE during the OLE. The percentage of patients with ≥1 TEAE considered treatment-related by the investigator during the OLE was 23% (). The most common TEAEs in the OLE were: nasopharyngitis (10%), upper respiratory tract infection (6%), and injection-site reaction (6%). Most TEAEs were mild or moderate in severity. There were no deaths. Serious adverse events (SAEs) were reported in 33 patients (3%). Five SAEs in 4 patients (urticaria, seizure, migraine, diverticulitis, and upper abdomen pain) were considered by the investigator to be related to the open-label treatment; the patient with seizure had a previous history of seizures. The incidence of discontinuations due to AEs was 5%. Of the 46 patients who discontinued due to an AE, 7 patients discontinued due to urticaria, and 2 patients each discontinued due to back pain, dyspnea, headache, increased hepatic enzymes, and rash. All other AEs causing discontinuation were reported by 1 patient each. Of the 7 patients who discontinued due to urticaria, one patient experienced a serious event of urticaria that occurred all over the body and was considered related to the investigational product. The other 6 events were of moderate severity, of which 5 events were possibly related to open-label treatment.
Table 3. Treatment-emergent adverse events during the open-label period.
Pooling all AE terms related to injection sites indicated that 14% of patients had an injection site-related TEAE during the OLE (11%, 17%, and 16% of patients in the previous placebo, galcanezumab 120-mg, and galcanezumab 240-mg treatment groups, respectively). The most frequent types of injection site-related TEAEs were injection site reaction (6%), injection site erythema (3%), and injection site pain (2%). Most injection site-related AEs were mild or moderate in severity. Four patients (0.4%) discontinued treatment due to an AE related to the injection site.
A total of 9% of patients experienced likely hypersensitivity events during the OLE (9% in the previous placebo group, 10% in the previous galcanezumab 120-mg group, and 10% in the previous galcanezumab 240-mg group), with the most frequent type being rash (2%). There were no cases of an anaphylactic reaction.
A total of 15 patients (1%) reported treatment-emergent constipation during the OLE (2% in the previous placebo, <1% in previous galcanezumab 120-mg group, and 2% in previous galcanezumab 240-mg group). Most cases were mild (11 patients) or moderate (3 patients) in severity. No patients discontinued due to constipation.
A total of 11 patients (1%) had hypertension reported as a treatment-emergent adverse event during the OLE (1% in the previous placebo, 1% in previous galcanezumab 120-mg group, and 2% in previous galcanezumab 240-mg group). All cases were mild (5 patients) or moderate (6 patients) in severity, and no patients discontinued due to hypertension.
A total of 7 patients became pregnant during treatment with galcanezumab (2 during double-blind exposure and 5 during open-label exposure). Of these, 3 resulted in normal births, 2 were lost to follow-up, and 2 were electively terminated. For comparison, 3 patients receiving placebo became pregnant during the double-blind period (1 normal birth, 1 premature birth, and 1 trauma-induced miscarriage).
There were no clinically meaningful mean changes in laboratory analyses, vital signs, weight, or electrocardiograms. There was also no signal with respect to treatment-emergent abnormalities on any of the safety parameters.
Assessment of anti-drug antibodies (ADA) to galcanezumab indicated that 11% of patients developed treatment-emergent ADA during treatment with galcanezumab in either the double-blind or OLE periods. However, no meaningful relationship was found between the presence of ADA and the effectiveness of galcanezumab nor any clinical sequelae.
Safety and efficacy in patients on concurrent prophylaxis
A total of 162 patients (15%) elected to remain on either concurrent topiramate (115/162, 71%) or concurrent propranolol (48/162, 30%) for migraine prevention during the study (note that 1 patient inadvertently remained on both). See Supplemental Table 2 for details regarding the baseline characteristics and study disposition of this subgroup of patients. Patient characteristics in this subgroup were generally similar to those of the patients who did not remain on concurrent prophylaxis during the study except for a slightly higher mean age and duration of migraine illness as well as having an average of 1 greater comorbid illness relative to the patients without concurrent prophylaxis. Two of these patients had protocol deviations related to changing or stopping the concurrent prophylaxis (1 switched from propranolol to atenolol during the baseline period and 1 discontinued their propranolol at month 1); otherwise, all concurrent prophylaxis patients continued their topiramate or propranolol throughout their double-blind period participation, with only 6 patients stopping their concurrent prophylaxis at some point during the OLE, which was allowed per protocol.
