Abstract
Objective
To describe the trends in epidemiology, healthcare resource use (HCRU), and costs associated with Lewy body dementia (LBD), dementia with Lewy bodies (DLB), and Parkinson’s disease dementia (PDD) in the United States.
Methods
This retrospective study used administrative claims data for Medicare fee-for-service (2010–2018) and commercially-insured beneficiaries (2010–2017). The annual prevalence and incidence were calculated among the Medicare beneficiaries by dividing the number of prevalent or incident LBD, DLB, and PDD patients by the total eligible population of that calendar year. Baseline patient characteristics, HCRU, and costs over time were described for Medicare and commercially insured patients with continuous health plan enrollment for ≥12 months before and ≥24 months after first cognitive impairment (CI) diagnosis.
Results
From 2010 to 2016, the incidence and prevalence rates of LBD among Medicare beneficiaries ranged from 0.21%–0.18% and 0.90%–0.83%, respectively. Of 9019 Medicare patients with LBD who met other inclusion criteria, 4796 (53.2%) had DLB and 4223 (46.8%) had PDD. The mean age was 78 years and the mean Charlson Comorbidity Index score was 1.6. On average, patients with LBD incurred $18,309 in medical costs during the 1-year pre-diagnosis and $29,174 and $22,814 at years 1 and 5 after diagnosis, respectively. The main cost drivers were inpatient and outpatient visits. Similar trends were observed for DLB and PDD as well as for commercially-insured patients.
Conclusions
Our findings highlight the substantial epidemiological and economic burden across the LBD spectrum and underscore a high unmet need for effective treatments to improve patient outcomes.
Introduction
Lewy body dementia (LBD) is a generalized term used to describe neurodegenerative disorders characterized by the pathological accumulation of the protein alpha-synuclein (i.e. Lewy bodies) throughout the brainCitation1. Two prominent subtypes of LBD include dementia with Lewy bodies (DLB) and Parkinson’s disease dementia (PDD)Citation2. Although DLB and PDD have overlapping clinical pathologies (i.e. impairment in cognition and movement, sleep disorders), they are differentiated by the chronology of specific symptomsCitation2. Specifically, people who experience parkinsonism prior to cognitive impairment are classified as having PDD while those experiencing cognitive impairment prior to or within one year of parkinsonism are classified as having DLBCitation3,Citation4.
LBD is the third most prevalent form of dementia, after Alzheimer’s disease (AD) and vascular dementiaCitation5, and is estimated to affect 1.4 million adults in the United States (US)Citation6. Currently, two medications have been approved by regulatory agencies for the treatment of LBD subtypes: donepezil (approved for DLB in JapanCitation7) and rivastigmine (approved for PDD in the USCitation8 and EuropeCitation9). However, no pharmacological agent has been broadly indicated to treat LBD, and the clinical management of LBD focuses on alleviating the severity of symptoms using pharmacologic agents indicated for the treatment of dementia and/or parkinsonism. As a result, people with LBD often require care from multiple different specialists which complicate treatment and increases the burden of illnessCitation10. Indeed, a retrospective claims-based study published in 2019 using Medicare fee-for-service (FFS) data from California found that LBD was associated with the highest annual costs per beneficiary ($22,514) relative to other forms of dementia, including AD ($13,935)Citation11.
However, there is a paucity of empirical evidence regarding the recent trends in prevalence and incidence of LBD and its subtypes; particularly in the USCitation12,Citation13. A longitudinal study published in 2013 that estimated the incidence of DLB and PDD from 1991 to 2005 found that the combined incidence rate of both LBD subtypes was 54.6 per 100,000 person-yearsCitation13. Moreover, the incidence rate of DLB was 31.6 per 100,000 person-years and the incidence of PDD was 23 per 100,000 person-yearsCitation13. However, estimates were based on 542 incident cases from a single county within the state of Minnesota that primarily consisted of individuals of European descent which limits the generalizability of the reported incidence rate. Other studies assessing the overall prevalence of LBD in the US have reported estimates of 0.02% in the general population in FloridaCitation12 to 4.4–5.4% of all dementia cases among Medicare beneficiariesCitation11,Citation14. Moreover, differences in study designs, particularly the source of data and timeframes analyzed, make comparisons across studies challenging.
In addition to limited evidence regarding the epidemiology of LBD and its subtypes, little is known about the similarities and differences between the profiles of patients with PDD and DLB. Prior studies have largely focused on outcomes of cognitive and neuropsychiatric assessments as opposed to demographic and comorbidity profilesCitation15–17. One study that assessed the comorbidity profiles of patients with Parkinson’s disease (PD) and cognitive impairment (CI) relative to patients with PD without CI found that PD with CI was associated with significantly higher odds of cerebrovascular disease (odds ratio: 1.24) and PD medication use (odds ratio: 1.46) (both p < .05)Citation18.
