Abstract
Lifelong treatment with levothyroxine (LT4) is the mainstay of management for individuals with hypothyroidism. Many hypothyroid patients start LT4 treatment at a low dose (e.g. 25–50 µg), especially the elderly, those with residual thyroid function, those with low body weight, and those with significant (especially cardiac) comorbidities. Almost half of patients on LT4 replacement therapy demonstrate either under- or over-treatment. Many LT4 preparations have relatively large intervals between tablet strengths at the lower end of their dose ranges (providing 25 µg, 50 µg, and 75 µg tablets), which may represent a barrier to achieving the optimum maintenance treatment for some patients. The availability of intermediate tablet strengths of LT4 in the 25–75 µg range may facilitate precise and effective dose titration of LT4 and may also enable convenient maintenance regimens based on a single LT4 tablet daily, to support adherence to therapy.
Introduction
Lifelong treatment with adequate replacement doses of levothyroxine (LT4) is the mainstay of management for individuals with overt hypothyroidism, and for some patients with subclinical hypothyroidismCitation1,Citation2. The usual daily replacement dose of LT4 is rather wide: ranging between 12.5 and 150 µg, depending on individual circumstances. Many of these patients, especially the elderly, those with residual thyroid function, those with low body weight, and those with significant comorbidities (especially cardiac disease) need to start LT4 treatment at a low dose, such as 25–50 µg. This review considers pragmatic solutions for applying TSH-driven LT4 therapy for people with hypothyroidism, especially for patients who need lower doses of LT4, focusing on the importance of – and barriers to – precise and careful initiation, titration, and maintenance of LT4.
Place of levothyroxine in the management of hypothyroidism
Thyroid hormone replacement therapy has been used for more than a century to treat hypothyroidismCitation3. Initially, natural animal-derived thyroid preparations containing both thyroxine (T4) and triiodothyronine (T3) were utilized for this purpose. Dose adjustments of these natural preparations were based on resolution of symptoms and normalization of basal metabolic rate. However, this frequently led to the use of supra-physiologic doses of thyroid hormones, causing multiple adverse effects associated with iatrogenic thyrotoxicosis. Subsequently, two developments in the 1970s changed the management landscape of hypothyroidism. First, the discovery that thyroid stimulating hormone (TSH) is the most sensitive marker of thyroid hormone status (and it subsequently has become easily accessible for testing) and the development of assays to detect TSH accurately and sensitively in serum led to the realization that many people on animal-derived thyroid extracts were being over-treatedCitation4. Second, the discovery that T4 is deiodinated to the active thyroid hormone, T3, in the periphery by deiodinase enzymes provided a physiologic basis to support the use of LT4, the synthetic form of T4, for the routine management of hypothyroidismCitation5.
Accordingly, oral thyroid hormone replacement with LT4 is the current standard of care for the management of hypothyroidismCitation1,Citation2. Treatment with oral LT4 monotherapy is applied at a dose which normalizes serum TSH levels to within a reference range derived from levels measured in a population of subjects without thyroid disease. Box 1 provides a brief overview of the management of hypothyroidismCitation1,Citation2. Adults with overt hypothyroidism and TSH >10 mIU/L require thyroid hormone replacement with LT4. The situation with subclinical hypothyroidism (elevated TSH with normal T3/T4) is more nuanced. TSH levels tend to increase with age, and randomized trials have not demonstrated clear benefits of intervention with LT4 in elderly people with mildly elevated TSHCitation6. Severe obesity is also associated with increased TSHCitation7. Not all individuals with mildly elevated TSH will require treatment with LT4, especially if they do not report symptoms of hypothyroidismCitation1. Also, these recommendations relate to non-pregnant adults; consideration of the details of thyroid care for preconception and pregnancy, and for children with thyroid dysfunction is beyond the scope of this review and covered in the appropriate guidelinesCitation1,Citation2.
This approach is effective in resolving the symptoms of overt hypothyroidism for most patients. Optimizing the dose of LT4 carefully is essential for achieving a good outcome for people with hypothyroidism. Under-treatment is likely to result in complaints of continuing troublesome hypothyroid-like symptoms as well as dyslipidaemia and higher cardiovascular risk, and over-treatment risks disabling, or life-threatening long-term complications related to iatrogenic thyrotoxicosis, particularly affecting the heart and bonesCitation8. Nevertheless, 30–50% of patients on LT4 replacement therapy demonstrate either under- or over-replacement, as evidenced by high or low serum TSH levels, respectively, according to one reportCitation9.