The study was not powered to detect a difference between treatment arms within the concurrent prophylaxis subgroup, and only the 120-mg galcanezumab group showed numeric superiority to placebo on the primary outcome measure of monthly migraine headache days. The mean change from double-blind baseline to month 3 in monthly migraine headache days (SE) among concurrent prophylaxis patients was −2.6 (1.1) for placebo, −2.9 (1.3) for galcanezumab 120 mg, and −1.9 (1.3) for galcanezumab 240 mg. Once all concurrent prophylaxis patients began open-label treatment with galcanezumab, monthly migraine headache days continued to decrease: −5.0 (1.1), −6.9 (1.4), and −4.4 (1.4) at month 6, and −6.8 (1.1), −9.5 (1.5), and −6.4 (1.5) at month 12 for the previous placebo, previous galcanezumab 120 mg, and previous galcanezumab 240 mg groups, respectively.
Similar to the general study population, a total of 72% of concurrent prophylaxis patients experienced at least 1 treatment-emergent AE in the OLE, with the most common of these being nasopharyngitis (8%), bronchitis (7%), upper respiratory tract infection (7%), and injection-site reaction (6%). There were no meaningful differences between the concurrent prophylaxis patients and the no concurrent prophylaxis patients with respect to safety parameters.
Discussion
The REGAIN study was an international, phase 3, multicenter, randomized, double-blind, placebo-controlled, 3-month study followed by a 9-month OLE in adults with CM. Results from the OLE extend and support the findings of efficacy and safety previously reported for the double-blind treatment periodCitation22. Adherence to treatment was high, with 81% of all patients who entered the OLE (825/1022) completing the 9-month treatment period. Among patients in the randomized galcanezumab groups, who had an opportunity to receive a full 12 months of galcanezumab treatment, adherence at 12 months was 74% (412/555).
Treatment effectiveness
With respect to treatment effectiveness in the OLE, patients in the previous placebo group showed a rapid mean improvement on all efficacy measures after the first open-label dose of galcanezumab, which paralleled the rapid response seen in the galcanezumab-treated groups during the double-blind treatment period. Although results should be interpreted with caution due to the open-label nature of this portion of the study, this parallel slope in the reduction of migraine headache days in the first month of treatment suggests the presence of a rapid and robust drug effect. Additionally, patients in the previous 120-mg or 240-mg galcanezumab groups continued to show mean improvements or maintenance of these responses during the OLE. Thus, results from the OLE demonstrated a sustained effect of galcanezumab in the preventive treatment of migraine in this CM population. At the end of the 9-month OLE, the change in monthly migraine headache days ranged from −8.5 to −9 (), with results starting to plateau. This finding is generally consistent with results over a similar timeframe from open-label studies of erenumab and fremanezumab in patients with chronic migraineCitation24,Citation25.
Other measures of treatment effectiveness indicated that many patients who had already received double-blind galcanezumab continued to improve in the OLE, with almost one-third of patients experiencing a 75% or more reduction from baseline in monthly migraine headache days. Reductions in headache hours and symptoms such as nausea, vomiting, phonophobia, and photophobia also point to important clinical gains over time. These clinically important changes are mirrored in the finding of substantial reductions in migraine-related disability and improvements in day-to-day functioning based on results from the MIDAS and MSQ measured throughout the studyCitation33.
Interestingly, efficacy appeared slightly lower among patients who elected to remain on concurrent topiramate or propranolol for migraine prophylaxis during the study. Comparison of baseline severity indicators for this subgroup relative to those who washed out of their concurrent prophylaxis was most notable for the similarity between the subgroups despite the fact that these patients were still receiving a standard-of-care migraine preventive treatment (i.e. one might have expected their illness severity to be slightly lower at baseline relative to the other patients). Although the reasons for the choice to remain on concurrent prophylaxis rather than risk washing out of the medication were not reported, it is possible that these reasons may have created a selection bias that influenced the efficacy outcomes in this subgroup. Data from a double-blind study of fremanezumab in chronic migraine which also allowed concurrent prophylaxis in a limited number of patients similarly showed a numerically greater reduction in monthly headache days in the patients who were not receiving a concurrent preventiveCitation16.
