Abstract
Background
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disorder characterized by disturbed cellular and humoral immune responses. Dysregulations of immune system and immunosuppressive medications predispose SLE patients to infection. This study aims to investigate the alterations and absolute concentrations of lymphocyte subpopulations in SLE patients with different infection and their responses of low-dose IL-2 therapy.
Methods
A total of 333 patients with SLE without recent infection, 162 patients suffering infection, and age and sex-matched 132 healthy controls (HCs) were recruited. Of them, 54 SLE patients (including 41 non-infected group and 13 infected group) received a 5-day course of low-dose IL-2 administration at a dose of 0.5 million IU per day. Lymphocyte subpopulations were analyzed by flow cytometry.
Results
Patients with SLE had lower levels of lymphocyte subpopulations in peripheral blood such as T, B, NK, CD4 + T, CD8+ T, Th1, Th2, Th17, and Treg cells, and the reduction in these cells was more obvious in patients with infection (p <.05 to p <.01). Low-dose IL-2 effectively expanded T (p <.001), B (p <.001), CD4 + T (p <.01), CD8 + T (p <.001), Th1 (p <.01), Th17 (p <.1), and Treg cells (p <.01) of SLE patients, these cells were comparable to that of HCs after the IL-2 treatment.
Conclusions
Patients with SLE had insufficiency of circulating lymphocyte subsets. This phenomenon was more obverse in those accompanying infection, suggesting the low concentration of lymphocytes may be used as indicators of high infection risk in SLE patients. Low-dose IL-2 induced expansion of Treg cells and NK cells, which may contribute to the restoration of immune homeostasis in SLE patients.
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disorder characterized by a variety of autoantibodies production that affects multiple systems in the body with a vast array of clinical manifestationsCitation1. Despite advanced awareness of this disease and multiple autoantibodies, infections are a most common cause of morbidity and mortality in this patient populationCitation2–4 and at least 50% of patients with SLE suffered with infections during the course of their diseaseCitation5 and around 30% of deaths are related to infectionsCitation6,Citation7. The deregulation of intrinsic and adaptive immune systems plays a central role in the susceptibility of patients to infections. Recent studies revealed that patients with SLE had immunologic abnormalities, especially those treated by immunosuppressive agentsCitation8–12.
Interleukin-2 (IL-2), a cytokine mediator with bacteriolytic activity of immune system, plays an important role in the proliferation and differentiation of lymphocyte cellsCitation13,Citation14. One study has shown that human IL-2 is able to exhibit bacteriolytic activityCitation13. IL-2 is capable of lysing Escherichia coli and Lactobacillus plantarum cells, but the mechanism of the bacteriolytic action of IL-2 still remains unknown. Based on this activity, it has entered clinical development for treating infectious diseasesCitation15. Recent studies revealed that low-dose IL-2 alleviates disease activity in SLE patients by electively modulates CD4 + T cell subsetsCitation16–19. However, most previous studies focused on the proportion of CD4 + T cell subsets, which can be affected by levels of their subsets (such as Th1, Th2, and Th17 cells), rather than the absolute counts, and did not include large amounts of clinical dataCitation20. Furthermore, the status of lymphocyte subpopulations in patients with SLE and the association of these cells and infection remain unclear, and the effect of low dose IL-2 on these subpopulations in these patients with infection and without infection is also unknown.
In this retrospective study, we mainly measured the absolute counts of peripheral blood (PB) lymphocytes and CD4 + T cell subsets of SLE patients with infection to those without infection and/or those of HCs to elucidate the immune mechanism of SLE, and further explored whether low-dose IL-2 could effectively correct the immunologic abnormalities in SLE, especially infected patients.
Methods
Recruitment of participants
Between July 2014 and December 2016, 495 patients with SLE (333 patients without recent infection, 162 patients suffering infection) from the inpatient population of the Second Hospital of Shanxi Medical College were enrolled in the study. All patients fulfilled the 1997 SLE revised classification criteria of the American College of RheumatologyCitation21. Age- and sex-matched healthy individuals (n = 132) were recruited from the physical examination center of the Second Hospital. The history, physical examination, and auxiliary examination were based to determine whether infection is bacterial or viral. The presence of an infection was confirmed by a positive pathogen test from various specimens (blood, sputum, pus, stool, and urine) or clear evidence of infection, such as an abscess on computed tomography. Patients who had fever (body temperature over 38.0 °C) for at least 3 days not caused by disease activity, and effectively reversed by anti-infective treatment, were also considered as infected. All of these patients who met the criteria for infection were enrolled in the study. All patients were on prednisone and/or other immunosuppressive medications to control disease activity ().