Regulators in both Europe and the US have classified LT4 as a “narrow therapeutic index” (NTI) drug, due to the fact that even small variations in exposure to LT4 can induce clinically significant variations in its biological effectsCitation10–14. The introduction of more stringent quality standards for LT4 tablets has reduced the potential for variations in the actual effectiveness of a given LT4 dose between batches of tablets, and when switching between LT4 preparationsCitation8. Hypothyroidism itself is a dynamic process, however, where the underlying severity of the condition may change over time for many patients, necessitating periodic thyroid function testing and adjustment of the LT4 doseCitation15,Citation16. Furthermore, LT4 requirements can also change with age, most likely due to the concomitant reduction in lean mass with ageingCitation16.
Following the diagnosis of hypothyroidism, achieving and maintaining optimal dosage levels of LT4 can therefore be a challenging, life-long processCitation1,Citation2. Moreover, each individual’s requirement of replacement LT4 differs and is influenced by multiple factors including age, body weight, smoking status, aetiology of the hypofunction, severity and duration of the hypothyroidism, and other conditions and medications. LT4 tablets are provided in a range of set dosage strengths which may differ between countries and providers; thus complicating further the task of delivering a precise and consistent level of exposure to the patient.
Practical issues in the application of LT4 therapy for hypothyroidism
Starting and maintaining LT4 treatment
Patients presenting with hypothyroidism are highly heterogeneous, requiring widely different starting doses of LT4Citation1,Citation2. The main factor determining the initial dosage is the residual level of thyroid function: patients range from subclinical hypothyroidism, with a small deficit in thyroid function requiring only modest additional supplementation with oral LT4, to patients with little or no residual function who require essentially full thyroid hormone replacementCitation1,Citation2. Not all individuals diagnosed with subclinical hypothyroidism require LT4 treatment, although it is a matter of intense debate which individuals may benefit: for example, very elderly or severely obese patients may present with mildly elevated TSH that is not caused by primary thyroid dysfunction, and thyroid hormone replacement therapy may not be of benefit to themCitation17,Citation18. For those with subclinical hypothyroidism who are commenced on LT4 treatment, typical starting doses of LT4 usually range between 25 and 50 µg, and the average daily dose of LT4 needed to normalize the TSH level has been found to range from below 50 µg to about 100 µg2. Patients with overt hypothyroidism and relatively mild elevation of TSH may also be started on LT4 at doses of 25–50 µg2. Other factors that influence the TSH level, and thus the starting dose of LT4, are weight/adiposity, pregnancy, age, and the presence of other medical conditions (e.g. a lower starting dose is recommended for patients with coronary heart disease [25 µg or less] or for elderly patients)Citation2. Middle-aged or younger and otherwise healthy patients may start on the dose of LT4 estimated to provide full thyroxine replacement (about 160–200 µg for a 100 kg patient)Citation1.
Furthermore, patients with the relatively rare secondary hypothyroidism (where the pathology is extra-thyroidal due to pituitary or hypothalamic dysfunction) also tend to require relatively smaller doses of LT4 replacement than patients with the primary form (where the thyroid itself is diseased) of the condition. Actual maintenance doses in routine practice are also variable, however: data from real-life studies have demonstrated that many patients receive relatively low average daily maintenance doses of LT4, e.g. of 100 µg (median, Italy)Citation19, 108 µg (mean, France)Citation20, or 116 µg (mean, Italy, after weight loss)Citation21. Thus, a substantial proportion of patients receive LT4 doses comprised of one or more smaller tablets in routine practice.