Furthermore, contemporary insight regarding the economic burden of LBD overall and by subtype is lacking. For example, a cross-sectional study published in 2003 that compared one year of direct costs among patients with AD without parkinsonism, AD with parkinsonism, and DLB found that patients with DLB and AD with parkinsonism incurred higher costs than patients with AD alone (average adjusted increases above the AD baseline costs were $19,564 and $7394, respectively)Citation19. However, that study was limited by small sample sizes (15 patients with DLB; 39 with AD with parkinsonism), and proxy cost data (e.g. estimates were based on caregiver utilization reports and national estimates of care)Citation19. A more recent retrospective, claims-based study focused on characterizing trends in healthcare resource use (HCRU) among patients with DLB found that increases in direct medical costs correlated with the complexity of DLB (e.g. dementia and higher number of core features); however, patients with PDD were not assessedCitation20. In contrast, the aforementioned study by Chandler et al. found that patients with PD with CI had more hospitalizations, visits to the emergency department (ED), and ultimately incurred higher costs compared with patients with PD without CI; DLB patients were not assessedCitation18.
In light of the aging US population and high costs related to dementia care, it is important to characterize recent trends in the epidemiology and economic burden of LBD in larger and more representative populations. In this study, we assessed the incidence, prevalence, HCRU, and costs over time of LBD overall as well as by DLB and PDD subtypes among adults in the US.
Methods
Data sources
This study used administrative claims data from two sources: 1) Standard Analytical Files for a 5% random sample of FFS Medicare beneficiaries, and 2) OptumHealth Care Solutions database for a commercially-insured population. Both data sources contain information regarding patients’ medical and enrollment histories although they differ with regard to the population covered and the time period of data availability as described below. Data from both sources were de-identified and compliant with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act; as a result, approval from an ethics committee was not required.
Medicare
The Medicare data used in this study were obtained from the Standard Analytical Files for a 5% random sample that includes >4 million beneficiaries drawn from the total Medicare population and covers services provided between 1999 and 2018. Approximately 97% of the US population aged ≥65 years receives health insurance from Medicare, a federal health insurance programCitation21. Among all Medicare beneficiaries, about half are 65–74 years, 36% are ≥75 years, and 14% are <65 years; the majority (79%) are whiteCitation22. Although pharmacy claims (i.e. Part D) are not available, the database includes enrollment history, patient demographics, and detailed medical claims, including inpatient and outpatient claims (e.g. diagnosis codes, procedure codes, place of service, quarter and year of service, provider type, provider specialty, physician identifiers, and paid amounts) and is generalizable to the Medicare FFS population in the US.
Commercial insurance
The OptumHealth Care Solutions commercial insurance database contains information on approximately 19.9 million beneficiaries with coverage from January 1999 to March 2017. The database includes individuals and their dependents who have commercial health insurance plans provided through their employers. While beneficiaries of all age groups are included, most are between the ages of 18 and 64 years. Medicare Supplemental data are available for a subset of beneficiaries (approximately 1.5 million) aged 65 years or older. The database includes medical (e.g. diagnosis codes, procedure codes, place of service, date of service, provider type, provider specialty, paid amounts) and pharmacy claims (e.g. National Drug Codes, quantity dispensed, date rendered, paid amounts). Data are provided by 84 companies (“employers”) across a broad spectrum of industries (e.g. financial services, manufacturing, telecommunications, energy, and food and beverage) and job classifications in the US.
Sample selection and study design
Prevalence and incidence
The annual prevalence and incidence of LBD among Medicare beneficiaries were evaluated over seven calendar years of available data (2010–2016) to allow sufficient time to observe the development of both CI and parkinsonian symptoms. Patients were required to have ≥2 diagnoses indicative of LBD (≥2 DLB diagnoses, or ≥1 DLB diagnosis and ≥1 non-DLB CI diagnosis plus ≥1 PD diagnosis, or ≥2 non-DLB CI diagnoses plus ≥2 PD diagnoses) to ensure the sample does not include people who were potentially misdiagnosed in the claims data (and had only one diagnosis record) (see Table S1 for a complete list of ICD-9 and ICD-10 codes used to identify CI and PD). Requiring more than one relevant diagnosis was informed by clinical expert opinion and is consistent with prior research using claims dataCitation23,Citation24. Patients were further required to be ≥65 years of age and have continuous enrollment in both Medicare Part A and B during the calendar year of estimation as well as the previous year (for incidence estimates only). Patients were included in the prevalence and incidence estimates for LBD in the year they first met the criteria for either DLB or both PD and CI. For the DLB subgroup, patients were included in the assessment in the year they had a diagnosis for DLB or a diagnosis of PD, with a diagnosis of CI in the years leading up to the PD diagnosis. Similarly, patients were included in the PDD prevalence and incidence estimation in the year they received their first CI diagnosis, following a PD diagnosis.
Economic burden
The criteria used to select patients are summarized in . Briefly, for Medicare assessment, the study population comprised patients who met the criteria for LBD as defined in the prevalence and incidence estimation above, and had the first observed CI diagnosis between January 2012 and December 2016 (index date). In addition, all patients were required to be ≥65 years of age and be continuously enrolled in Medicare Part A and B for ≥12 months before and ≥24 months after the index date.
