Abstract
Objective
To describe the design of the CLARION post-approval safety study (EU PAS Register number, EUPAS24484) and provide a status update, including characteristics of patients included up to 1 May 2021.
Methods
CLARION aims to further evaluate adverse events of special interest in patients who are newly initiating treatment with cladribine tablets for relapsing multiple sclerosis (MS). The study population consists of two cohorts: patients newly initiating cladribine tablets (cladribine cohort) and patients newly initiating oral fingolimod tablets (comparator fingolimod cohort), with an aim to include 8000 patients (4000 patients per cohort). The study relies on secondary use of data from pre-existing MS registries/data sources (except in Germany, where primary data collection is performed). The study is projected to last 15 years, with an anticipated 5-year inclusion period. Study outcomes are: malignancies; severe infections; tuberculosis; progressive multifocal leukoencephalopathy; other opportunistic infections; herpes zoster; severe lymphopenia (Grade ≥ 3); and treatment discontinuation.
Results
As of 1 May 2021, 2393 patients were included in CLARION from seven participating MS registries/data sources (cladribine cohort, n = 1266; fingolimod cohort, n = 1127). The majority of patients are female (cladribine cohort, 72.5%; fingolimod cohort, 68.0%), with mean age at onset of MS of 31.5 years for the cladribine cohort and 30.9 years for the fingolimod cohort. The majority of patients in both cohorts had relapsing MS (cladribine cohort, 92.1%; fingolimod cohort, 93.5%).
Conclusion
By providing further information on adverse events of special interest during long-term follow-up, CLARION will assist neurologists and patients regarding treatment decision-making for management of relapsing MS.
Introduction
Patients with multiple sclerosis (MS) require life-long treatment with disease-modifying therapies (DMTs); as such, it is important to understand the long-term safety of such therapies in routine clinical practiceCitation1–3. This can be achieved through collection and assessment of high-quality drug utilization and safety data. The European Medicines Agency (EMA) Patient Registry Initiative provides guidance for a standardized approach to evaluating the benefit-risk of medicines in the European Economic Area using data from registriesCitation4. In response, MS registries have begun to collect safety data using a more systematic approach, including harmonization of data collection across different sourcesCitation5.
In recent years, cladribine tablets 10 mg (3.5 mg/kg cumulative dose over 2 years) have been approved in many countries for the treatment of adult patients with relapsing MS. The mechanism of action of cladribine tablets in MS involves selective, transient reduction of lymphocytes; consequently, lymphopenia is an expected event during treatmentCitation6–10. Long term safety data showed that, apart from herpes zoster, there was no increased risk of infections (including severe infections or opportunistic infections) in patients receiving cladribine tabletsCitation9,Citation10.
In placebo-controlled clinical trials of cladribine tablets, a numerical imbalance in the rate of malignancies was notedCitation10. While this was not statistically significant, regulatory guidelines state that appropriate pharmacovigilance activities should be used to estimate the incidence rate of such rare events and generate long-term safety dataCitation11. Regulatory authorities consequently requested a post-approval safety study to further characterize the safety profile of cladribine tablets in the real-world setting. The CLARION study (EU PAS Register number, EUPAS24484Citation12) was therefore designed to compare adverse events of special interest (AESI) among patients with relapsing MS newly initiating either cladribine tablets or fingolimod tablets. AESI are defined as malignancies, severe infections, herpes zoster, tuberculosis, progressive multifocal leukoencephalopathy (PML), and other opportunistic infections. Fingolimod has been selected as the primary comparator for this study given that both cladribine tablets and fingolimod tablets are orally administered and have a similar indication, thereby minimizing possible indication and selection bias.
The objective of this manuscript is to describe the design of the CLARION study and provide a status update, including characteristics of included patients up to 1 May 2021. The main results of the first interim analysis are summarized elsewhereCitation13.
Methods
Study design
CLARION is an observational, comparative cohort study involving multiple countries, mostly across Europe, where cladribine tablets have been launched. It is planned to obtain US data from the Department of Defense data source, pending results of an ongoing feasibility assessment. The study population consists of two cohorts: patients newly initiating cladribine tablets and patients newly initiating fingolimod tablets (hereafter referred to as the cladribine and fingolimod cohorts, respectively). In addition to the fingolimod control cohort, at completion of the study, an external comparator cohort of ocrelizumab-treated patients, obtained from the ongoing VERISMO studyCitation14, will be used to better contextualize the risk of malignancies.
