Abstract
Objective
To evaluate real-world annualized bleeding rates (ABRs), dosing frequency, and factor consumption of four recombinant FVIII (rFVIII) products using pooled data from centers in the US, Germany, and Italy.
Methods
De-identified patient medical chart data were collected from 48 hemophilia treatment centers in the US, Germany, and Italy. Patients included in this analysis had hemophilia A and were treated with rVIII-SingleChain, rFVIIIFc, octocog alfa, or BAY 81-8973 for ≥12 weeks. Where possible, patient selection considered age and disease severity in order to balance patient groups across products. Summary statistics were presented descriptively by product for dosing frequency, consumption, ABR/annualized spontaneous bleeding rate (AsBR), and corresponding percentage of patients with zero bleeds. Logistic regression was performed for patients with zero bleeds or zero spontaneous bleeds (vs. patients with any such bleeds). Generalized linear model regression was performed for ABR, AsBR, and consumption. All regression models included the product variable for comparison as well as additional independent variables for adjustment (age, weight, severity, and country for the consumption model, with the addition of consumption for the bleeding outcomes models).
Results
Overall, 616 patients were included (rVIII-SingleChain, n = 129; rFVIIIFc, n = 159; octocog alfa, n = 181; BAY 81-8973, n = 147). Dosing frequency was ≤2 times a week for 65.9%, 75.5%, 25.4%, and 40.1% of patients treated with rVIII-SingleChain, rFVIIIFc, octocog alfa, and BAY 81-8973, respectively. ABRs were not significantly different among products, with mean (median) values of 1.1 (0.0), 1.0 (0.0), 1.4 (1.0), and 1.9 (1.0) for rVIII-SingleChain, rFVIIIFc, octocog alfa, and BAY 81-8973, respectively. The percentage of patients with zero bleeds was comparable between rVIII-SingleChain and rFVIIIFc (59.7% vs. 62.3%; p =.916) and significantly higher for rVIII-SingleChain compared with octocog alfa (p <.001) and BAY 81-8973 (p =.003). Comparison of mean weekly consumption showed: rVIII-SingleChain (83.0 IU/kg/week) vs. rFVIIIFc (96.9; p =.055) and significantly lower for rVIII-SingleChain vs. octocog alfa (108.6; p <.001) and BAY 81-8973 (104.3; p =.001). The median values for weekly consumption were 85.7, 90.1, 100.1, and 98.5 IU/kg/week for rVIII-SingleChain, rFVIIIFc, octocog alfa, and BAY 91-8973, respectively. Similar trends were observed for all outcomes when analyzing the subgroups of patients aged ≥12 years and patients with severe disease (all age and ≥12 years).
Conclusions
rVIII-SingleChain prophylaxis may provide improved bleed protection, less frequent dosing, and lower consumption compared with standard-acting FVIII products, and comparable protection and consumption to the other long-acting FVIII product, in patients with hemophilia A.
Introduction
Hemophilia A is an X-linked bleeding disorder, occurring in approximately 25 in 100,000 live male birthsCitation1,Citation2. People with hemophilia A have a deficiency or absence of factor VIII (FVIII), an essential component of the blood coagulation pathwayCitation1,Citation3,Citation4. This results in spontaneous or excessive internal bleeding, damage to muscles and joints, and significantly impacts the mobility and quality-of-life of people with hemophiliaCitation1,Citation4,Citation5.
The World Federation of Hemophilia (WFH) recommends regular long-term prophylaxis in people with hemophilia A, with the aim of preventing hemarthrosis, maintaining musculoskeletal health, and promoting quality-of-lifeCitation1,Citation6. Multiple recombinant FVIII (rFVIII) products that mimic the function of endogenous FVIII are available for the treatment of hemophilia A, with varying half-lives. Typically, treatment with standard half-life rFVIII products requires dosing 3–4 times per week; this can place a high burden on patients, potentially impacting adherence and resulting in suboptimal careCitation1,Citation6,Citation7. The development of longer acting rFVIII products, which have improved pharmacokinetics and an extended half-life compared to standard-acting products, has meant that dosing intervals can be extended whilst retaining efficacyCitation8,Citation9. These longer dosing intervals allow patients to reduce the dosing frequency, improving convenience and potentially increasing adherence to treatmentCitation8,Citation10.
In addition to dosing frequency, another important consideration in hemophilia management is factor consumption. With some extended half-life FVIII products, patients can achieve similar or potentially lower bleed rates to those achieved with standard-acting FVIII while reducing factor consumptionCitation11. Lowering consumption may reduce treatment costs in clinical practice, providing economic benefits in addition to clinical benefits.