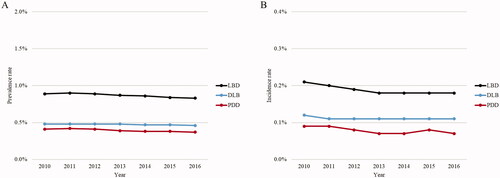
Figure 1. PrevalenceCitation1–6 and incidenceCitation7 rates of LBD, DLB, and PDD in the Medicare population. Abbreviations: CI: Cognitive impairment; DLB: Dementia with Lewy bodies; LBD: Lewy body dementia; PDD: Parkinson’s disease dementia. Notes: 1Annual prevalence was estimated by dividing the number of prevalent LBD patients by the total number of patients in the eligible population of that calendar year. Prevalent LBD patients in a given calendar year were identified as the subset of patients who met the criteria for LBD based on all claims observed in the database, and whose index date was prior to or within the calendar year of interest.
2Patients were considered to have met all criteria of LBD if they had any of the following: 2 DLB diagnoses; or 1 DLB diagnosis, 1 non-DLB CI diagnosis, and 1 PD diagnosis; or 2 CI diagnoses and 2 PD diagnoses. 3Patients were considered to have DLB if their first-ever CI event was before or concurrent with the first-ever PD event on record. Their index date was the date of the first PD event. 4Patients were considered to have PDD if their first-ever CI event was after the first-ever PD event on record. Their index date was the date of the first CI event. 5CI events were defined as DLB diagnoses and non-DLB CI diagnoses. PD events were defined as DLB diagnoses and PD diagnoses. 6See Table S1 for ICD-9-CM and ICD-10-CM codes used to identify DLB, non-DLB CI, and PD diagnoses. Non-DLB CI diagnoses considered included mild cognitive impairment, memory loss, and dementia. 7Annual incidence was estimated by dividing the number of incident LBD patients by the total number of patients at risk in the eligible population of that calendar year. Incident LBD patients in a given calendar year were identified as the subset of patients who met all criteria for LBD based on all claims observed in the database, and whose index date was in the calendar year of interest.
For commercial insurance, in addition to the diagnosis codes noted in Table S1, prescription claims were also used to ascertain the presence of CI and PD. Specifically, patients were considered to have LBD if they had any of the following: 1) ≥2 DLB diagnoses; 2) 1 DLB diagnosis plus ≥1 non-DLB CI diagnosis or antidementia medication fill, and ≥1 PD diagnosis or antiparkinson medication fill; 3) ≥2 CI diagnoses or antidementia medication fills plus ≥2 PD diagnoses or antiparkinson medication fills. Patients were required to be ≥50 years old and be continuously enrolled in non-HMO health plans for ≥12 months before and ≥24 months after the first indication of CI from March 2010 to March 2015 (index date).
For both data sources, the baseline period was defined as the 12 months before the index and the follow-up period was defined as the time interval that spanned from the index date to the end of data availability or continuous insurance enrollment, whichever occurred first. Analyses were conducted for the overall study cohort of all LBD patients as well as stratified by subtype (i.e. DLB, PDD). Specifically, patients were included in the DLB subgroup if their earliest observed CI diagnosis or antidementia medication fill was before or concurrent with the first observed PD diagnosis or antiparkinson medication fill. Patients were included in the PDD subgroup if their earliest observed CI was after their first PD diagnosis or antiparkinson medication fill.
Study measures
Prevalence and incidence
The annual prevalence and incidence were calculated by dividing the number of prevalent or incident LBD patients (or the relevant subtype) by the total number of beneficiaries aged ≥65 years who were continuously enrolled in Medicare Part A and B for that calendar year. For incidence estimation, all beneficiaries were required to have no indication of LBD (or the relevant subtype) prior to the start of the calendar year of estimation.
Baseline characteristics
Patient characteristics were assessed for the overall LBD cohort and DLB and PDD subgroups on the index date or during the baseline period. The characteristics evaluated on the index date included demographics (e.g. age, sex, race/ethnicity, US census region), index year, type of diagnosis (i.e. DLB, PDD), type of CI diagnosis (e.g. mild cognitive impairment [MCI], senile dementia), physician specialty (e.g. internal medicine, neurologist, geriatrician) at the first observed CI diagnosis, and setting of the first diagnosis (e.g. inpatient, outpatient). Additional assessments included comorbidity profile (e.g. the Charlson Comorbidity Index [CCI]Citation25), HCRU (e.g. inpatient admissions, ED visits, outpatient visits), prescription drug use for commercially insured (e.g. cholinesterase inhibitors, memantine, antiparkinson agents, antipsychotics, antidepressants, sedatives and hypnotics) during the baseline period. Total costs from a payer perspective (i.e. medical costs for the 5% Medicare sample; a sum of medical costs and pharmacy costs for the commercially-insured) were also assessed during the baseline period.