CLARION relies on secondary use of data from pre-existing MS registries/data sources except in Germany, where primary data collection is performed. Data collection began from the launch of cladribine tablets in each participating country. The study is projected to last 15 years, with an anticipated 5-year inclusion period. Patient follow-up starts on the date a patient newly initiates cladribine tablets or fingolimod tablets, and continues for a period of 10 years (or until the patient is lost to follow-up, withdraws consent, or dies). Study objectives and corresponding outcomes are shown in .
Table 1. Objectives and cohort level outcomes in CLARION.
Study population and data sources
Patients eligible for inclusion are those who newly initiated treatment with cladribine tablets or fingolimod tablets in line with the local labels for the respective treatment, after the date of cladribine tablets launch in the relevant country. Patients are considered as newly initiating their treatment if they have not previously received a prescription for cladribine tablets or fingolimod tablets. In Germany (primary data collection), patients are included after written informed consent is obtained at the date of treatment initiation or, at the latest, 24 weeks after treatment initiation.
Patients who have received fingolimod tablets before newly initiating cladribine tablets, or cladribine tablets before newly initiating fingolimod tablets, are not eligible for the study. The population coverage and data elements planned for inclusion in the study are shown in .
Table 2. Planned data sources, population coverage, and data elements to be included in CLARION.
Outcomes and covariables
Study outcomes are shown in . Study-specific data will be registered for each of the following events by international classification of disease (ICD)-10 or MedDRA codes: malignancies, grouped and by individual type; severe infections, tuberculosis, PML, other opportunistic infections, and herpes zoster (regardless of severity). Severe lymphopenia (Grade ≥ 3) will also be recorded, either on the basis of laboratory test results (lymphocyte cell count <0.5 × 109/L) or in Germany recorded as an adverse event in the electronic case report form.
Treatment discontinuation (including date and reasons for discontinuation) will also be evaluated.
The following variables will be collected at inclusion:
Demographics.
MS disease history and medical history, including infection status (e.g. hepatitis B virus [HBV]).
Concomitant medicines and vaccines (type and date), including immunosuppressive/immunomodulatory agents (other than DMTs) such as corticosteroid treatment.
Any personal and family history of malignancy.
Use of sex hormones prior to inclusion (among women).
Radiation exposure (e.g. UV light), alcohol consumption, smoking, and obesity.
The following variables will be collected during the follow-up period:
Concomitant medications, including immunosuppressive/immunomodulatory agents (other than DMTs) such as corticosteroid treatment.
Other infections (e.g. hepatitis B and C status).
Use of sex hormones (among women).
Radiation exposure (e.g. UV light), alcohol consumption, smoking, and obesity.
Date of death, if applicable.
Regarding the use of DMT, the following data will be collected over the period prior to inclusion and during follow-up: type of DMT, as well as start and stop date. This will allow for presentation of data according to DMTs used and the two most recent DMTs used (including reasons for discontinuation). For each cohort, DMTs will be presented by drug type and grouped as immunosuppressive/immunomodulatory agents (according to influence of the agent on the immune system) and short-to-mid-term or long-term DMTs, according to the duration of the effect.
Definition of exposure
The primary definition of exposure for all outcomes (except for severe lymphopenia) is intention to treat (ITT), in which patients are classified according to treatment received at inclusion. Regarding severe lymphopenia and as part of sensitivity analysis for other outcomes, the definition of exposure is as-treated (AT) in which exposure is time-dependent and classified per on-going treatment at a given time: current exposure to cladribine tablets; current exposure to fingolimod tablets; or no current exposure to study treatment. At treatment discontinuation or switch from initial study treatment to another study treatment or to any other DMT, current exposure will be extended by a washout period of 6 months (or 12 months) for the discontinued treatment after which the exposure to the newly initiated treatment will start. Other definitions of exposure to cladribine tablets or fingolimod tablets will also be explored ().
Table 3. Additional exposure definitions.