Table 1. Comparison of baseline characteristics between the non-infected group and infected group in patients with SLE.
Low-dose IL-2 (recombinant human interleukin-2, Beijing, China) was administered in 54 patients (including 41 in the non-infected group and 13 in the infected group) at a dose of 0.5 million IU per day for 5 consecutive days subcutaneously. The IL-2 was approved by the State Food and Drug Administration of China and had comparable bioactivity to Proleukin (aldesleukin). The exclusion criteria included an IL-2 allergy or intolerance; any other autoimmune disease; a malignant tumor; any heart, kidney, or liver dysfunction. All subjects recruited for this study provided written the prior informed consent and agreed to be assessed for absolute number of lymphocyte subsets. This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical College (Taiyuan, China).
Flow cytometric analysis
Materials
Phorbol myristate acetate (PMA), Anti-human FoxP3-PE and IL-17-PE, Permeabilization Buffer 10× Fixation/Permeabilization Diluent, and Fixation/Permeabilization Concentrate were from Invitrogen, eBioscience, Affymetrix Inc. (by Thermo Fisher Scientific, San Diego, CA). GolgiStop was from BD Biosciences Pharmingen (San Diego, CA). Monoclonal antibodies including CD3FITC/CD8PE/CD45PercP/CD4APC, CD3FITC/CD16 + 56-PE/CD45 PercP/CD19APC, CD4-FITC, IL-4PE, IFN-γ-APC, CD25-APC, and Trucount tube (contain a set number of beads) are from Becton-Dickinson (BD; Franklin Lakes, NJ, USA).
Absolute lymphocyte count
Briefly, the absolute number of T cell subsets (cell/µl) were established from fresh blood samples using reference beads in a Trucount tube from BD as an internal standard for cell concentration. This BD Trucount Tube with beads is ready to use and avoid variation produced by adding standard beads one by one.
Analysis of lymphocyte surface and intracellular markers
The total absolute number of CD4 + T cells was assessed by flow cytometry (FACS Calibur, BD) according to our modified stain and then lyse and wash protocol in manufacturers’ directions of BD Trucount TM tubes. Briefly, 50 μl EDTA-anticoagulated venous blood was added into Trucount tubes A and B separately by reverse pipetting and stained by 20 μl antiCD3-FITC/CD8-PE/CD45-PercP/CD4-APC antibodies in tube A and 20 μl CD3-FITC/CD16 + 56-PE/CD45-PercP/CD19-APC antibodies in tube B (do not touch the blood). Then cells were mixed with 450 μl 1X FACS. Fifteen thousand cells were acquired and detected by MultiSET software within 24 h. The absolute number of T, B, CD8 + T, and NK cells could also be counted in the same way.
Analysis of Th1/Th2/Th17 cells
Cells in 80 μl heparin-anticoagulated venous blood were stimulated by 10 μl PMA, 10 μl Ionomycin (final concentration was 750 ng/ml), and 1 μl GolgiStop in 37 °C for 5 h and then divided into two tubes (tube A and tube B) followed by staining with human anti-CD4-FITC antibodies at room temperature away from light for 30 min. One milliliter fresh Fixation/Permeabilization was used to fix and permeabilize cells and then they were stained by IL-4-PE and IFN-γ-APC in tube A and human anti-IL-17-PE in tube B. Cells were washed with PBS and analyzed using flow cytometry.
Analysis of CD4Treg cells
Cells in 80 μl of heparin-anticoagulated vein blood were surface-labeled with anti-CD4-FITC and anti-CD25-APC and fixed and permeabilized by 1 ml fresh Fixation/Permeabilization followed by staining with human anti-FOXP3-PE.