Clinical trials have demonstrated that the rFVIII products rVIIISingleChain (Afstyla, CSL Behring), rFVIIIFc (Eloctate/Elocta, Sobi), octocog alfa (Advate, Takeda), and BAY 81-8973 (Kovaltry, Bayer) effectively control bleeding events, result in low annualized bleeding rates (ABRs) in patients on routine prophylaxis, and have favorable safety profilesCitation8,Citation9,Citation12–16. Real-world data regarding efficacy and cost effectiveness are vital for efficient utilization of public healthcare resources. Previous studies have reported on real-world outcomes of different standard-acting and long-acting FVIII products using data from the US, Germany, and ItalyCitation17–20. Here we reported real-world dosing frequency, ABRs/annualized spontaneous bleeding rate (AsBR), and consumption associated with prophylactic use of rVIII-SingleChain compared with two standard-acting (octocog alfa and BAY 81-8973) and one long-acting (rFVIIIFc) rFVIII products using pooled data from centers in the US, Germany, and Italy.
Methods
Data collection
Retrospective chart review studies were conducted for patients with hemophilia A receiving prophylaxis with commonly prescribed FVIII products in the US, Germany, and Italy. De-identified patient medical chart data were collected during 2018/2019 from 48 hemophilia treatment centers in the US (n = 11, data collected May–August 2018), Germany (n = 21, data collected September–November 2018), and Italy (n = 16, data collected June–August 2019); the results for this data from the individual countries have previously been publishedCitation17–20. This current analysis combined pre-existing data from patients using the four products that were studied in all three countries: rVIII-SingleChain, rFVIIIFc, octocog alfa, and BAY 81-8973. Following evaluation by an institutional review board in each country, the study was determined to be exempt from patient informed consent under category 4 as secondary research for which consent is not required.
Data collected included: age, sex, weight, disease severity, dosing frequency, bleeding rates, and consumption. Where possible, patient selection took age and disease severity into account in order to balance patient groups across products as closely as possible. Patients were required to have received at least 12 weeks of treatment with FVIII for inclusion in the analysis. Both adult and pediatric patients were included; for the purposes of this study, patients ≥12 years were defined as adult/adolescents, and patients <12 years were defined as pediatric patients. Patients with <1% of normal FVIII blood levels were defined as severe, patients with 1–5% were defined as moderate, and patients with >5–40% were defined as mildCitation21.
Descriptive analysis
Data from the three countries were pooled and analyzed across the four FVIII products for all patients, patients aged ≥12 years and for patients with severe disease, as well as by country or region (Germany and Italy grouped as Europe region). Overall patient characteristics were summarized. Summary statistics were compared descriptively among the four products for dosing frequency, ABR/AsBR and corresponding percentage of patients with zero bleeds, and consumption. Prescribed dosing and infusion frequencies were taken from the most recent prescription for each product. The ABR/AsBR was calculated as the number of reported bleeding events divided by the number of months in the reported time window (3–12 months) and multiplied by 12. Prophylactic factor consumption was reported as international units of product per kilogram of bodyweight per week (IU/kg/week), calculated as dose per infusion (IU) multiplied by the number of infusions per week and divided by the patient’s weight (kg).
Regression analysis and statistical comparison
To assess the effect of the different products, the following analyses were performed: Logistic regression was performed for patients with zero bleeds or zero spontaneous bleeds (vs. patients with any such bleeds), including age, weight, severity, consumption, and country as adjustment variables. Generalized linear model regression was performed for ABR and AsBR, including age, weight, severity, consumption, and country as adjustment variables. Since the bleeding rates were highly skewed and not normally distributed, a negative binomial distribution was specified in the model, with a log link. Generalized linear model regression with a Gaussian distribution and an identity link was performed for consumption, which was approximately normally distributed, including age, weight, severity, and country as adjustment variables. Adjusted p values from these regression models were obtained for comparisons between products, with statistical significance defined as p <.05. These regression analyses were performed separately for all patients, all patients with severe disease, patients aged ≥12 years, and patients aged ≥12 years with severe disease. The software used to perform all analyses was Stata, version 15.1.
Results
Study cohort
A total of 616 patients were included in this analysis (rVIII-SingleChain, n = 129; rFVIIIFc, n = 159; octocog alfa, n = 181; BAY 81-8973, n = 147). Patient characteristics are described in . The percentage of patients with severe disease ranged from 65.9% (rVIII-SingleChain) to 73.6% (rFVIIIFc). The majority (84.9%) of patients were adults/adolescents (≥12 years old).