Data management and flow
Three types of data transfer to the clinical research organization (CRO; IQVIA) supporting Merck Healthcare KGaA for this non-interventional study will be used, depending on the participating MS registry/data source (): Patient-level data transfer of primary data (Multiple Sclerosis Management System 3D and NeuroTransData), patient-level data transfer of secondary data (Finnish and Norwegian registries and Swiss MS Cohort-Study [SMSC]), and aggregate-level data transfer of secondary use of data (Danish, Italian, and Swedish registries, MSBase, and Observatoire Français de la Sclérose en Plaques [OFSEP]).
Figure 1. Overview of data flow from collection to the consolidated database in the CLARION study. CRO, clinical research organization; MSDS 3D, Multiple Sclerosis management system 3 Dimension; MSBase, Multiple Sclerosis database: NTD, NeuroTransData database; OFSEP, Observatoire Français de la Sclérose en Plaques; SMSC, Swish MS Cohort.
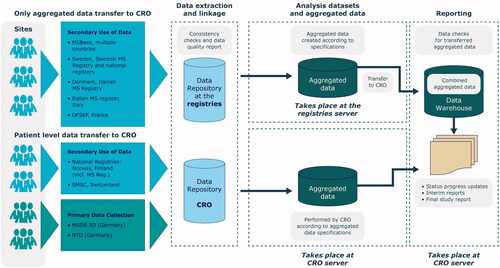
For Nordic countries (secondary data), data from MS registries are linked with national registers. In Finland and Norway, data are anonymized before each transfer. In Denmark and Sweden, data will be transferred already aggregated and linked.
For harmonization purposes, and to allow merging and analysis of data across the individual registries, a common data model (CDM) is used. Based on the list of outcomes and variables, study data elements are cleaned, mapped to common terminology, and transformed into a CDM by each registry providing aggregated-level data or by the CRO for those providing patient-level data. Registries providing aggregated-level data create aggregated datasets from the recorded patient-level data in a predefined standard format. This CDM defines the level of aggregation and minimum information required to enable the statistical analysis of study objectives. The CRO will combine the datasets from registries and data sources and perform the statistical analysis of the whole study.
Study size and data analyses
The study aims to include 8000 patients (4000 patients per cohort). Considering the primary study objective to further evaluate the risk of AESI, the study size estimation was based on simulated data and driven by malignancy as the AESI with the lowest incidence rate among the list of AESI being evaluated in CLARION. Four interim study analyses are planned at 3, 6, 9, and 12 years from the start of data collection, and a final analysis after study completion. shows an overview of the planned analyses.
Table 4. Overview of planned analyses.
The primary analysis will be the crude and adjusted incidence rates of AESI along with 95% confidence intervals (CI) in both cohorts. Incidence rates will be calculated as the number of events divided by the risk time in person-years. To compare the incidence of AESI between cohorts, the incidence rate ratios of AESI (together with the 95% CIs) will be estimated by Poisson regression models adjusted for key prognostic factors. For malignancies, time-to-event analysis using a weighted Cox proportional hazard model with inverse probability of treatment weights will be used to obtain a hazard ratio (HR) estimate and its corresponding 95% CIs using the ITT exposure definition. Specifically, time-to-malignancy will be analyzed using a meta-analytical approach in three steps: Step 1: estimation of propensity score (PS) weights within each data source. Step 2: estimation of the data source specific confounder-adjusted HRs from the time-to-event analysis using Cox proportional hazards model, incorporating the PS weights estimated in step 1. Step 3: estimation of the overall pooled confounder-adjusted HR from a meta-analysis of data source specific confounder-adjusted HRs.
The primary analysis described above will be repeated for the subset of patients with relapsing remitting MS only.
Secondary analyses will include the incidence rate and corresponding 95% CI of severe lymphopenia in patients treated with cladribine tablets; its duration will also be described. The effect of prior use of immunomodulatory/immunosuppressive agents on the incidence of AESIs in the two cohorts will be assessed by subgroup analysis. Also included are the number and percentage of patients that switch in the cladribine cohort as well as the type of DMT given after cladribine tablets.