HCRU and costs during the follow-up period
For each year of the follow-up period, the number of events and proportions of patients with ≥1 event of the following types was reported for the overall LBD cohort as well as the two subgroups: inpatient admissions, inpatient days, ED visits, physician office/outpatient visits, and other visits (e.g. home healthcare). In addition, for each cohort, annual per-patient costs during the follow-up period were assessed from a payer perspective, excluding patient contributions (e.g. copayment, coinsurance, deductible). The annual costs included medical costs, overall and by place of service (e.g. inpatient, emergency room, outpatient, other), as well as pharmacy costs (for commercial insurance only). All costs were inflated to 2019 US dollars using the medical care component of the Consumer Price IndexCitation26.
Statistical analysis
Patient characteristics, HCRU, and costs were summarized using means and standard deviations (SDs) for continuous measures and frequencies and percentages for categorical measures. The differences in characteristics and outcomes were compared between the DLB and PDD subgroups and statistical significance was assessed using Chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous measures. All analyses were conducted using SAS Enterprise Guide version 7.15 and R version 3.6.1. Unless otherwise indicated, statistical significance was defined as p < .05.
Results
Prevalence and incidence
Overall, the prevalence and incidence of LBD, DLB, and PDD among Medicare beneficiaries were stable over time (). From 2010 to 2016, the prevalence rate of LBD ranged from 0.90% to 0.83%. The prevalence rate was slightly higher for DLB (0.48%–0.46%) than for PDD (0.42%–0.37%) (). From 2010 to 2016, the incidence rate of LBD ranged from 0.21% to 0.18%. The incidence rate was slightly higher for DLB (0.12%–0.11%) than PDD (0.09%–0.07%) ().
Study sample and baseline characteristics for economic burden assessment
Of the 9019 patients from the Medicare database who met all inclusion criteria for LBD, 4796 and 4223 were included in the DLB and PDD subgroups, respectively (). In the overall cohort of Medicare patients with LBD (), the mean age at the index date was 77.8 years and about half of all patients were male (53.2%). The most common CI diagnoses at the index date were memory loss, other dementia, MCI, and AD. Significantly more patients with DLB were diagnosed with memory loss relative to patients with PDD on the index (47.9% vs. 31.2%; p < .001); significantly fewer patients with DLB were diagnosed with other dementia relative to patients with PDD (30.9% vs. 45.9%; p < .001).
Figure 2. Sample selection flowchart. Abbreviations. CI, cognitive impairment; DLB, dementia with Lewy bodies; GPI, generic product identifier; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; LBD, Lewy body dementia; PD, Parkinson's disease; PDD, Parkinson's disease dementia Notes: 1See Table S1 for ICD-9-CM and ICD-10-CM codes used to identify DLB, non-DLB CI, and PD diagnoses. Non-DLB CI diagnoses considered included mild cognitive impairment, memory loss, and dementia. 2Antidementia medications considered included memantine and cholinesterase inhibitors. 3CI events were defined as DLB diagnoses, non-DLB CI diagnoses, and anti-dementia medication fills. PD events were defined as DLB diagnoses, PD diagnoses and antiparkinson medication fills. Note that medication use was only evaluated in the commercial database, not the Medicare database. 4Patients with 1 DLB diagnosis were required to have the following: ≥1 non-DLB CI diagnosis or antidementia medication fill; ≥1 PD diagnosis or antiparkinson medication fill. Patients with no DLB diagnoses were required to have the following: ≥2 CI diagnoses; or ≥2 antidementia medication fills; or ≥1 CI diagnosis and ≥1 antidementia medication fill; ≥2 PD diagnoses; or ≥1 PD diagnosis and ≥1 antiparkinson medication fill.
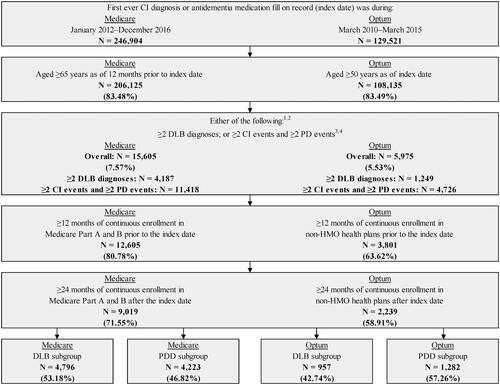
Table 1. Baseline characteristics of the Medicare population.