Available tablet strengths and precision of LT4 dosing
As described above, many people with primary hypothyroidism start LT4 on doses of 25–50 µg, especially those with mild (or subclinical) hypothyroidism or significant comorbidity, and the dose of LT4 is titrated until the TSH level is within a predefined reference range (Box 1). Numerous generic formulations of LT4 are available that provide a range of tablet strengths for this purpose. As an example, the British National Formulary (BNF) used by primary care prescribers in that country lists formulations of LT4 (presented as tablets, capsules or oral solution) in dosage strengths of 12.5 µg, 25 µg, 50 µg, 75 µg, 100 µg, and 125 µgCitation22. Formulations listed by the National Competent Authorities responsible for licensing medicines in the European Union and the European Economic Area () also provide multiple dosage strengths, with additional higher strengths (150–200 µg) and some intermediate strengths (63 µg, 88 µg, 112 µg, 137 µg), compared with the BNF (although not all of these formulations will be available in all countries in the region, or elsewhere)Citation23. According to information from the US for 2019, the 50 µg tablet was most often prescribed (19% of prescriptions), followed by the 75 µg tablet (16%); the 25 µg tablet accounted for 9% of prescriptions, while other tablet strengths ranged from 5% of prescriptions for the 137 µg tablet to 12% of prescriptions for the 100 µg tabletCitation24. Although we do not know to what extent these tablets were prescribed as monotherapy or as part of a regimen incorporating tablets of different strengths, these data suggest that lower strength LT4 tablets (<100 µg) are used frequently in routine clinical practice.
Table 1. Tablet strengths of levothyroxine (LT4) available in the European Union.
Having a range of closely spaced tablet strengths of LT4 is likely to be helpful in titration, as small increments in the LT4 dose can alter serum TSH levels to a clinically significant extent, as described above. The distance between tablet strengths becomes less marked in relative terms, as the tablet strength increases: for example, titrations from 25 µg to 50 µg and from 175 µg to 200 µg involve increases in LT4 doses of 100% and 13%, respectively. The availability of intermediate tablet strengths at the lower end of the dosage range facilitates more cautious titration from a starting dose of 25 µg to a final dose of 75 µg or higher. It is important to note here that the current tight regulatory requirements for manufacturing LT4 tablets means that even relatively small increments in tablet strength, for example from 25 µg to 38 µg or from 50 µg to 63 µg, are likely to be meaningful clinically, without overlaps in actual delivered doses between tablet strengths or batches, within and between different LT4 preparations.
Moreover, the patient’s final optimal dose may reside between two available dosage strengths: this may require ingestion of more than one tablet of different strengths, particularly for preparations with large gaps between tablet strengths (). The availability of intermediate tablet strengths across the usual dosage range for LT4 increases the likelihood that the patient may achieve maintenance treatment on a straightforward regimen of a single tablet. It is well known that simplification of drug regimens supports patients’ ability to adhere to therapeutic drug regimens for chronic, non-communicable conditionsCitation25–27. A recent observational study found that about two-thirds of a population with hypothyroidism demonstrated “low” or “medium” adherence to LT4 tabletsCitation28. Other data support the existence of a variable, but often substantial, proportion of patients who do not adhere well to LT4 therapy for hypothyroidismCitation29–33. Unsurprisingly, non-adherence to LT4 therapy increases healthcare utilization and is associated with adverse long-term clinical outcomesCitation34. For completeness, other approaches to optimizing LT4 adherence have been proposed to take advantage of the relatively long half-life of LT4, including weekly LT4 dosingCitation16 and taking “a week’s worth of LT4 in a week,” i.e. taking missed doses to ensure that the overall weekly dose is correctCitation35. Current guidance does not support these approaches, however. Maintenance treatment on a single tablet/day is also likely to help to restrain treatment costs, compared with prescription of multiple tablet strengths to achieve a desired dose.
Switching between LT4 products
Generic LT4 preparations are evaluated for bioequivalence with a marketed reference product before entering therapeutic practice, so in principle it should be possible to switch between different products in search of an optimum dose for maintenance treatment for a given patient. Indeed, a consensus statement from the British Thyroid Association from 2016 considered prescribing within one LT4 preparation to be unnecessary for most patients (weak recommendation based on low quality evidence)Citation36. Nevertheless, concerns have been raised about switching between brands (or formulations) of NTI drugs, including LT4, due to observations of loss of control of thyroid function or thyroid-related side-effects following such a switchCitation37–40. Switching from branded to generic LT4 has also been associated with increased healthcare costs that effectively balanced out the reduced cost of the medication itself, according to a database study from the USCitation41. Generic or branded LT4 is similarly effective for the initiation of thyroid replacement therapy, howeverCitation42.