Statistical analyses
All statistical analyses were performed with SPSS 22.0 software (IBM, Armonk, NY, USA). The categorical demographic characteristics of the patients were compared with the use of the χ2 test. Continuous data that satisfy the homogeneity of normality and variance are presented as mean (±SD). An independent Student’s t-test was used for comparisons between two groups, and one-way analysis of variance (ANOVA) was used for comparisons among three or more groups. Paired-sample t-test was used for paired comparison of differences in immunological features between values at baseline and those after IL-2. Data that dose note satisfy the homogeneity of normality or variance are presented as median (range) and compared by Mann–Whitney U test. A p value of less than .05 was considered statistically significant.
Results
The baseline demographic and clinical characteristics of the patients
Of a total of 495 SLE patients, 302 (60.1%) were female and 162 (32.7%) were suffering from infection. As shown in , there was no significant difference in gender between the patients with and without infection (p >.05). The patients with infection were older than those without infection (p <.05). The average duration of disease in the infected patients was longer than that of the non-infected, but no significant difference was observed (p =.098). All the patients received conventional steroids and immunosuppressive therapies and a higher dosage of prednisone was used in the infection groups (p <.01). Compared with the non-infected SLE, infected patients had a lower level of platelets (p <.01), but a higher proportion of neutrophils (p <.001).
Regarding localization of infection, the respiratory tract was most frequently involved (its infection was found in 71.6% patients), followed by the gastrointestinal (18.5%) and urinary tract (13.0%). Concerning pathogens, bacterial infection was more common (59.9%); viral (10.5%) including Epsteim-Barr virus (6.8%), cytomegalovirus (3.7%), and respiratory syncytial virus (0.6%); 33 patients had unknown pathogens (20.4%) ().
Table 2. Localization and cause of infection (n = 162).
The absolute numbers of T and CD4 + T cells in the non-infected group were significantly lower than that of HCs (p <.001), but still dramatically higher than that in infected patients (p <.001). The levels of B, CD8 + T, and NK cells in the infected patients were significantly decreased when compared with that of both non-infected patients and healthy controls (p <.01), while there was no significant difference in these cells between healthy controls and the non-infected patients (p >.05) ().
Figure 1. Multiple subpopulations of lymphocytes decreased in peripheral blood of SLE patients with infection. Absolute numbers of peripheral lymphocytes subpopulations were analyzed by flow cytometry. Data were presented as mean ± SD and statistical analysis was determined by one-way ANOVA. *p <.05, **p <.01, ***p <.001. (a) SLE patients (n = 495) have lower levels of T, B, NK, CD4 + T, and CD8 + T cells in PB compared with healthy controls. The numbers of PB lymphocytes in the infected groups (n = 162) was also much lower than those in non-infected patients (n = 333). (b) Comparison of numbers of CD4 + T cell subsets among different groups. Patients with SLE had lower levels of Th1 cells, Th2 cells, Th17 cells, as well as Treg cells compared with healthy donors (n = 132), especially the infected groups.
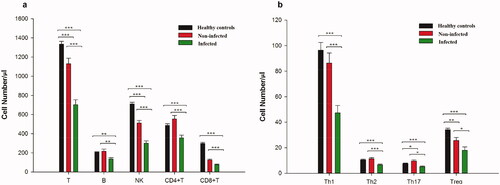
As for CD4 + T cell subsets, the numbers of Th1 and Th2 cells in the infected group were lower than that of non-infected patients and healthy controls (p <. 001), though there was no significant difference in these cells between the non-infected patients and HCs (p >.05). The non-infected patients had a higher level of Th17 cells than that of HCs (p <.05), but the absolute numbers of Th17 cells in infected patients was the lowest among the three groups (p <.05). The number of Treg cells in SLE patients was significantly lower than that of HCs (p <.01), and the infected patients had the fewest Treg cells among all these group (p <.05) ().