The most common physician specialties at the index date were neurology (DLB: 20.1%; PDD: 27.2%; p < .001) and internal medicine (DLB: 19.5%; PDD: 11.0%; p < .001). For both subgroups, the physician's office/outpatient setting was the most commonplace service (DLB: 82.3%; PDD: 75.0%; p < .001). The mean CCI score was 1.6 (SD = 1.8) for both DLB and PDD subgroups (). Almost all patients had doctor's office/outpatient visits during baseline (DLB: 98.9%; PDD: 99.8%; p < .001). All baseline HCRU was significantly lower among patients in the DLB subgroup relative to the PDD subgroup, including the proportion of patients with any ED visits (DLB: 51.0%; PDD: 58.1%; p < .001) and inpatient visits (DLB: 31.1%; PDD: 37.7%; p < .001). Similarly, baseline total medical costs were significantly lower for the DLB vs. PDD subgroup ($15,903 vs. $21,040; p < .001).
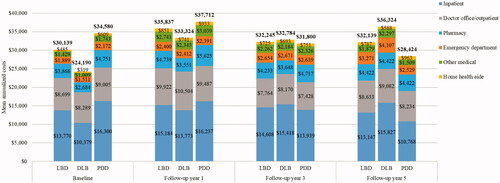
For commercial insurance, 2239 patients met all inclusion criteria and of these patients, 957 and 1282 were included in the DLB and PDD subgroups, respectively. In general, the baseline characteristics were similar to the Medicare population (see ). For the commercially insured, information on prescription medications was also available. The proportion of patients with DLB who used different medications during the baseline period was significantly lower than the proportion of patients with PDD (antiparkinson agents: 0.0% vs. 69.3%; antidepressants: 31.5% vs. 42.6%; antipsychotic agents: 6.4% vs. 12.6%; hypnotic/sedative agents: 6.9% vs. 10.6%; all p < .01). At the index date, the DLB subgroup also had lower use of antidementia medications than the PDD subgroup (cholinesterase inhibitors: 14.5% vs. 21.8%, p < .001; memantine use: 2.4% vs. 3.8%, p = .078). The baseline total costs, including medical and pharmacy costs, were also significantly lower for the commercially-insured DLB vs. PDD subgroup ($24,190 vs. $34,580; p < .001) ().
Table 2. Baseline characteristics of the commercially-insured population.
HCRU and costs during follow-up period
Overall, the proportions of patients in the LBD cohort with any inpatient, physician office/outpatient, and home health aide visits as well as the number of such visits were generally highest during the first year following the index date for both the Medicare and commercially-insured populations (Tables S2 and S3).
For the Medicare population, during the first year of follow-up, significant differences were observed between the DLB and PDD subgroups in the proportions of patients with any home health aide visits (DLB: 34.2%; PDD: 40.9%; p < .001) and any skilled nursing facility visits (DLB: 24.2%; PDD: 28.8%; p < .001). The most common sites of care were the physician's office/outpatient setting (DLB: 99.9%; PDD: 99.9%; p = .713) followed by the ED (DLB: 58.7%; PDD: 60.6%; p = .073) and inpatient setting (44.1%; 47.5%; p = .001) (Table S2). The HCRU declined in the subsequent years and remained relatively stable through year five post-index. Similar trends were observed among commercially-insured patients (Table S3).
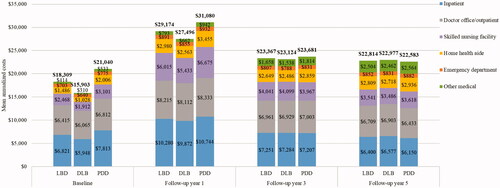
The total healthcare costs were also highest in the first year post-index and remained high three and five years following the index date compared to the baseline period in both databases ( and ). The mean total medical costs in the first year were $29,174 for Medicare patients with LBD (). The costs were significantly lower for the DLB vs. PDD subgroup during the first year post-index ($27,496 vs. $31,080, p < .001). However, the mean total healthcare costs were similar across the patient groups three years post-index (LBD: $23,367; DLB: $23,124; PDD: $23,681) and five years post-index (LBD: $22,814; DLB: $22,977; PDD: $22,583). The main drivers of total medical costs for LBD, DLB, and PDD were inpatient costs, followed by physician office/outpatient costs, and skilled nursing facility costs across all years ().
Figure 3. Healthcare costs over time in the Medicare population. Abbreviations. DLB, Dementia with Lewy bodies; LBD, Lewy body dementia; PDD, Parkinson’s disease dementia.
The mean total medical costs were higher for the commercially-insured patients compared with Medicare patients: $31,098, $29,773, and $32,088 in the first year post-index for LBD, DLB, and PDD, respectively (). The mean total costs, including pharmacy costs, were $35,837, $33,324, and $37,712, respectively. Similar to the Medicare cohort, the main drivers of total costs for LBD, DLB, and PDD were inpatient costs, followed by physician office/outpatient costs, and pharmacy costs across all years (). The trends in costs for commercially-insured patients with LBD were similar to those for Medicare patients. However, unlike Medicare patients, commercially-insured PDD patients experienced a decrease in costs after the first year post-index, whereas DLB patients experienced a decrease in the second year post-index followed by an increase in costs over time. As a result, the costs for the two subgroups were similar at year 3 (DLB: $32,784; PDD: $31,800, p = .821) and slightly higher for DLB than for PDD at year 5, albeit not statistically significantly different (DLB: $36,324; PDD: $28,424, p = .252). During the five years of follow-up, the DLB subgroup had lower use of antiparkinson agents (26.9–35.6%) compared with the PDD subgroup (43.8–67.3%) but higher use of antidementia medications (Table S3). These differences were statistically significant for some but not for all follow-up years. At year 5, the only statistically significant differences in medication use were for cholinesterase inhibitors (DLB: 40.7%; PDD: 24.3%, p = .003) and memantine (DLB: 23.3%; PDD: 8.3%, p < .001).