Table 1. Baseline patient characteristics.
Dosing frequency
Across all patient groups, dosing frequency was ≤2 times a week for 65.9% of patients treated with rVIII-SingleChain, 75.5% of patients treated with rFVIIIFc, 25.4% of patients treated with octocog alfa, and 40.1% of patients treated with BAY 81-8973 (). Similar patterns in dosing frequencies among the products were observed for patients with severe disease (60.0% rVIII-SingleChain; 72.6% rFVIIIFc; 17.5% octocog alfa; 34.0% BAY 81-8973; ). When analyzing patients aged ≥12 years old only, dosing frequency of ≤2 times a week was observed in 65.2%, 75.0%, 29.1%, and 36.4% of patients for rVIII-SingleChain, rFVIIIFc, octocog alfa, and BAY 81-8973, respectively (). A similar trend was seen for patients with severe disease aged ≥12 years old (60.0%, 70.5%, 22.4%, and 31.3%, respectively; ).
Figure 1. Frequency of prophylaxis infusions in (a) all patients, (b) patients with severe hemophilia A, (c) patients ≥12 years, and (d) patients ≥12 years with severe hemophilia A.
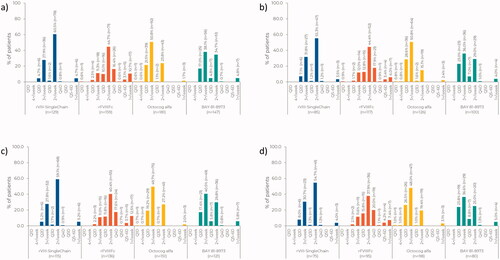
When analyzing dosing frequency by country or region, a similar pattern across the products was observed (Supplementary Figure S1).
Bleeding rates
For all patients, ABRs were comparable between all products (mean ± SD: rVIII-SingleChain, 1.1 ± 2.0; rFVIIIFc, 1.0 ± 1.9; octocog alfa, 1.4 ± 2.1; BAY 81-8973, 1.9 ± 3.1; ). The same pattern was seen for the subgroups of patients with severe disease, patients aged ≥12 years, and those aged ≥12 years with severe disease (). AsBRs were similar for all FVIII products across all patient groups analyzed (mean ± SD: rVIII-SingleChain, 0.7 ± 1.6; rFVIIIFc, 0.5 ± 1.3; octocog alfa, 0.7 ± 1.7; BAY 81-8973, 0.8 ± 2.5; ). Differences in ABR and AsBR were not statistically significant among products (p values >.05). Median data for ABR and AsBR are also available in .
Table 2. Bleeding rates in patients using one of four commonly used products in the US, Germany, and Italy.
The percentage of patients with zero bleeds was comparable between rVIII-SingleChain and rFVIIIFc (59.7% vs 62.3%; p =.916) and significantly higher for rVIII-SingleChain compared with octocog alfa (45.3%; p <.001) and BAY 81-8973 (44.9%; p =.003). For AsBR, the percentage of patients with zero bleeds was similar between rVIII-SingleChain (76.0%) and rFVIIIFc (77.4%; p =.976) as well as BAY 81-8973 (73.5%; p =.529). The percentage of patients with zero spontaneous bleeds was significantly higher for rVIII-SingleChain compared with octocog alfa (68.5%; p =.025).
In the subgroup of patients with severe disease, the percentage of patients with zero bleeds was comparable between rVIII-SingleChain and rFVIIIFc (54.1% vs 59.0%; p =.788) and significantly higher for rVIII-SingleChain compared with octocog alfa (42.9%; p =.004) or BAY 81-8973 (47.0%; p =.041). The percentage of patients with zero spontaneous bleeds was comparable between rVIII-SingleChain (72.9%) and rFVIIIFc (75.2%; p =.549) as well as BAY 81-8973 (72.0%; p =.263). The percentage of patients with zero spontaneous bleeds was significantly higher for rVIII-SingleChain compared with octocog alfa (64.3%; p =.009).