Two expert groups, the European Thyroid Association and Thyroid Federation International, provided a more recent consensus statement on LT4 treatment in 2018, noting that even testing for bioequivalence has not always maintained patients free of symptoms after a change of formulationCitation40. They recommended that patients should be maintained on the same brand or formulation of LT4 where possibleCitation40. They also recommend that pharmaceutical sponsors should work with all key stakeholders in managing hypothyroid patients (healthcare professionals, pharmacists, regulators, patient associations) in advance of any unavoidable changes in LT4 preparations such as those mandated recently by regulators (see above), with a plan for monitoring the clinical impact of the changeCitation40.
The expert recommendations also state that LT4 content and bioequivalence should be declared “in an unambiguous way”Citation40. The introduction of updated LT4 formulations is an opportunity to do this. For example, one formulation that meets the latest regulatory requirements was demonstrated to be formally bioequivalent to a marketed reference product in two pharmacokinetic studies conducted in healthy volunteersCitation43. One of these studies also confirmed dose proportionality between tablet strengths by demonstrating that exposure of subjects to LT4 was consistent for 600 µg doses of LT4 given as 12 × 50 µg, 6 × 100 µg, or 3 × 200 µg tabletsCitation43. Thus, any overall dose of this preparation can be constructed from any combination of tablet strengths, with confidence that its potency will be predictable (i.e. unambiguous) from the labelled content of the tablets. Importantly, no new pharmacokinetic evaluation of the new 38 µg and 63 µg tablet strengths for this LT4 preparation is needed, as they are within the range of tablet strengths already available.
Other practical issues in the application of optimized levothyroxine therapy
This review has focused mainly on how the potential impact of the changing pharmaceutical regulatory environment has influenced the precise application of optimized LT4-based therapy for hypothyroidism. A number of other barriers to achieving this goal exist that are encountered commonly in routine endocrinological practice, and are discussed here briefly. For example, a minority (10–15%) of patients continue to report symptoms suggestive of hypothyroidism, despite successful return of TSH to within its reference range after titrated LT4 therapyCitation7,Citation44,Citation45. In most (but not all) cases, careful clinical evaluation reveals another explanation for these symptoms, without increasing the LT4 doseCitation46. A number of drugs, food products, and endogenous substances are known to interfere with the TSH test and may lead to aberrant results. More speculatively, it has been proposed that each patient has their own “set point” for thyroid hormone homeostasis, implying that the ideal TSH level for that individual may lie outside the range of “normal” values determined in the reference population used to validate the TSH testCitation47,Citation48. Peripheral deiodinases activate and deactivate thyroid hormones, and possible roles for polymorphisms in these enzymes, or iatrogenic or age-related changes in their activity, in determining an individual’s thyroid hormone status is a current active area of researchCitation49. Identifying and correcting issues such as these, where present, will provide clarity in interpreting the results of TSH tests, and facilitate achievement of the individual patient’s optimum LT4 dosage.
Conclusions and future perspectives
Many patients with hypothyroidism, especially the elderly, those with residual thyroid function, and those with significant comorbid (especially cardiac) disease need to start treatment with LT4 at a low dose, such as 25–50 µg, which is then titrated to a maintenance dose defined by the level TSH and amelioration of symptoms. Given the status of LT4 as a NTI, providing a broader range of tablet strengths at the lower end of the dosage range may facilitate the achievement and application of long-term maintenance treatment with LT4 for people with hypothyroidism.
The clinical utility of this approach will need to be proven. At present, we face a paradox where the clinical utility of smaller titration steps for lower doses of LT4 has not been evaluated for lack of such tablets. Real world studies are contributing increasingly to our understanding of how medicines work in routine clinical practice. Observational studies in large populations will be needed to evaluate the impact of smaller LT4 titration steps on TSH control, quality-of-life, cost-effectiveness, adherence, and convenience for patients and physicians.
Transparency
Declaration of funding
Merck Healthcare KGaA, Darmstadt, Germany, funded editorial support (see below) and FastTrack review. Authors did not receive payment for co-authoring this article, and no other funding applied.
Declaration of financial/other relationships
UG-H is an employee of Merck Healthcare KGaA, Darmstadt, Germany. SR has acted as a speaker and member of advisory boards for Merck Healthcare KGaA, Darmstadt, Germany.
Box 1 Brief overview of the management of primary hypothyroidism in non-pregnant adults.a
A small change in serum T4 causes a large change in TSH due to the log-linear relationship between the two hormones and the feedback mechanisms between the thyroid and TSH secreting cells in the pituitary
Therefore, serum TSH (not T4) is used to guide thyroid hormone replacement therapy
Main objectives of treatment:
Resolve symptoms of hypothyroidism;
Bring serum TSH within a predefined normal reference rangea and improve levels of other thyroid hormones; and
Avoid iatrogenic thyrotoxicosis (over treatment).