Discussion
Dementia is associated with a substantial burden to society, similar to the burden of heart disease and cancerCitation27. Although AD is the most prevalent type of dementia, prior evidence suggests that the economic burden of LBD is even higherCitation11. However, there is a paucity of recent evidence on the epidemiology of the LBD disease spectrum and its burden. To address this knowledge gap, this study characterized the prevalence and incidence of LBD overall and by subtype (DLB vs. PDD) among a large representative population of Medicare FFS beneficiaries. In addition, this study assessed the patient profiles for the two subtypes and described trends in HCRU and costs in both Medicare and commercially-insured adults.
Results showed that the prevalence and incidence rates of LBD were relatively stable from 2010 to 2016 (prevalence rate: 0.90%–0.83%; incidence rate: 0.21%–0.18%). The prevalence and incidence rates were slightly higher for the DLB vs. PDD subgroup (prevalence rate: 0.48%–0.46% vs. 0.42%–0.37%, respectively; incidence rate: 0.12%–0.11% vs. 0.09%–0.07%, respectively). In general, the profiles of the DLB and PDD patients were similar in both Medicare and commercially-insured databases. The average age at the time of the first observed CI was 77–78 years, and less than half were females. The comorbidity profiles, HCRU and costs during baseline were also similar for the two subgroups in both databases. During the five years of follow-up, commercially insured patients with DLB were less likely to be treated with antiparkinson agents compared with PDD patients but more likely to be treated with memantine and cholinesterase inhibitors. Healthcare costs were highest in the first year following the index date for both Medicare and commercially-insured patients with LBD, DLB, and PDD, and remained high three and five years post-index. For the Medicare population, the main drivers of healthcare costs were costs associated with inpatient, physician office/outpatient, and skilled nursing facility services. For commercially-insured patients, the main drivers of healthcare costs were inpatient, physician office/outpatient, and pharmacy costs.
There is limited research on the epidemiology of LBD to date and our estimates of prevalence and incidence are higher than those reported previously in the literature. For instance, a retrospective analysis using statewide data from the One Florida Clinical Research Consortium found that the prevalence of LBD from 2012 to 2018 was 0.02%Citation12, which is lower than the prevalence estimates of 0.90%–0.83% in the present study. A different study published by Savica and colleagues in 2013 found that from 1991 to 2005, the incidence rates for LBD, DLB, and PDD were 54.6 per 100,000, 31.6 per 100,000, and 23 per 100,000 person-years, respectivelyCitation13. However, neither of these estimates could be compared directly with the present study due to differences in data sources, study designs, and time periods for assessment. In particular, although the time interval for the study by Patel overlapped with our study, only DLB diagnosis codes were used to identify cases of LBD in the prior studyCitation12, while the present study considered the sequence of CI and parkinsonism, in addition to the use of DLB diagnosis codes. In contrast, the analysis by Savica et al. was based on the identification of LBD, DLB, and PDD via medical records and clinical confirmation among 542 patients (of which 64 had DLB and 46 PDD) in a single county within the state of Minnesota, whereas the present study used nationally representative data for all Medicare FFS beneficiaries but could only rely on the diagnosis codes reported in Medicare claims.
The results from this study further suggest that although there are two subtypes of LBD based on the sequencing of CI and PD symptoms, there is little difference between the patient profiles before or after the onset of cognitive decline. As noted earlier, the baseline demographics, comorbidity profiles, as well as HCRU (with the exception of some symptomatic medication use) and costs during the follow-up period were largely similar between subtypes. Moreover, when comparing the burden of illness between DLB and PDD subtypes, no statistically significant differences were observed in HCRU and costs after the first year following the initial CI diagnosis, for both Medicare and commercially insured patients. The pharmacy costs, available only for the commercially insured, were slightly lower for patients with DLB relative to PDD.