In patients aged ≥12 years only, the percentage of patients with zero bleeds for rVIII-SingleChain (58.3%) was similar compared with rFVIIIFc (61.0%; p =.967) and was significantly higher than octocog alfa (45.0%; p =.002) and BAY 81-8973 (42.1%; p =.006). The percentage of patients with zero spontaneous bleeds in this subgroup of patients was not significantly different among the products (rVIII-SingleChain, 73.0%; rFVIIIFc, 77.2%; octocog alfa, 68.2%; BAY 81-8973, 69.4%; overall p =.130):
In patients aged ≥12 years with severe disease, the percentage of patients with zero bleeds for rVIII-SingleChain (52.0%) was similar compared with rFVIIIFc (55.8%; p =.882), and was significantly higher than octocog alfa (42.9%; p =.032) and BAY 81-8973 (40.0%; p =.032). The percentage of patients with zero spontaneous bleeds in this subgroup of patients was not significantly different among the products (rVIII-SingleChain, 69.3%; rFVIIIFc, 73.7%; octocog alfa, 63.3%; BAY 81-8973, 66.3%; overall p =.166).
Corresponding data on bleeding by country follow similar patterns across products and are provided in Supplementary Table S1.
Consumption
In all patients, overall mean consumption was lowest for rVIII-SingleChain (83.0 IU/kg/week) followed by rFVIIIFc (96.9 IU/kg/week; p =.055), BAY 81-8973 (104.3 IU/kg/week; p =.001), and octocog alfa (108.6 IU/kg/week; p <.001; ). A similar trend was observed in the subset of patients with severe disease; those who received rVIII-SingleChain had the lowest mean consumption (92.8 IU/kg/week) followed by rFVIIIFc (104.9 IU/kg/week; p =.181), BAY 81-8973 (113.4 IU/kg/week; p =.013), and octocog alfa (120.0 IU/kg/week; p =.001). In patients aged ≥12 years, the mean consumption was lowest for rVIII-SingleChain (81.6 IU/kg/week) followed by rFVIIIFc, BAY 81-8973, and octocog alfa (88.6 IU/kg/week, p =.119; 96.2 IU/kg/week, p =.001; and 97.5 IU/kg/week, p <.001, respectively) with a similar trend observed for patients with severe disease aged ≥12 years (rVIII-SingleChain, 92.0 IU/kg/week; rFVIIIFc 95.3 IU/kg/week, p =.408; BAY 81-8973, 102.2 IU/kg/week, p =.045 IU/kg/week; and octocog alfa, 105.5 IU/kg/week, p =.011). Median values for weekly consumption are also presented in .
Table 3. Weekly factor consumption in patients using one of four commonly used products in the US, Germany, and Italy.
Consumption by country or region showed similar patterns across the products (Supplementary Table S2).
Discussion
This analysis provides real-world data on the use and effectiveness of four widely used FVIII products in the US, Germany, and Italy. This retrospective patient medical chart review analysis shows that patients treated with rVIII-SingleChain have comparable protection and consumption to a long-acting FVIII product, and that both rVIII-SingleChain and rFVIIIFc have improved bleed protection, less frequent dosing, and lower consumption compared with standard-acting FVIII products.
The highest proportion of patients with a dosing frequency of ≤2 times a week was reported in patients treated with rFVIIIFc. The proportion of patients treated with rVIII-SingleChain with a dosing frequency of ≤2 times a week (65.9%) was slightly lower than rFVIIIFc (75.5%) but greater than for patients treated with octocog alfa (25.4%) or BAY 81-8973 (40.1%); this remained true when considering the sub-groups of patients with severe disease, patients ≥12 years, and patients ≥12 years with severe disease. This supports findings from previous studies that have observed prolonged dosing frequency in FVIII products with longer half-lives than standard-acting productsCitation8,Citation9.
Furthermore, bleed protection was shown to be improved in FVIII products with longer half-lives than standard-acting products. The percentage of patients with no bleeds was significantly higher for rVIII-SingleChain compared with both octocog alfa and BAY 81-8973 in all patients and all subgroup comparisons (all patients with severe disease, patients aged ≥12 years, and patients aged ≥12 years with severe disease). The percentage of patients with no spontaneous bleeds was significantly higher for rVIII-SingleChain compared with octocog alfa in all patients and all patients with severe disease. When compared to the long-acting product rFVIIIFc, the ABR, AsBR, and percentage of patients with zero bleeds were not significantly different for patients treated with rVIII-SingleChain. The lower dosing frequency reported here for rVIII-SingleChain in combination with improved bleed protection compared to standard-acting products may increase treatment adherence and thus treatment effectiveness and quality-of-life for patients with hemophilia ACitation22,Citation23.