Data quality
The validity of the primary outcome across all participating MS registries and data sources is acceptable, although some variation across registries may exist. For example, in the Nordic countries (Denmark, Finland, Norway, and Sweden), the ascertainment of malignancies is established through linkage with national cancer registries for the purpose of the study. In these countries, reporting a cancer event to the cancer registries is mandatory and the registries are considered as achieving high-quality standards in terms of completeness and accuracy of the registered dataCitation19. Close to 100% completeness of incidence of solid malignancies have been reported in each of the cancer registriesCitation20,Citation21. When compared to morphologically verified cases (i.e. biopsy or autopsy), the validity of the cancer registries ranges from 93% in FinlandCitation20 to 98% in SwedenCitation19.
In Germany, where primary data collection is used, as well as in the OFSEP (France) and SMSC (Switzerland) data sources, the completeness and validity of malignancies can be ascertained through data source verification at site level and feedback to centers with queries in case of missing data. This source verification process is not possible in the remaining data sources (MSBase [multi-country] and Italian MS Registry [Italy]).
The quality of data is assessed yearly using pre-specified data quality indicators (DQIs) and summarized in data quality assessment reports. Each participating data source is responsible for the quality and integrity of collected study data according to existing standard operating procedures or internal guidelines. A set of DQIs has been developed to monitor the quality of study data in terms of consistency, accuracy, completeness, and representativeness. These DQIs are based on the European Medicines Agency’s Patient Registries Initiative’s report on multiple sclerosis workshop held on 7 July 2017Citation1. This approach helps to identify potential data inconsistencies and methodological differences across registries early, including outcome definitions such as treatment discontinuation. To date, the application of data quality indicators in the CLARION study has improved data quality over time in terms of completeness and accuracyCitation22. Detailed data quality results will be published in a separate manuscript.
Management and reporting of adverse events
The management and reporting of adverse events (AEs) vary according to the study model in the countries involved following EU GVP module VI revision 3. Non-interventional studies based on secondary use of data do not require reporting of suspected adverse reactions in the form of Individual Case Safety Reports, where the reporting obligation rests with the investigator. In Germany where primary data are collected, the reporting of AEs is based on additional forms as appropriate, including: (serious) AE, pregnancy, parent-child/fetus AE, and AE of special interest report forms.
Ethical considerations
The study is conducted according to Guidelines for Good Pharmacoepidemiology Practice (GPP) and the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) Code of Conduct, which ensures compliance with General Data Protection Regulation (GDPR). The study protocol was approved in Germany by the Ethikkommission of the Technische Universität Dresden (reference number: EK 338092018) and in Switzerland by the Ethikkommission Nordwest- und Zentralschweiz (EKNZ) (reference number: 2019-01949). Data permits are granted by approval bodies in each Nordic country.
Study status
As of May 2021, 2393 patients were included in the CLARION study (); registry participation in the study, over time, is summarized in . Of the 2393 patients included, 1266 patients were in the cladribine cohort and 1127 in the fingolimod cohort; their characteristics are summarized in . The majority of patients are female (cladribine cohort, 72.5%; fingolimod cohort, 68.0%), with mean age at onset of MS of 31.5 years for the cladribine cohort and 30.9 years for the fingolimod cohort. The majority of patients included in each cohort had relapsing MS (cladribine cohort, 92.1%; fingolimod cohort, 93.5%). Approximately 75% of patients have at least one annual visit with their treating physician, on average, over the follow-up period.
Figure 2. Inclusion status in the CLARION study over time. The figure shows the number of patients included in the study at different data cut-offs, and this is regardless of the initial treatment time (some patients added in 2020 were already treated in 2019).
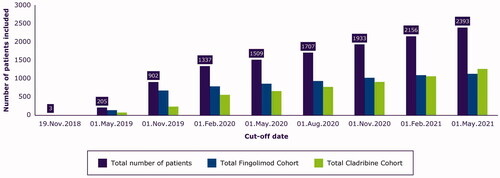
Table 5. Registry participation in the CLARION study, over time.
Table 6. Demographics and disease characteristics of patients in CLARION (cut-off date: 1 May 2021).
Discussion
Data generated with cladribine tablets in the clinical development program have demonstrated the efficacy and safety profile in the treatment of patients with relapsing MSCitation9,Citation10,Citation23–27, findings that have been confirmed in follow-up reportsCitation28 and real-life studies of the safety and effectiveness of such treatmentCitation29. As a large, non-interventional study reflecting real-world treatment and management of such patientsCitation12, CLARION will provide additional evidence relevant to clinical practice given that it will involve a larger number of patients and a longer observation period than the clinical development program and the aforementioned studies. Furthermore, it’s design – using mainly pre-existing data – makes it an invaluable resource to potentially address new safety issues in patients treated with cladribine tablets.