Low-dose IL-2 corrected the decreased lymphocyte subpopulations in SLE patients
Fifty-four patients with SLE with or without infection received the treatment of IL-2 at 0.5 million IU per day for 5 days subcutaneously (Supplementary Table S1). Of note, low-dose IL-2 treatment also raised the absolute numbers of these lymphocyte subpopulations in the patients with infection as efficiently as those without infection (). After the treatment, compared with the baseline, there was a significant increase in the number of studied lymphocyte subpopulations except CD4 + T cells: T (p <.001), B (p <.001), CD8 + T (p <.01), NK (p <.001), Th1 (p <.01), Th17 (p <.1), and Treg cells (p <.01) ( and ). CD4 + T cells in SLE patients were still lower than that of healthy subjects (p <.001). Furthermore, low-dose IL-2 raised the cell numbers of these subpopulations in SLE patients to a comparable level of those in healthy controls and an even higher level of Treg cells was detected in SLE as compared to healthy donors (p <.001) ( and ).
Figure 2. Multiple subpopulations of peripheral lymphocytes of SLE patients were significantly upregulated after low-dose IL-2 treatment (n = 54). Absolute numbers of lymphocyte subpopulations in peripheral blood were analyzed by flow cytometry. (a–i) Changes in the numbers of T, B, NK, CD4 + T, CD8 + T, Th1, Th2, Th17, and Treg cells. Comparison between amounts of cells at baseline and after the treatment. Data were presented as mean ± SD and were calculated and compared by paired-sample t-test. *p <.05, **p <.01, ***p <.001.
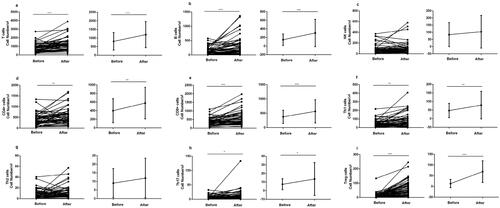
Figure 3. Lymphocyte subpopulations in patients with SLE (n = 54) were upregulated by IL-2 treatment and got to levels near healthy controls (n = 132). Absolute numbers of lymphocyte subpopulations in peripheral blood were analyzed by flow cytometry. Data were presented as mean ± SD and statistical analysis was determined by one-way ANOVA. *p <.05, ***p <.001. (a) SLE patients had a comparable level of T, B, CD4 + T, and CD8 + T cells in PB to healthy controls. (b) Comparison of the number of CD4 + T cell subsets among different groups. Patients with SLE had a comparable number of Th1, Th2, and Th17, and even higher Treg cells than healthy donors, especially the infected groups.
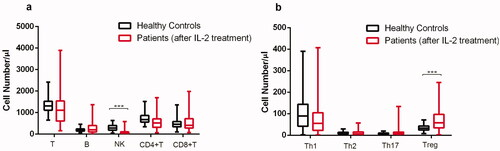
Table 3. Comparison of the number of PB lymphocyte subpopulations in patients with SLE before and after IL-2 treatment (n = 54).
Table 4. Comparison of peripheral lymphocyte subsets in patients with SLE before and after IL-2 treatment (mean ± SD).
Table 5. Comparison of peripheral lymphocyte subsets in patients with SLE after IL-2 treatment and HCs (mean ± SD).
In addition, some of the patients received blood routine and inflammatory indicators both before and after the treatments. Paired-sample t-test suggested a decrease in erythrocyte sedimentation rate (p <.001) and an increase in blood routine measures such as platelets (p <.05), WBC, and lymphocyte neutrophils (p <.001) after the treatment ().
Table 6. Comparison of baseline characteristics in patients with SLE before and after IL-2 treatment.
Discussion
Infection remains a major cause of mortality and morbidity in SLECitation2,Citation6,Citation22. Epidemiological studies found the hospitalization rate of SLE patients for infections was 11–23%Citation23–25, which was 12-times higher than that of patients without SLECitation26. Our result showed that the total incidence of infection in SLE (32.7%) was not very different from previous reports (11–45%)Citation6,Citation23,Citation27–32. Our patients had a high dose of Prednisone (30 mg/Day median). Considering that, this study was only a short-term course of treatment for patients with newly-diagnosed SLE, and 5-days observation is not sufficient to adjust glucocorticoid dose. During the course of diagnosis and treatment, the dose of prednisone depends on the patient’s condition. At each visit, the investigators assessed patients to determine whether it was necessary to adjust the dose of glucocorticoids. Some viruses, bacteria, and protozoa were revealed to cause immune dysfunction in a variety of waysCitation33. Our data confirm respiratory infection and bacteria pathogens were the most common in SLE patients with infection, which were consistent with previous studiesCitation6,Citation23,Citation34.