Tolerability and safety
Among all patients, the incidence of discontinuation due to AEs in the open-label period was low (5%) and similar to the incidence found in another 1-year galcanezumab study (5%)Citation26. Incidence of TEAEs related to injection site appeared to be generally manageable, with most being mild or moderate in severity and only a few leading to treatment discontinuation. Incidence of constipation in the OLE (1%) did not appear to differ from the incidence occurring in the placebo group during the double-blind period (0.5%) when accounting for the 3-times longer duration of the OLE relative to the double-blind period. The use of concurrent topiramate or propranolol did not appear to affect the safety profile for galcanezumab based on the 15% of patients who continued to use one of those medications during the trial.
Accounting for the longer exposure time, results from the REGAIN OLE indicated a safety profile for galcanezumab, which was generally consistent with the findings previously observed in the 3-month double-blind treatment periodCitation22, as well as another open-label galcanezumab studyCitation26. The 1-year study completion rate was high (81%), which was consistent with the 78% completion rate previously observed in the other 1-year open-label galcanezumab study in patients with EM or CMCitation26. This high level of treatment persistence compares favorably with the existing oral standard-of-care migraine prevention treatments given that the majority of patients treated with a standard-of-care preventive medication were shown to be non-adherent after 6 months with discontinuation rates of 73% for antidepressants, 70% for antiepileptics, and 68% for beta-blockers when used as migraine preventivesCitation34.
Limitations
During the OLE, both investigators and patients were not blinded to galcanezumab dosages. This may have affected patients’ response to treatment and reporting of subjective events. Furthermore, because a loading dose (2 injections of 120 mg each) was re-instituted at the beginning of the OLE, patients in the previous 120-mg group essentially received a temporary dose increase. At the next visit, however, all patients had their dose decreased to a single injection (120 mg). This fixed dose of 120 mg at month 4 represented a dose decrease for patients previously receiving the 240 mg dose. Also, the visible switch from 2 injections to 1 may have had a psychological effect on all groups’ responses and may account for the small, transient bump seen in the efficacy figures at month 5. This real and/or perceived dose decrease may have had an impact not only on patients’ response in the month 4–5 timeframe but also may have had an impact on the dosing decision of the following visits. During the flexible dosing period, the higher dose may have been used in an attempt to achieve better efficacy results, especially when AEs were tolerable. However, based on the double-blind data, there is no indication that the use of the 240-mg dose provides significantly different results relative to the 120-mg dose with respect to efficacyCitation22.
Conclusions
Galcanezumab was effective for the preventive treatment of chronic migraine during this 1-year clinical trial. Patients had substantial and sustained reductions in the number of monthly migraine headache days and other important indicators of efficacy such as headache hours and days with symptoms such as nausea, vomiting, phonophobia, and photophobia. Galcanezumab was well tolerated, with low rates of discontinuation and a favorable safety profile. No new safety findings were identified with longer exposure.
Transparency
Declaration of funding
This work was supported by Eli Lilly and Company. Eli Lilly and Company funded the study in a whole and its employees and assignees were involved in study design, data collection, data analysis, data interpretation, and writing of all related reports and publications. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication. Editorial assistance provided by Syneos Health was also funded by Eli Lilly and Company.
Declaration of financial/other relationships
HCD and LQL are full-time employees and minor stockholders of Eli Lilly and Company. SW is a full-time employee of Sarepta Therapeutics. SKA is an employee of Impel NeuroPharma. PPR has received honoraria as a speaker and consultant for Allergan, Amgen, Biohaven, Eli Lilly and Company, Medscape, Novartis, and Teva. She does not have stocks from any pharmaceutical company. UR has received honorarium for the participation in advisory boards, consultation or presentations from Allergan, Amgen, CoLucid, Electrocore, Eli Lilly and Company, Medscape, Novartis, StreaMedUp, and Teva. DD has received honoraria for the participation in advisory boards, consultation, or as a speaker from Novartis, Amgen, Eli Lilly and Company, and Teva. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conception of the work: HCD, SW; Design of the work: HCD, SW; Data acquisition: PPR, UR, DD; Data analysis: SW; Interpretation of data: all authors (HCD, SW, PPR, UR, DD, LQL, SKA); Drafting of the manuscript: HCD; Critical revision of the manuscript for important intellectual content: all authors (HCD, SW, PPR, UR, DD, LQL, SKA).