Over time, national and international MS registries have improved data coverage and established collaborations with regulatory authorities and the pharmaceutical industry in the conduct of post-approval safety studies such as CLARION. The existing framework to combine data from multiple registries is expected to contribute to the advancement of knowledge and management of patients with MSCitation5. Patient counts by cohort are obtained every 4 months from each participating MS registry/data source. This allows tracking of the number of patients included and implementation of mitigation plans as needed. For example, the feasibility of adding further MS registries to the CLARION study has been examined, so that the range of data sources can be broadened to increase the rate of patient inclusion at a later date if needed.
Limitations of the study and mitigation plans
Selection bias
To minimize selection bias, the inclusion criteria for the study were selected to be as broad as possible for this study population.
Generalizability of results
Since the study is based on pre-existing MS registries and conducted mainly via secondary use of data, some countries where no registry or data source was identified were not eligible for this study. However, countries with the highest MS incidence in Europe, such as Finland, Denmark, France, and Norway, are included or plannedCitation30. Per protocol, those patients receiving fingolimod tablets before initiation of cladribine tablets were also excluded. The proportion of excluded patients is 19.5% overall, and varies across MS registries/data sources and countries.
Information bias
This is a distortion in the estimate of association between risk factor and disease that is due to systematic measurement error or misclassification of patients on one or more variables, either risk factor or outcome. To minimize this type of bias in the current study, instructions on variables and outcome definitions are provided to all neurologists/physicians in the centers participating in the study with primary data collection. In the Nordic national registries, the study outcomes and covariables were defined using validated algorithms when available.
Channeling bias
Cladribine tablets was a new drug on the market at the start of this study while fingolimod was well established. Thus, patients newly initiating cladribine tablets might be different from patients treated with fingolimod tablets, despite similar therapeutic indications, especially at the very beginning of the study. To address this potential channeling biasCitation31, baseline characteristics in the two cohorts will be checked over time to identify any differences between the two cohorts. If such differences are identified, sub-group analysis will be performed.
Confounding by indication
This bias can arise because of the presence of an underlying diagnosis or other clinical features that trigger preferred use of either cladribine tablets or fingolimod and are related to patient outcomes. For example, patients on annual skin screening due to risk of basal cell carcinoma might be prescribed cladribine tablets more often than fingolimod, and this may impact study outcomes.
Limitations related to differences across data sources
In all Nordic countries, the national healthcare registers have a lag time of 3–12 months. Different categories may also be used by the data sources for recording the type of MS disease. For example, Norway’s NMSRB does not include primary and secondary progressive MS in their categorization. Different data sources may have different missing variables, such as date of diagnosis. The magnitude of this limitation, and impact on study results, will be assessed using analyses for Germany in which fewer missing variables are expected as it is primary data collection versus other data sources. Due to data protection restrictions in Denmark, a “tiny cell rule” applies for database cells with fewer than five observations (e.g. rare events). If fewer observations are contained in the aggregated data, a further aggregation might be applied. Alternatively, cells with fewer than five observations are clouded before the aggregated data is transferred. In CLARION, the inclusion period and number of patients included also varies across registries/data sources. This might introduce differences in patient characteristics that may be associated with, or reflect, the effect of underlying determinants and bring potential bias and/or confounding. To account for this potential impact, descriptive analysis of patient characteristics by cohort, country, and calendar time will be performed. Further analysis of study outcomes will be performed if the description suggests that imbalances or differences in patient characteristics have the potential to introduce bias and/or confounding.
Limitations related to the aggregated data model
Compared with patient-level data, the use of aggregate-level data will potentially limit the performance of additional analyses for the overall population.
Limitation related to missing variables
As an example, this may be an issue when measuring the duration of lymphopenia. Given that no regular blood tests are requested per protocol to confirm recovery of lymphocyte counts, there is a risk to overestimate the duration of lymphopenia. However, for patients with severe lymphopenia, which is the outcome of interest in CLARION, this risk will likely be minimized as the physician would be expected to perform a closer follow-up with more frequent blood testing.