The possible cause of the infection accounts for the abnormality in immune system by disturbed lymphocytes and its signalingCitation1,Citation12,Citation35–37. Our results revealed that patients with SLE had a lower absolute number of T cells, B cells, NK cells, CD4 + T cells, CD8 + T cells, Th1 cells, Th2 cells, Th17, or Treg cells in PB, and these decreases were more obvious in patients suffering infection. The decrease in these cells suggested disturbances of both innate and adaptive immune systems and thereby more vulnerability to infection. Wu et al.Citation38 reported a lower level of CD4 + T cells but a greater percentage of CD8 + T cell levels in SLE patients with infection, however, the increased CD8 + T cell percentage did not mean a higher level of these cells; as a matter of fact, the absolute number of all these lymphocyte cells are lower in SLE patients with infection. Infected SLE patients had a lower level of platelets. Thrombocytopenia is the most common manifestation of blood system damage in SLECitation39, which is associated with the presence of anti-platelet antibodies, anti-phospholipid antibodies in serum, and impaired bone marrow megakaryocyte maturationCitation40. The level of platelet <100 × 109/L at least once is considered thrombocytopeniaCitation41,Citation42. Therefore, physicians should carefully consider the possible effects of low absolute numbers of lymphocyte subpopulations on infection. The low absolute number of these cells may be more susceptible to infection in SLE patients.
IL-2 is known as a T cell growth factor, playing a central role in maintaining proper function of the immune system of the hostCitation43. Though concanavalin A-generated blast cells in SLE patients responded normally to exogenous IL-2, the ability of lupus lymphocyte cells to produce IL-2 significantly decreasedCitation44. This inability of producing adequate amounts of IL-2 may account for the increased rates of infections. Recently, IL-2 treatment represents a novel strategy to control immune tolerance by influencing functions and survival of Treg cells and to control inflammation and autoimmune diseasesCitation45,Citation46. The IL-2 pathway is an attractive target for the treatment in acute and chronic phases of Chagas’ disease, dampening T cell activation through the expansion/maintenance of Treg cells and regulation of IL-17 productionCitation47. IL-2 therapy has been used to stimulate an immune response to increase T cell numbers in vivo, particularly in treating cancers and human immunodeficiency virus-infected patientsCitation48,Citation49. In this study, we observed an increase in various lymphocyte subsets, most of them comparable with that of HCs, suggesting a potential restoration of abnormal immune function and responses in patients with SLE, which may not only benefit the alleviation of disease activity by the increase of Treg cellsCitation16, but also help in enhancing the potential of anti-infection.
Besides expansion of regulatory T cells, low-dose IL-2 may also sustain cellular immunity with enhanced natural killer cellsCitation18. In our study, though total CD4+ T cells remained unchanged after IL-2 injection, low-dose IL-2 therapy induced a significant expansion of Treg cells and CD8+ T cells. Some findings suggest that sequential IL-2 treatment can restore Hepatitis B virus-specific CD8+ T cell responses, showing efficacy in rescuing immune function in non-responder patients with refractory chronic hepatitis BCitation50.
IL-2 treatment may enhance virus-specific CD8+ T cell responses and promote the activity of NK cells against infectionsCitation51,Citation52. Similar to our clinical observation, He etc.Citation18 observed significantly increased expression of IFNγ and NKp46 by NK cells in response to low-dose IL-2 treatment, which implicated potential augmentation of anti-infectious cellular immunity. In fact, NK cells are important in protection against various infectionsCitation51,Citation52. Infection is a major cause of relapse, hospitalization, and death in patients with SLECitation23,Citation53, and that low-dose IL-2 might increase anti-infectious immune responseCitation54,Citation55 in agreement with previous studiesCitation16. Whether low-dose IL-2 treatment could decrease viral and bacterial loads in infected patients should be carried out in the future.