Additionally, FVIII consumption was lowest for all age groups and disease severity subgroups for patients treated with rVIII-SingleChain compared to all other products analyzed, and the difference was statistically significant vs. both octocog alfa, and BAY 81-8973 in all patients and in all subgroup analyses. The rVIII-SingleChain consumption reported here for all patients was similar to consumption recorded previously (83.0 IU/kg/week in the current study vs. 86.4 IU/kg/week in the clinical trial)Citation8. The calculated mean annual consumption for rVIII-SingleChain, rFVIIIFc, octocog alfa, and BAY 81-8973 is 4,316 IU/kg, 5,038.8 IU/kg, 5,647.2 IU/kg, and 5,423.6 IU/kg, respectively. When considering treatment cost, these differences in annual consumption may impact FVIII product preference.
Limitations of this analysis include the relatively short assessment period (approximately a year) and the lack of detailed information on the location, severity, treatment, and outcomes of bleeding events. Nonetheless, this study included pooled data from multiple countries, reflecting the range of management practices used in the real-world treatment of patients with hemophilia, with similar findings between countries. Potential selection bias may exist in the study cohort, and we were not able to assess the effect of potentially differing patient management practices from the centers contributing data. As such, patients included here may not be representative of the entire population of patients with hemophilia A. Additionally, adjustments for patient characteristics and activity levels prior to initiation of the FVIII products were not made in the analysis. Further research is therefore needed in larger sample sizes to confirm real-world experience and identify treatment patterns in patients with hemophilia A. Moreover, consumption calculations were based on collected prescription information which may not be a true representation of factor consumption. However, measuring compliance to record true factor consumption is difficult and, given that the ABRs reported here reflect those previously reported and that the study cohort likely included overusers and underusers, we believe that the consumption calculations used here reflect real-world use of FVIII products.
As the most recently approved product among the four products compared, it is also noted that the mean observation period for rVIII-SingleChain was shorter (47 weeks) than for the other FVIII products (52 weeks). The proportion of patients treated with rVIII-SingleChain with zero bleeds reported here may therefore be slightly higher than if the duration of observation had been the same as for the other FVIII products. Lastly, the sample sizes for patients <12 years of age were small and not suitable for subgroup analysis or statistical comparisons; however, the pooled data for this age group for the three countries showed similar trends in general to the overall data.
Conclusion
In conclusion, this analysis compared pooled real-world patient chart data on FVIII products used for prophylaxis treatment of hemophilia A, from the US, Germany, and Italy. The results indicate that prophylaxis with rVIII-SingleChain may provide improved bleed protection, less frequent dosing, and lower consumption compared with standard-acting FVIII products (octocog alfa and BAY 81-8973), and comparable protection and consumption to long-acting FVIII products (rFVIIIFc) in all patients with hemophilia A, including those aged ≥12 years and those with severe disease. Further research with a longer observation period and using a larger sample size may help to confirm real-world experience in patients with hemophilia A.
Transparency
Declaration of funding
This study was funded by CSL Behring.
Declaration of financial/other relationships
MO: grants/research support from Bayer, Biotest, CSL Behring, Octapharma, Pfizer, Shire/Takeda and Swedish Orphan Biovitrium, consultancy for Bayer, Biotest, Novo Nordisk, CSL Behring, Pfizer and Swedish Orphan Biovitrium; MS: consultancy for Bayer, CSL Behring, Genentech, Novo Nordisk, Octapharma, and Takeda, speakers bureau for Novo Nordisk; SY, XZ, RT, and KP: employees of CSL Behring; JF: employee of Adivo Associates; MEM: advisory boards for Bayer Healthcare, Biomarin, CSL Behring, Novo Nordisk, Pfizer, Roche, Sanofi, Sobi, Takeda, and UniQure, consultancy for Bayer Healthcare, CSL Behring, Novo Nordisk, Roche, Sobi, Grifols, Takeda, and Kedrion, speakers bureau for Bayer Healthcare, Biomarin, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Sparks Therapeutics, and Sobi. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
SY, XZ, RT, and KP contributed to the conception, design, and analysis of the study. JF provided substantial contributions to the data collection and analysis and drafting the manuscript. MO, MS, and MEM were integral to reviewing the manuscript for important intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
AFSTYLA_pooled_data_comparison_manuscript_draft_7_Feb_2022_Suppl.docx
Download MS Word (313.7 KB)Acknowledgements
Analyses were conducted by Adivo Associates. Medical writing support was provided by Meridian HealthComms (Plumley, UK) and funded by CSL Behring.