This is one of the few studies to describe the patient characteristics and economic outcomes over time among LBD patients overall, as well as its subtypes in the real world. However, several aspects of our findings are supported by prior studies evaluating the burden of dementia, including LBD. For example, using a large national health plan, Chandler and colleagues found that the median total healthcare costs for patients with PD and newly diagnosed CI peaked during the year immediately following the CI diagnosis and were $8224 in the 1-year pre- and $10,590 in the 1-year post-diagnosis (2019 USD)Citation18. Although it is not feasible to directly compare costs between that study and the current analysis due to differences in study design and populations, it is noteworthy that a similar peak in costs was observed during the first year post-CI diagnosis in both studies. Similar trends in costs are also reported in other studies evaluating the economic burden of AD and related dementias (ADRD). For example, a retrospective claims-based study by Lin and colleagues reported Medicare expenditures for ADRD were $15,091 in the 1-year pre-diagnosis and increased to $27,126 in the 1-year immediately after diagnosisCitation28. In contrast, we found that for Medicare patients, costs in the 1-year baseline period were $18,309 and $29,174 in the 1-year post-index. The high costs associated with LBD found in our study are also consistent with findings from Chen et al. who reported that LBD is the costliest subtype of dementia among California Medicare FFS beneficiariesCitation11. As a comparison, the national health care spending in the US in 2019 was $11,462 per personCitation29, about three times lower than the 1-year post-index mean healthcare costs for both Medicare and commercially insured people with LBD.
The clinical management of LBD is particularly challenging for healthcare stakeholders due to the absence of effective disease-modifying therapies and the need for multiple different specialties, which can affect the quality of careCitation10,Citation30. By providing a comprehensive assessment of the trends in the epidemiology of LBD, as well as the patient profiles, HCRU, and costs stratified by subtype, this study makes an important contribution to the LBD literature. In particular, the epidemiology estimates are generalizable to the entire Medicare FFS population and not limited to a certain region or county. Furthermore, trends in HCRU and costs were assessed among both Medicare and commercially insured patients, allowing to capture the economic burden across different payers. Taken together, these findings have the potential to assist healthcare stakeholders to identify patients across the LBD spectrum, support assessments related to population estimates, inform trial design for studies focused on symptomatic treatments and project costs of care over time.
However, this study should be considered within the context of certain limitations. First, claims data may be subject to potentially incorrect records or inaccurate diagnostic codes. While there are specific codes for DLB, a meaningful proportion of patients with DLB, and all patients with PDD were identified based on the sequence of events related to evidence of PD and CI, which may have been misclassified in some patients, particularly if there were delays in their diagnoses. Second, the epidemiology findings only represent diagnosed prevalence and incidence within the Medicare FFS population and may be also subject to inaccurate diagnosis or records. Furthermore, no clinical measures to ascertain diagnosis and assess disease severity were available in the data. Third, all analyses were descriptive in nature and did not adjust for differences in baseline characteristics among patients or compare the outcomes relative to patients without LBD. Future studies should evaluate the impact of different patient characteristics on clinical and economic outcomes associated with LBD. Fourth, patients were not required to have complete data for the full five-year follow-up period following the index date. Therefore, results for HCRU and costs may have been skewed by survival bias as patients with more severe disease are likely to be lost to follow-up earlier than patients with mild disease progression. Further research is warranted to examine potential differences in survival across different levels of disease severity and assess outcomes of patients with complete data over the full study period. Fifth, it is not known how the study results may be extrapolated to patient populations other than Medicare and commercial insurance. Lastly, as noted earlier, the Medicare database used in this study does not contain pharmacy claims. Although the analysis in the commercially-insured population suggests pharmacy costs are among the top three drivers of cost, further research is warranted to assess pharmacy costs for Medicare patients with LBD.
Conclusions
The results of this analysis demonstrated that the prevalence and incidence rates of LBD overall as well as by subtype remained relatively stable from 2010 to 2016. In addition, the demographic and comorbidity profiles of patients with DLB and PDD in the real world were generally similar. Furthermore, LBD and its subtypes are associated with a high disease burden, particularly during the first year following cognitive decline diagnosis. While HCRU and costs generally declined in the subsequent years, both remained high over the five-year time interval evaluated. These findings highlight the need for effective treatments and improved care for this historically difficult-to-treat population. They may also serve as a useful benchmark to assist healthcare stakeholders in determining the clinical and financial impact of therapies aimed at reducing the burden of illness of LBD.
Transparency
Declaration of funding
This study was funded by Eli Lilly.
Declaration of financial/other relationships
JC and KB are employees of Eli Lilly and are minor stockholders. UD, NK, MG, and HL are employees of Analysis Group, Inc., which received consulting fees from the study sponsor to conduct this research. HCC and BW were employees of Analysis Group, Inc. at the time this study was conducted. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
JC, KB, UD, NK, and MG contributed to the study design. Formal analyses were conducted by HCC, BW, and HL. All authors contributed to the critical interpretation of data as well as drafting/editing the manuscript, have approved the final version of this manuscript, and take responsibility for the integrity of this research study.
LBD_claims_-_Supplementary_Tables.docx
Download MS Word (29.5 KB)Acknowledgements
Medical writing assistance was provided by Gloria DeWalt, PhD, who was an employee of Analysis Group, Inc. at the time this study was conducted.
Data availability statement
The datasets analyzed during the current study are not publicly available, as they are subject to a data use agreement between Analysis Group, Inc. and the Centers for Medicare & Medicaid Services and OptumHealth.