IL-2 is a regulatory protein that plays a role in oncological and infectious diseases. It is reported that IL-2 had the properties of a bacteriolytic agent via the mechanism of bacteriolytic enzymeCitation13. In current medical practice, IL-2 is used as a regulator of the immune system but not as a bacteriolytic factor, since its bacteriolytic properties had not been previously known. However, it is possible that antimicrobial properties also play an important role in some cases when the effectiveness of IL-2 is confirmed. IL-2 is used both in the case of sepsis, where the role of bacteria is obvious, and in the treatment of cancer, where the role of bacteria is less obvious but there may be a combination of bacterial tissue damage and the underlying diseaseCitation13,Citation56,Citation57. In a randomized, double-blind, placebo-controlled trial of Ld-IL2 therapy in SLE, no serious infections were observed in the IL-2 group compared with the control groupCitation16,Citation58. Zhou et al.Citation58 showed that Ld-IL2 therapy reduced the risk of infections in SLE patients and enhanced the control of viral infection. Saadoun et al.Citation54 reported an in vivo expansion of potent suppressive Tregs in response to low-dose IL-2 immunotherapy in patients with autoimmune-related diseases in the absence of concomitant use of glucocorticoids or immunosuppressive treatments. Besides, low-dose IL-2 was associated with clinical improvement in patients of mixed cryoglobulinemic vasculitis associated with HCV infection and had a modest effect on viral load. These results give a new understanding of the biological functions of IL-2. The mechanism of bacteriolytic action of IL-2 has not yet been established and the mechanism of action of effectors on IL-2 activity also requires further investigation.
This study provides supportive data to confirm the therapeutic effects of low-dose IL-2 in SLE treatment. However, some limitations of this study must be acknowledged. First, several descriptions such as measures of disease activity and damage were not assessed. Furthermore, detailed comparison of lymphocyte subsets in different etiologic agents and locations was not presented due to the unmatched samples. Finally, only 5-days observation is not enough to estimate the direct effect of IL-2 treatment on infection and whether IL-2 therapy could reduce infection rates will be studied further.
Safety
Low-dose IL-2 was well tolerated in SLE patients. None of them displayed severe adverse effects. Non-severe adverse events were characterized by skin rashes at the injection site that resolved spontaneously without special intervention.
Conclusions
Patients with SLE had a disturbance in immune system by decreased number of various lymphocyte subsets, especially those suffering infections. This preliminary finding suggests that the low absolute numbers of these cells may be used as indicators of possible infection in SLE patients and low-dose IL-2 may enhance the ability to resistant infection in SLE patients by restoring the decreased number of lymphocyte subpopulations. Further studies are needed.
Transparency
Declaration of funding
This project was supported by the National Natural Science Foundation of China (No. 82001740) and Graduate Students Outstanding Innovation Project Foundation of Shanxi Province (2C592020079). The funding body had a role in the design of the study and collection, analysis, and interpretation of data.
Declaration of financial/other relationships
The authors declare that there is no conflict of interest. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Study design and manuscript writing: JZ and SZ. Data extraction, quality assessment, analysis, and interpretation of data: JW. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Xiao-Feng Li had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical approval
This study was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (2016 KY-007).
2022.3.30concise_description_of_supplementary_material_file.doc
Download MS Word (32 KB)Acknowledgements
Some of this work was presented as a poster in the International Journal of Rheumatic Diseases and published as an abstract.
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
- Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. J 2016;2:16039.
- Fors Nieves CE, Izmirly PM. Mortality in systemic lupus erythematosus: an updated review. Curr Rheumatol Rep. 2016;18(4):21.
- Pan Q, Liu Z, Liao S, et al. Current mechanistic insights into the role of infection in systemic lupus erythematosus. Biomed Pharmacother. 2019;117:109122.
- Kedves M, Kosa F, Kunovszki P, et al. Large-scale mortality gap between SLE and control population is associated with increased infection-related mortality in lupus. Rheumatology. 2020;59(11):3443–3451.
- Bouza E, Moya JG, Munoz P. Infections in systemic lupus erythematosus and rheumatoid arthritis. Infect Dis Clin North Am. 2001;15(2):335–361.
- Rua-Figueroa I, Lopez-Longo J, Galindo-Izquierdo M, et al. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2017;47(1):38–45.
- Wang J, Niu R, Jiang L, et al. The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine. 2019;98(33):e16798.
- Dorner T, Jacobi AM, Lee J, et al. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J Immunol Methods. 2011;363(2):187–197.
- Becker AM, Dao KH, Han BK, et al. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PLOS One. 2013;8(6):e67003.