Limitations relating to the comparison with an external cohort, regarding malignancy
Results of the comparison with the external cohort of ocrelizumab-treated patients need to be interpreted with caution due to potential selection bias and other differences in patient characteristics and their management. Inclusion and exclusion criteria for the ocrelizumab study may differ from the CLARION study population, and ocrelizumab administration is via intravenous infusion while cladribine and fingolimod are both oral. However, ocrelizumab is approved for a similar indication to cladribine tablets and its approval date will allow comparison of incidence of malignancies for patients included during the same time period as CLARION; moreover, both cohorts are expected to have a similar size.
Data representativeness and generalization of results
From the data collected currently in CLARION, the percentage of women and mean age of onset of MS is representative of the general MS population. The majority of included patients had previously received MS treatment, with a similar proportion of treatment-naïve patients across the two cohorts. However, patients included in the study are new users for cladribine tablets and fingolimod tablets with no prior fingolimod use. Potential exclusion of a number of patients with prior fingolimod use may limit the generalization of results to patients treated with cladribine tablets. Early findings from CLARION indicate that 18% of patients were excluded from the cladribine cohort because of prior fingolimod useCitation13.
Conclusions
By providing further information on AESIs during long-term follow-up, CLARION will assist neurologists and patients regarding treatment decision-making for management of relapsing MS.
Transparency
Declaration of funding
The CLARION study is sponsored by Merck [CrossRef Funder ID: 10.13039/100009945].
Declaration of financial/other relationships
HB’s institution (Monash University) received compensation for consulting, talks, and advisory/steering board activities from Alfred Health, Biogen, Merck, Novartis, and Sanofi; research support from Biogen, Merck, MS Research Australia, National Health and Medical Research (Australia), Novartis, the Oxford Health Policy Forum, and Roche. He has received personal compensation for steering group activities from Oxford Health Policy Forum and Merck. NM has consulted with Merck. AA is an employee of EMD Serono Research & Development Institute, Inc. Billerica, MA, USA (an affiliate of Merck KGaA). JS was an employee of IQVIA at the time of this study, and is currently an employee of CATO SMS, Durham, NC, USA. IB is an employee of IQVIA, a contract research organization that performs commissioned pharmacoepidemiological studies for several pharmaceutical companies. PK was an employee of IQVIA at the time of this study, and is currently an employee of REWODAT Oy, Lohja, Finland. MS is an employee of Merck Healthcare KGaA, Darmstadt, Germany. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Nicholas Moore, Aida Aydemir, Jaak Sõnajalg, Pasi Korhonen and Meritxell Sabidó were involved in the conception and design of the study whilst all authors were responsible for the analysis/interpretation of data, drafting and revision of the intellectual content and final approval of the paper.
Data availability statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the Data Sharing Policy of Merck. All requests should be submitted in writing to the data sharing portal of Merck https://www.merckgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials/commitment-responsible-data-sharing.html. When Merck has a co-research, co-development, or co-marketing or co-promotion agreement, or when the product has been out-licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavour to gain agreement to share data in response to requests.
Acknowledgements
Medical writing assistance (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Mark O’Connor and Joseph Ward of inScience Communications, Springer Healthcare Ltd, Chester, UK, and funded by Merck Healthcare KGaA, Darmstadt, Germany.
References
- Dirks P, Zingler V, Leemhuis J, et al. Design of a non-interventional post-marketing study to assess the long-term safety and effectiveness of ocrelizumab in German real world multiple sclerosis cohorts - the CONFIDENCE study protocol. BMC Neurol. 2020;20(1):95.
- Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91(6):660–668.
- Chisari CG, Toscano S, D’Amico E, et al. An update on the safety of treating relapsing-remitting multiple sclerosis. Expert Opin Drug Saf. 2019;18(10):925–948.
- European Medicines Agency. Use of disease registries for benefit-risk evaluation of medicines: a regulatory perspective 2018. Available from: www.ema.europa.eu/en/documents/presentation/presentation-registry-initiative-april-2018_en.pdf.