Data availability statement
CSL Behring will only consider requests to share data that are received from systematic review groups or bona-fide researchers. CSL will not process or act on data requests until 12 months after article publication on a public website. A data request will not be considered by CSL unless the proposed research question seeks to answer a significant and unknown medical science or patient care question. Applicable country specific privacy and other laws and regulations will be considered and may prevent sharing of data.
Requests for use of the data will be reviewed by an internal CSL review committee. If the request is approved, and the researcher agrees to the applicable terms and conditions in a data sharing agreement, data that has been appropriately anonymized will be made available. Supporting documents including study protocol and Statistical Analysis Plan will also be provided.
For information on the process and requirements for submitting a voluntary data sharing request for data, please contact CSL at [email protected].
References
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26 Suppl 6:1–158.
- Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med. 2019;171(8):540–546.
- Franchini M, Mannucci PM. Hemophilia a in the third millennium. Blood Rev. 2013;27(4):179–184.
- Mannucci PM, Tuddenham EG. The hemophilias-from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773–1779.
- Brown TM, Lee WC, Joshi AV, et al. Health-related quality of life and productivity impact in haemophilia patients with inhibitors. Haemophilia. 2009;15(4):911–917.
- Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125(13):2038–2044.
- Saxena K. Barriers and perceived limitations to early treatment of hemophilia. J Blood Med. 2013;4:49–56.
- Mahlangu J, Kuliczkowski K, Karim FA, et al. Efficacy and safety of rVIII-SingleChain: results of a phase 1/3 multicenter clinical trial in severe hemophilia A. Blood. 2016;128(5):630–637.
- Shapiro AD, Ragni MV, Kulkarni R, et al. Recombinant factor VIII Fc fusion protein: extended-interval dosing maintains low bleeding rates and correlates with von Willebrand factor levels. J Thromb Haemost. 2014;12(11):1788–1800.
- Schwartz CE, Powell VE, Su J, et al. The impact of extended half-life versus conventional factor product on hemophilia caregiver burden. Qual Life Res. 2018;27(5):1335–1345.
- Iorio A, Krishnan S, Myrén KJ, et al. Indirect comparisons of efficacy and weekly factor consumption during continuous prophylaxis with recombinant factor VIII Fc fusion protein and conventional recombinant factor VIII products. Haemophilia. 2017;23(3):408–416.
- Stasyshyn O, Djambas Khayat C, Iosava G, et al. Safety, efficacy and pharmacokinetics of rVIII-SingleChain in children with severe hemophilia A: results of a multicenter clinical trial. J Thromb Haemost. 2017;15(4):636–644.
- Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325.
- Saxena K, Lalezari S, Oldenburg J, et al. Efficacy and safety of Bay 81-8973, a full-length recombinant factor VIII: results from the LEOPOLD I trial. Haemophilia. 2016;22(5):706–712.
- Mahlangu J, Lopez Fernandez MF, Santagostino E, et al. Bay 81-8973 demonstrated efficacy, safety and joint status improvement in patients with severe haemophilia a in the LEOPOLD I extension for ≤2 years. Eur J Haematol. 2020;104(6):594–601.
- Dhillon S. Octocog alfa, antihaemophilic factor (recombinant), plasma/albumin free method (Advate®): a review of its use in the management of patients with haemophilia A. Drugs. 2012;72(7):987–1007.
- Olivieri M, Sommerer P, Maro G, et al. Assessing prophylactic use and clinical outcomes in hemophilia a patients treated with rVIII-SingleChain and other common rFVIII products in Germany. Eur J Haematol. 2020;104(4):310–317.
- Simpson ML, Desai V, Maro GS, et al. Comparing factor use and bleed rates in U.S. hemophilia a patients receiving prophylaxis with 3 different long-acting recombinant factor VIII products. JMCP. 2020;26(4):504–512.
- Yan S, Maro GS, Desai V, et al. A real-world analysis of commonly prescribed FVIII products based on U.S. medical charts: consumption and bleeding outcomes in hemophilia a patients. J Manag Care Spec Pharm. 2020;26(10):1258–1265.
- Mancuso ME, Santoro C, Maro G, et al. Retrospective analysis of rVIII-SingleChain and other commonly used recombinant FVIII products comparing prophylaxis treatment regimens and associated clinical outcomes in haemophilia A patients in Italy. Haemophilia. 2021;27:122.
- White GC, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85(3):560.
- Lambert T, Benson G, Dolan G, et al. Practical aspects of extended half-life products for the treatment of haemophilia. Ther Adv Hematol. 2018;9(9):295–308.
- Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677–1686.