When a patient presents with suspected primary hypothyroidism: 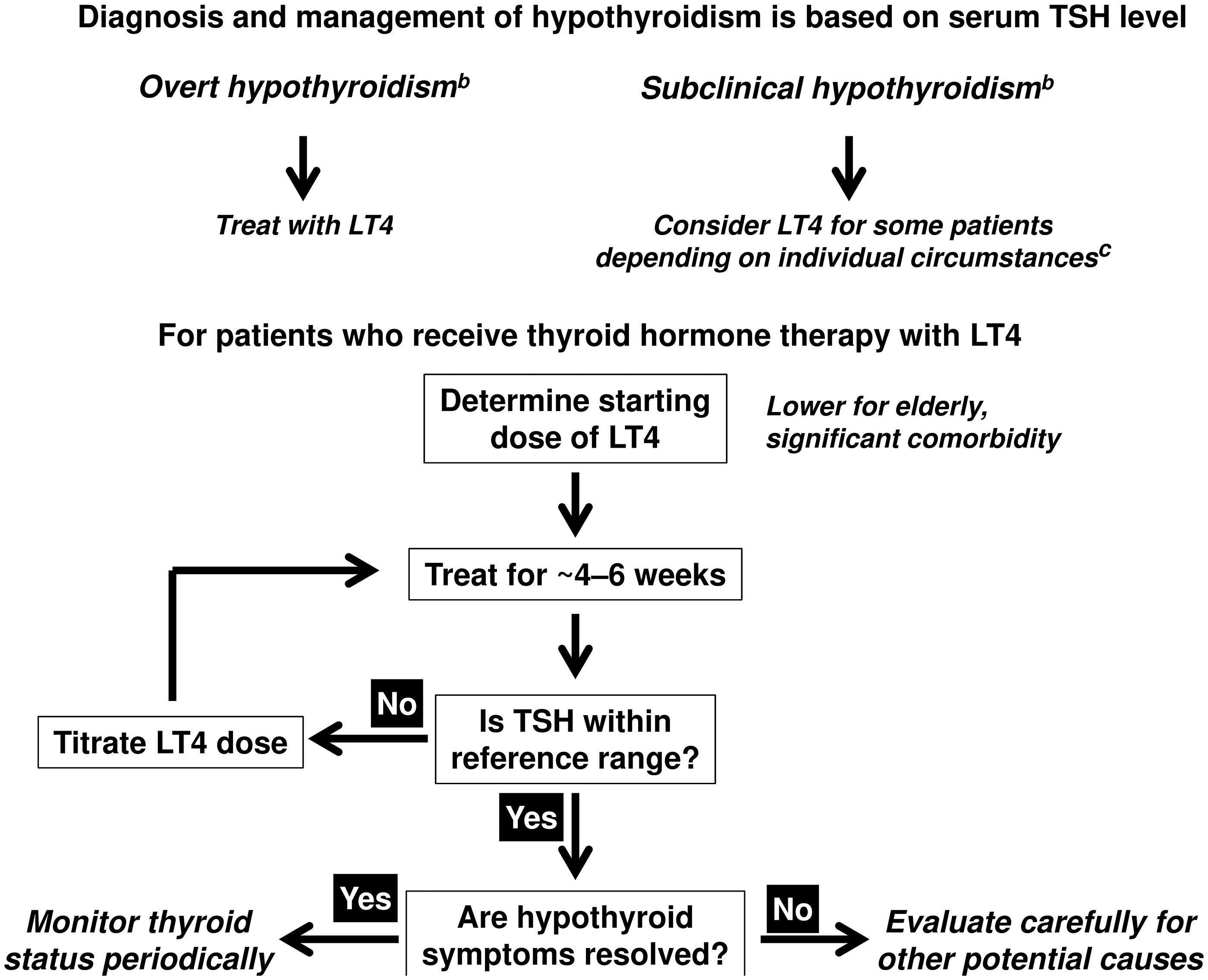
aVaries between tests and regions, but a typical reference range is between 0.45 and 4.0 or 4.5 mU/L.
bNote that this overview relates to the management of primary hypothyroidism in nonpregnant adults, diagnosed according to current guuidelinesCitation1,Citation2. Women planning or conducting a pregnancy and children are managed differentlyCitation1,Citation2.
cMildly elevated TSH is not thought to be pathological in certain groups of patients such as the very elderly, people with morbid obesity, etc.: LT4 therapy may not be beneficial for such cases (see text).