- Hillert J, Trojano M, Vukusic S, et al. Big multiple sclerosis data – a registry basis for post authorization safety studies (PASS) for multiple sclerosis [P1381]. Mult Scler. 2019;25(2 suppl):761–762.
- Baker D, Pryce G, Herrod SS, et al. Potential mechanisms of action related to the efficacy and safety of cladribine. Mult Scler Relat Disord. 2019;30:176–186.
- Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168–174.
- Stuve O, Soelberg Soerensen P, Leist T, et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther Adv Neurol Disord. 2019;12:1756286419854986.
- Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult Scler Relat Disord. 2019;29:157–167.
- Leist T, Cook S, Comi G, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord. 2020;46:102572.
- European Medicines Agency. Guideline on Risk Management Systems for Medicinal Products for Human Use [EMEA/CHMP/96268/2005] 2005. [Feb 2021]. Available from: https://www.pharmacoepi.org/pub/?id=1c2a4382-2354-d714-51bd-b9fdecdb3d0c.
- European Network of Centres for Pharmacoepidemiology and Pharmacovigilance registration number, EUPAS24484 Available from: www.encepp.eu/encepp/viewResource.htm?id=28797.
- Hillert J, Butzkueven B, Soilu-Hänninen M, et al. Incidence of infections and severe lymphopenia in patients newly initiating cladribine tablets or fingolimod for treatment of multiple sclerosis: CLARION study [P742]. Mult Scler. 2021;27(2 suppl):622–623.
- Wormser D, Evershed J, Ferreira G, et al. VERISMO: a post-marketing safety study to determine the incidence of all malignancies and breast cancer in patients with multiple sclerosis treated with ocrelizumab (P4.2-043). Neurology. 2019;92(15 Supplement):P4.2–043.
- Flachenecker P, Buckow K, Pugliatti M, et al. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523–1532.
- Myhr KM, Grytten N, Torkildsen Ø, et al. The Norwegian Multiple Sclerosis Registry and Biobank. Acta Neurol Scand. 2015;132(199):24–28.
- Hillert J, Stawiarz L. The Swedish MS registry – clinical support tool and scientific resource. Acta Neurol Scand. 2015;132(199):11–19.
- Persson R, Lee S, Yood MU, et al. Multi-database study of multiple sclerosis: Identification, validation and description of MS patients in two countries. J Neurol. 2019;266(5):1095–1106.
- Pukkala E, Engholm G, Højsgaard Schmidt LK, et al. Nordic cancer registries - an overview of their procedures and data comparability. Acta Oncol. 2018;57(4):440–455.
- Leinonen MK, Miettinen J, Heikkinen S, et al. Quality measures of the population-based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017;77:31–39.
- Maret-Ouda J, Tao W, Wahlin K, et al. Nordic registry-based cohort studies: possibilities and pitfalls when combining Nordic registry data. Scand J Public Health. 2017;45(17_suppl):14–19.
- Magyari M, Moore N, Wergeland S, et al. Novel use of harmonized data quality indicators in long-term safety studies using multiple sclerosis registries: approach in CLARION study. ICPE. 2021; 23–25 August.
- Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426.
- Leist TP, Comi G, Cree BA, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol. 2014;13(3):257–267.
- Freedman MS, Leist TP, Comi G, et al. The efficacy of cladribine tablets in CIS patients retrospectively assigned the diagnosis of MS using modern criteria: Results from the ORACLE-MS study. Mult Scler J Exp Transl Clin. 2017;3(4):2055217317732802.
- Montalban X, Leist TP, Cohen BA, et al. Cladribine tablets added to IFN-beta in active relapsing MS: the ONWARD study. Neurol Neuroimmunol Neuroinflamm. 2018;5(5):e477.
- Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: Results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24(12):1594–1604.
- Moccia M, Lanzillo R, Petruzzo M, et al. Single-center 8-years clinical follow-up of cladribine-treated patients from phase 2 and 3 trials. Front Neurol. 2020;11:489.
- Disanto G, Moccia M, Sacco R, et al. Monitoring of safety and effectiveness of cladribine in multiple sclerosis patients over 50 years. Mult Scler Relat Disord. 2022;58:103490.
- Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816–1821.
- Strom BL, editor. Pharmacoepidemiology. 4th ed. Chichester (UK): John Wiley & Sons Ltd; 2006.