- Abud-Mendoza C, Cuevas-Orta E, Santillan-Guerrero EN, et al. Decreased blood levels of B lymphocytes and NK cells in patients with systemic lupus erythematosus (SLE) infected with papillomavirus (HPV). Arch Dermatol Res. 2013;305(2):117–123.
- Ferreira VdS, Mesquita FV, Mesquita DJ, et al. The effects of freeze/thawing on the function and phenotype of CD4(+) lymphocyte subsets in normal individuals and patients with systemic lupus erythematosus. Cryobiology. 2015;71(3):507–510.
- Doaty S, Agrawal H, Bauer E, et al. Infection and lupus: which causes which? Curr Rheumatol Rep. 2016;18(3):13.
- Levashov PA, Sedov SA, Belogurova NG, et al. Bacteriolytic activity of human interleukin-2. Biochemistry. 2012;77(11):1312–1314.
- Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: biology, design and application. Trends Immunol. 2015;36(12):763–777.
- Siegel JP, Rook AH, Djeu JY, et al. Interleukin 2 therapy in infectious diseases: rationale and prospects. Infection. 1985;13(Suppl 2):S219–S223.
- He J, Zhang X, Wei Y, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22(9):991–993.
- Ballesteros-Tato A, Papillion A. Mechanisms of action of low-dose IL-2 restoration therapies in SLE. Curr Opin Immunol. 2019;61:39–45.
- He J, Zhang R, Shao M, et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2020;79(1):141–149.
- Zhao C, Chu Y, Liang Z, et al. Low dose of IL-2 combined with rapamycin restores and maintains the long-term balance of Th17/treg cells in refractory SLE patients. BMC Immunol. 2019;20(1):32.
- Dulic S, Vasarhelyi Z, Sava F, et al. T-Cell subsets in rheumatoid arthritis patients on long-term anti-TNF or IL-6 receptor blocker therapy. Mediators Inflamm. 2017;2017:6894374.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725.
- Thomas G, Mancini J, Jourde-Chiche N, et al. Mortality associated with systemic lupus erythematosus in France assessed by multiple-cause-of-death analysis. Arth Rheumatol. 2014;66(9):2503–2511.
- Goldblatt F, Chambers S, Rahman A, et al. Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus. 2009;18(8):682–689.
- Petri M, Genovese M. Incidence of and risk factors for hospitalizations in systemic lupus erythematosus: a prospective study of the Hopkins lupus cohort. J Rheumatol. 1992;19(10):1559–1565.
- Edwards CJ, Lian TY, Badsha H, et al. Hospitalization of individuals with systemic lupus erythematosus: characteristics and predictors of outcome. Lupus. 2003;12(9):672–676.
- Tektonidou MG, Wang Z, Dasgupta A, et al. Burden of serious infections in adults with systemic lupus erythematosus: a national population-based study, 1996–2011. Arthritis Care Res. 2015;67(8):1078–1085.
- Iliopoulos AG, Tsokos GC. Immunopathogenesis and spectrum of infections in systemic lupus erythematosus. Semin Arthritis Rheum. 1996;25(5):318–336.
- Noël V, Lortholary O, Casassus P, et al. Risk factors and prognostic influence of infection in a single cohort of 87 adults with systemic lupus erythematosus. Ann Rheum Dis. 2001;60(12):1141–1144.
- Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European working party on systemic lupus erythematosus. Medicine. 1999;78(3):167–175.
- Jacobsen S, Petersen J, Ullman S, et al. A multicentre study of 513 Danish patients with systemic lupus erythematosus. II. Disease mortality and clinical factors of prognostic value. Clin Rheumatol. 1998;17(6):478–484.
- Halberg P, Alsbjorn B, Balslev JT, et al. Systemic lupus erythematosus. Follow-up study of 148 patients. II: predictive factors of importance for course and outcome. Clin Rheumatol. 1987;6(1):22–26.
- Hernández-Cruz B, Tapia N, Villa-Romero AR, et al. Risk factors associated with mortality in systemic lupus erythematosus. A case-control study in a tertiary care center in Mexico City. Clin Exp Rheumatol. 2001;19(4):395–401.