Compiled from information presented in Pearce et al.1 and Jonklaas et al.2.
Acknowledgements
A medical writer (Dr Mike Gwilt, GT Communications) provided editorial support, funded by Merck Healthcare KGaA, Darmstadt, Germany.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/03007995.2022.2107767)
References
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215–228.
- Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751.
- Kahaly GJ. Therapeutic use of levothyroxine: a historical perspective. In: Kahaly GJ, editor, 70 Years of levothyroxine. Switzerland: Springer Nature Switzerland AG; 2021. pp 1–11.
- Utiger RD. Thyrotrophin radioimmunoassay: another test of thyroid function. Ann Intern Med. 1971;74(4):627–629.
- Braverman LE, Ingbar SH, Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970;49(5):855–864.
- Razvi SR. Levothyroxine in the older patient. In: Kahaly GJ, editor, 70 Years of levothyroxine. Switzerland: Springer Nature Switzerland AG; 2021. pp 75–84.
- Razvi S, Hostalek U. Therapeutic challenges in the application of serum thyroid stimulating hormone testing in the management of patients with hypothyroidism on replacement thyroid hormone therapy: a review. Curr Med Res Opin. 2019;35(7):1215–1220.
- Thayakaran R, Adderley NJ, Sainsbury C, et al. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ. 2019;366:l4892.
- Eligar V, Taylor PN, Okosieme OE, et al. Thyroxine replacement: a clinical endocrinologist's viewpoint. Ann Clin Biochem. 2016;53(Pt 4):421–433.
- Habet S. Narrow therapeutic index drugs: clinical pharmacology perspective. J Pharm Pharmacol. 2021;73(10):1285–1291.
- Committee For Medicinal Products For Human Use (CHMP). Draft guideline on the investigation of bioequivalence; [cited 2022 Mar]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-investigation-bioequivalence_en.pdf.
- European Medicines Agency. Committee for Proprietary medicinal Products (CPMP). Note for guidance on the investigation of bioavailability and bioequivalence. 2009; [cited 2022 Mar]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003519.pdf.
- US Department of Health and Human Services. Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry: levothyroxine sodium tablets – in vivo pharmacokinetic and bioavailability studies and in vitro dissolution testing; CDER; 2000; [cited 2022 Mar]. Available from: www.fda.gov/cder/guidance/index.htm.
- Lipp HP, Hostalek U. A new formulation of levothyroxine engineered to meet new specification standards. Curr Med Res Opin. 2019;35(1):147–150.
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87(7):3221–3226.
- Duntas LH, Jonklaas J. Levothyroxine dose adjustment to optimise therapy throughout a patient's lifetime. Adv Ther. 2019;36(Suppl 2):30–46.
- Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006.
- Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320(13):1349–1359.
- Trimboli P, Scappaticcio L, De Bellis A, et al. Different formulations of levothyroxine for treating hypothyroidism: a real-life study. Int J Endocrinol. 2020;2020:4524759. 2020:4524759.
- Richou M, Gilly O, Taillard V, et al. Levothyroxine dose adjustment in hypothyroid patients following gastric sleeve surgery. Ann Endocrinol. 2020;81(5):500–506.
- Fierabracci P, Martinelli S, Tamberi A, et al. Weight loss and variation of levothyroxine requirements in hypothyroid obese patients after bariatric surgery. Thyroid. 2016;26(4):499–503.
- British National Formulary. Levothyroxine sodium; [cited 2022 Mar]. Available from: https://bnf.nice.org.uk/medicinal-forms/levothyroxine-sodium.html.
- Heads of Medicines Agencies; [cited 2022 Mar]. Available from: https://www.hma.eu.
- ClinCalc.com. Levothyroxine. Drug Usage Statistics, United States, 2013–2019; [cited 2022 Mar]. Available from: https://clincalc.com/DrugStats/Drugs/Levothyroxine.