Ethics statement
This study was reviewed and approved by appropriate institutional review boards and was conducted according to the Declaration of Helsinki. All participants gave written informed consent. The ethical review boards included Quorum Review, Inc., Dean Foundation for Health Research and Education, Baylor, Scott, & White, IRB Services, Montreal Neurological Institute and Hospital, West Midlands - Edgbaston REC, Isala Klinieken METC, Hospital Universitari Vall d'Hebron, Comitato Etico Irccs San Raffaele Pisana, Comitato Etico Interaziendale Bologna-IMOLA, Comitato Etico Area Vasta Centro Presso AOU, Comitato Etico della Provincia di Modena, Comitato Etico Ospedale San Raffaele, Eticka Komise IKEM a Thomayerovy Nemocnice, Eticka Komise Clintrial, s.r.o., Eticka Komise FN u sv. Anny v Brne, Ethikkommission der Landesärztekammer Hessen, Comite de Etica Independiente en Invest. Clinica Dr. C Barclay, Comite de Etica del Centro de Osteopatias Medicas, Sanatorio Allende-Cordoba, Instituto Reumatologico Strusberg, Hillel Yaffe Medical Center, Chaim Sheba Medical Center, Maccabi Healthcare Services, Kfar Saba, Tel Aviv Sourasky Medical Center, Chi-Mei Medical Center - Yung Kang, Institutional Review Board, Kaohsiung Medical University Chung-Ho Memorial Hospital, Sin-Lau Hospital, Taipei Veterans General Hospital, Institutional Review Board, Far Eastern Memorial Hospital, Research Ethics Review Committee, Grupo Médico CAMINO S.C., Hospital Angeles de Culiacan, Hospital Hispano S.A. de C.V.
Supplemental Material
Download MS Word (19.2 KB)Acknowledgements
The authors thank all the study participants, site investigators, and personnel involved in the REGAIN study. Editorial assistance was provided by Regina E. Burris (Syneos Health). Additional statistical support was provided by Hai-An Hsu (TechData Service Company).
Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
- Diener HC, Solbach K, Holle D, et al. Integrated care for chronic migraine patients: epidemiology, burden, diagnosis and treatment options. Clin Med. 2015;15(4):344–350.
- Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache. 2012;52(10):1456–1470.
- Silva-Néto RP, Almeida KJ, Bernardino SN. Analysis of the duration of migraine prophylaxis. J Neurol Sci. 2014;337(1–2):38–41.
- Kowacs F, Roesler CAP, Piovesan EJ, et al. Consensus of the Brazilian headache society on the treatment of chronic migraine. Arq Neuropsiquiatr. 2019;77(7):509–520.
- Bhoi SK, Kalita J, Misra UK. Is 6 months of migraine prophylaxis adequate? Neurol Res. 2013;35(10):1009–1014.
- Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478–488.
- Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22–33.
- Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470–485.
- Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–655.
- Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622.
- Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543–559.
- Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–434.
- Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–1037.
- Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–2132.
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–2122.
- Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319(19):1999–2008.
- Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241–254.
- Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94(13):e1365–e1377.
- Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–1454.
- Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–1088.
- Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91(24):e2211–e2221.
- Reuter U. A review of monoclonal antibody therapies and other preventative treatments in migraine. Headache. 2018;58 Suppl 1:48–59.
- Tepper SJ, Ashina M, Reuter U, et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: results from a 52-week, open-label extension study. Cephalalgia. 2020;40(6):543–545.
- Goadsby PJ, Silberstein SD, Yeung PP, et al. Long-term safety, tolerability, and efficacy of fremanezumab in migraine: a randomized study. Neurology. 2020;95(18):e2487–e2499.
- Camporeale A, Kudrow D, Sides R, et al. A phase 3, long-term, open-label safety study of galcanezumab in patients with migraine. BMC Neurol. 2018;18(1):188.
- Benschop RJ, Collins EC, Darling RJ, et al. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage. 2014;22(4):578–585.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808.
- Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the quality standards subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337–1345.
- Guy W. ECDEU assessment manual for psychopharmacology, revised. 1976. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch. p 217–222.
- Jhingran P, Osterhaus JT, Miller DW, et al. Development and validation of the Migraine Specific Quality of Life Questionnaire. Headache. 1998;38(4):295–302.
- Stewart WF, Lipton RB, Kolodner K, et al. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia. 1999;19(2):107–114.
- Ford J, Tassorelli C, Leroux E, et al. Changes in patient functioning and disability: results from a phase 3, double-blind, randomized, placebo-controlled clinical trial evaluating galcanezumab for chronic migraine prevention (REGAIN). Qual Life Res. 2021;30(1):105–115.
- Berger A, Bloudek LM, Varon SF, et al. Adherence with migraine prophylaxis in clinical practice. Pain Pract. 2012;12(7):541–549.