- Jung JY, Suh CH. Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J Intern Med. 2017;32(3):429–438.
- Feldman CH, Hiraki LT, Winkelmayer WC, et al. Serious infections among adult medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 2015;67(6):1577–1585.
- Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384(9957):1878–1888.
- Kim HA, Jung JY, Suh CH. Usefulness of neutrophil-to-lymphocyte ratio as a biomarker for diagnosing infections in patients with systemic lupus erythematosus. Clin Rheumatol. 2017;36(11):2479–2485.
- Li Z, Xiao Y, Zhang L. Application of procalcitonin, white blood cell count and neutrophil-to-lymphocyte ratio in the diagnosis of systemic lupus erythematosus with a bacterial infection. Ann Palliat Med. 2020;9(6):3870–3876.
- Wu L, Wang X, Chen F, et al. T cell subsets and immunoglobulin G levels are associated with the infection status of systemic lupus erythematosus patients. Braz J Med Biol Res. 2017;51(2):e4547.
- Sturrock RD. Hematologic disorders in rheumatic disease. Curr Opin Rheumatol. 1991;3(1):172.
- Lazarus AH, Ellis J, Semple JW, et al. Comparison of platelet immunity in patients with SLE and with ITP. Transfus Sci. 2000;22(1-2):19–27.
- Guo Q, Ma XX, Gao H, et al. Association of semaphorin 3A with thrombocytopenia in systemic lupus erythematosus. Beijing Da Xue Xue Bao Yi Xue Ban. 2020;52(5):892–896.
- Yang F, Tian J, Peng L, et al. Thrombocytopenia is an independent risk factor for the prognosis of thrombotic microangiopathy in Chinese patients with systemic lupus erythematosus. Front Med. 2021;8:772607.
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190.
- Miyasaka N, Nakamura T, Russell IJ, et al. Interleukin 2 deficiencies in rheumatoid arthritis and systemic lupus erythematosus. Clin Immunol Immunopathol. 1984;31(1):109–117.
- Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15(5):283–294.
- Bayer AL, Yu A, Adeegbe D, et al. Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J Exp Med. 2005;201(5):769–777.
- Nihei J, Cardillo F, Mengel J. The blockade of interleukin-2 during the acute phase of Trypanosoma cruzi infection reveals its dominant regulatory role. Front Cell Infect Microbiol. 2021;11:758273.
- Bayer AL, Pugliese A, Malek TR. The IL-2/IL-2R system: from basic science to therapeutic applications to enhance immune regulation. Immunol Res. 2013;57(1–3):197–209.
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–5458.
- Wang D, Fu B, Shen X, et al. Restoration of HBV-specific CD8(+) T-cell responses by sequential low-dose IL-2 treatment in non-responder patients after IFN-alpha therapy. Signal Transduct Target Ther. 2021;6(1):376.
- Zwirner NW, Domaica CI. Cytokine regulation of natural killer cell effector functions. Biofactors. 2010;36(4):274–288.
- Littwitz-Salomon E, Dittmer U, Sutter K. Insufficient natural killer cell responses against retroviruses: how to improve NK cell killing of retrovirus-infected cells. Retrovirology. 2016;13(1):77.
- Kang I, Park SH. Infectious complications in SLE after immunosuppressive therapies. Curr Opin Rheumatol. 2003;15(5):528–534.
- Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365(22):2067–2077.
- Zhang R, Xi X, Wang C, et al. Therapeutic effects of recombinant human interleukin 2 as adjunctive immunotherapy against tuberculosis: a systematic review and meta-analysis. PLOS One. 2018;13(7):e0201025.
- Levashov PA, Ovchinnikova ED, Morozova OA, et al. Human interleukin-2 and hen egg white lysozyme: screening for bacteriolytic activity against various bacterial cells. Acta Naturae. 2016;8(1):98–102.
- Levashov PA, Matolygina DA, Ovchinnikova ED, et al. Bacteriolytic activity of human interleukin-2, chicken egg lysozyme in the presence of potential effectors. Acta Naturae. 2017;9(2):82–87.
- Zhou P, Chen J, He J, et al. Low-dose IL-2 therapy invigorates CD8+ T cells for viral control in systemic lupus erythematosus. PLoS Pathog. 2021;17(10):e1009858.