References
- Coughlin DG, Hurtig HI, Irwin DJ. Pathological influences on clinical heterogeneity in Lewy body diseases. Mov Disord. 2020;35(1):5–19.
- Galvin JE, Balasubramaniam M. Lewy body dementia: the under-recognized but common FOE. Cerebrum. 2013;2013(13):13.
- Gomperts SN. Lewy body dementias: dementia with Lewy bodies and Parkinson disease dementia. Continuum. 2016;22(2 Dementia):435–463.
- McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89(1):88–100.
- Alzheimer's Association. Lewy Body Dementia. [cited 2021 July 26]. Available from: https://www.alz.org/alzheimers-dementia/what-is-dementia/types-of-dementia/lewy-body-dementia.
- Lewy Body Dementia Association. About LBD. [cited 2021 July 8]. Available from: https://www.lbda.org/about-lbd/.
- Eisai Co. Ltd. ARICEPT® approved in Japan as treatment for dementia with Lewy bodies [press release]. [cited 2021 July 20]. Available from: https://www.eisai.com/news/news201452.html.
- U.S. Food and Drug Administration. Exelon®: Highlights of prescribing information. [cited 2021 July 15]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/022083lbl.pdf.
- European Medicines Agency. Rivastigmine Actavis. [cited 2021 July 15]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/rivastigmine-actavis.
- Taylor JP, McKeith IG, Burn DJ, et al. New evidence on the management of Lewy body dementia. Lancet Neurol. 2020;19(2):157–169.
- Chen Y, Wilson L, Kornak J, et al. The costs of dementia subtypes to California Medicare fee-for-service, 2015. Alzheimers Dement. 2019;15(7):899–906.
- Patel B. Lewy body dementia (LBD) prevalence and cholinesterase inhibitor use in Florida (abstract 6-011). In Paper presented at: The 71st American Academy of Neurology Annual Meeting2019; Philadelphia, Pennsylvania.
- Savica R, Grossardt BR, Bower JH, et al. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013;70(11):1396–1402.
- Goodman RA, Lochner KA, Thambisetty M, et al. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011-2013. Alzheimers Dement. 2017;13(1):28–37.
- Petrova M, Mehrabian-Spasova S, Aarsland D, et al. Clinical and neuropsychological differences between mild Parkinson's disease dementia and dementia with Lewy bodies. Dement Geriatr Cogn Disord Extra. 2015;5(2):212–220.
- Smirnov DS, Galasko D, Edland SD, et al. Cognitive decline profiles differ in Parkinson disease dementia and dementia with Lewy bodies. Neurology. 2020;94(20):e2076–e2087.
- Aldridge GM, Birnschein A, Denburg NL, et al. Parkinson's disease dementia and dementia with Lewy bodies have similar neuropsychological profiles. Front Neurol. 2018;9(123):123.
- Chandler J, Nair R, Biglan K, et al. Characteristics of Parkinson's disease in patients with and without cognitive impairment. J Parkinsons Dis. 2021;11(3):1381–1392.
- Murman DL, Kuo SB, Powell MC, Colenda CC. The impact of parkinsonism on costs of care in patients with AD and dementia with Lewy bodies. Neurology. 2003;61(7):944–949.
- Espinosa R, Davis M, Johnson S, et al. Direct medical costs of dementia with Lewy bodies by disease complexity. J Am Med Dir Assoc. 2020;21(11):1696–1704 e1695.
- De Lew N. Medicare: 35 years of service. Health Care Financ Rev. 2000;22(1):75–103.
- Chronic Conditions Warehouse (CCW) Medicare Enrollment Charts. 2021. [cited 2022 March 22]. Available from: https://www2.ccwdata.org/web/guest/medicare-charts/medicare-enrollment-charts.
- Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of medicare + choice health plans that have chronic medical conditions. Health Serv Res. 2004;39(6 Pt 1):1839–1857.
- Desai U, Kirson NY, Ye W, et al. Trends in health service use and potentially avoidable hospitalizations before Alzheimer's disease diagnosis: a matched, retrospective study of US Medicare beneficiaries. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2019;11(1):125–135.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139.
- United States Department of Labor: Bureau of Labor Statistics: Consumer Price Index—All Urban Consumers. Medical Care. https://data.bls.gov/timeseries/.
- Hurd MD, Martorell P, Delavande A, et al. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334.
- Lin PJ, Zhong Y, Fillit HM, et al. Medicare expenditures of individuals with Alzheimer's disease and related dementias or mild cognitive impairment before and after diagnosis. J Am Geriatr Soc. 2016;64(8):1549–1557.
- Hartman M, Martin AB, Washington B, et al. The national health expenditure accounts T. National health care spending in 2020: growth driven by federal spending in response to the COVID-19 pandemic. Health Aff. 2022;41(1):13–25.
- Hershey LA, Coleman-Jackson R. Pharmacological management of dementia with Lewy bodies. Drugs Aging. 2019;36(4):309–319.