- Elnaem MH, Irwan NA, Abubakar U, et al. Impact of medication regimen simplification on medication adherence and clinical outcomes in patients with long-term medical conditions. Patient Prefer Adherence. 2020;14:2135–2145.
- Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31(3):213–224.
- Ayele AA, Tegegn HG, Ayele TA, et al. Medication regimen complexity and its impact on medication adherence and glycemic control among patients with type 2 diabetes mellitus in an ethiopian general hospital. BMJ Open Diabetes Res Care. 2019;7(1):e000685.
- Kumar R, Shaukat F. Adherence to levothyroxine tablet in patients with hypothyroidism. Cureus. 2019;11:e4624.
- Crilly M. Thyroxine adherence in primary hypothyroidism (letter). Lancet. 2004;363(9420):1558.
- El Helou S, Hallit S, Awada S, et al. Adherence to levothyroxine among patients with hypothyroidism in Lebanon. East Mediterr Health J. 2019;25(3):149–159.
- Machado-Alba JE, Machado-Duque ME. Adherence to levothyroxine prescription in patients with hypothyroidism. Rev Med Chil. 2020;148(6):740–745.
- Topaloğlu Ö, Yavuz A, Tiryaki Aylıkcı AB. Evaluation of adherence to levothyroxine and out-of-range thyroid-stimulating hormone levels in pregnant women with primary hypothyroidism. Int J Clin Pract. 2021;75(8):e14312.
- Cappelli C, Castello R, Marini F, et al. Adherence to levothyroxine treatment among patients with hypothyroidism: a northeastern Italian survey. Front Endocrinol. 2018;9:699.
- Hepp Z, Lage MJ, Espaillat R, et al. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018;21(9):912–919.
- Davoren P. Modern management of thyroid replacement therapy. Aust Prescr. 2008;31(6):159–161.
- Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British thyroid association executive committee. Clin Endocrinol. 2016;84(6):799–808.
- Flinterman LE, Kuiper JG, Korevaar JC, et al. Impact of a forced dose-equivalent levothyroxine Brand switch on plasma thyrotropin: a cohort study. Thyroid. 2020;30(6):821–828.
- New Zealand Medicines and Medical Devices Safety Authority (MEDSAFE). Some medicines need to be prescribed by brand; [cited 2022 Mar]. Available from: https://www.medsafe.govt.nz/profs/PUArticles/December2019/Some-medicines-need-to-be-prescribed-by-brand.htm.
- Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(Suppl 2):59–71.
- Fliers E, Demeneix B, Bhaseen A, et al. European thyroid association (ETA) and thyroid federation international (TFI) joint position statement on the interchangeability of levothyroxine products in EU countries. Eur Thyroid J. 2018;7(5):238–242.
- Katz M, Scherger J, Conard S, et al. Healthcare costs associated with switching from Brand to generic levothyroxine. Am Health Drug Benefits. 2010;3(2):127–134.
- Brito JP, Ross JS, Sangaralingham L, et al. Comparative effectiveness of generic vs Brand-Name levothyroxine in achieving normal thyrotropin levels. JAMA Netw Open. 2020;3(9):e2017645.
- Gottwald-Hostalek U, Uhl W, Wolna P, et al. New levothyroxine formulation meeting 95-105% specification over the whole shelf-life: results from two pharmacokinetic trials. Curr Med Res Opin. 2017;33(2):169–174.
- Jonklaas J. Persistent hypothyroid symptoms in a patient with a normal thyroid stimulating hormone level. Curr Opin Endocrinol Diabetes Obes. 2017;24:356–363.
- Saravanan P, Chau WF, Roberts N, et al. Psychological well-being in patients on 'adequate' doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol. 2002;57(5):577–585.
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: randomised double blind placebo controlled crossover trial. BMJ. 2001;323(7318):891–895.
- Hoermann R, Midgley JE, Larisch R, et al. Homeostatic control of the Thyroid-Pituitary axis: Perspectives for diagnosis and treatment. Front Endocrinol. 2015;6(177):177.
- Li E, Yen PM, Dietrich JW, et al. Profiling retrospective thyroid function data in complete thyroidectomy patients to investigate the HPT axis set point (PREDICT-IT). J Endocrinol Invest. 2021;44(5):969–977.
- Bianco AC, Kim BS. Pathophysiological relevance of deiodinase polymorphism. Curr Opin Endocrinol Diabetes Obes. 2018;25(5):341–346.
