Abstract
Objectives
Hypertension is a major cause of morbidity and mortality worldwide. Although current drug therapies can be effective, management of hypertension is closely linked to patient adherence to therapy. A fixed-dose combination (FDC) of bisoprolol and amlodipine has shown to be effective and convenient, and to significantly improve patient adherence.
Methods
This narrative review evaluates recent evidence from four studies which explore the efficacy, safety, and adherence of FDC bisoprolol and amlodipine in patients with hypertension: one observational study; two randomized clinical trials (RCTs); and one indirect comparison analysis.
Results
All four studies support the efficacy of FDC bisoprolol and amlodipine in the management of hypertension, highlighting clinically meaningful reductions in systolic and diastolic blood pressure (BP), and high adherence. In a large cohort study exploring FDC bisoprolol and amlodipine in daily practice, high adherence improved BP and heart rate control versus baseline. In a Phase 3 RCT, FDC bisoprolol and amlodipine demonstrated superiority over monotherapies in BP control and a positive tolerability profile, further supporting its use to manage hypertension in second line following monotherapy. In another Phase 3 RCT, the combination of bisoprolol and amlodipine led to significant BP reductions versus monotherapy with amlodipine with a comparable safety profile. Finally, in the indirect treatment comparison, a low dose combination of bisoprolol and amlodipine showed a similar decrease in systolic BP compared with a maximum dose of amlodipine.
Conclusions
This review adds to growing evidence supporting the efficacy, safety, and tolerability of FDC bisoprolol and amlodipine in managing hypertension.
Introduction
Hypertension is a global health burden, affecting approximately 1.13 billion people worldwideCitation1. Current treatment options for hypertension include five drug classes: beta-blockers, angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers (CCB), and diuretics (including thiazides and thiazide-like diuretics)Citation2. Studies have shown that monotherapies fail to adequately control blood pressure (BP) in up to 75% of patientsCitation3. These patients require a combination of drug classes to achieve their target BPCitation3,Citation4. Most hypertension management guidelines recommend initiating antihypertensive treatment with a drug combination, preferably a fixed-dose combination (FDC)Citation2,Citation5.
In long-term treatment of chronic diseases, such as hypertension, patient adherence is a challengeCitation6,Citation7. BP is often poorly controlled if patients do not fully adhere to the prescribed pharmacologic therapyCitation2, a problem commonly observed in patients with a high pill burdenCitation2. A single-pill drug regimen is therefore the preferred strategy to manage hypertensionCitation2, as it simplifies treatment and can improve adherenceCitation8,Citation9.
A large cohort analysis showed adherence rates declined with an increasing number of medicationsCitation6. In a meta-analysis that included patients with hypertension, the use of a fixed-dose single-pill therapy improved adherence to treatment regimens by 24% compared with free-dose combinationsCitation10. Additionally, a recent meta-analysis showed superior BP control under a fixed-dose single-pill compared with equivalent free-dose combinationsCitation11.
The use of FDC in preference to free-dose combinations in the treatment of patients with chronic conditions, such as hypertension, could result in improved medication adherence and better clinical outcomesCitation10.
Bisoprolol, a beta-blocker, and amlodipine, a CCB, are frequently used as monotherapies to manage hypertension. These two antihypertensive agents have different, complementary modes of action to reduce BP, and demonstrate an additive effect, making them suitable for combination therapyCitation3,Citation7,Citation12. The FDC of bisoprolol and amlodipine (Concor AM, Merck Healthcare KGaA, Darmstadt, Germany) is indicated for the treatment of hypertension as substitution therapy in patients adequately controlled with individual products given concurrently at the same dose as in the combination, but as separate tabletsCitation13. Administration of these two drugs as a FDC improves patient adherence compared with the free-dose combination of bisoprolol and amlodipineCitation7, and may reduce the burden of hypertension on health care systemsCitation7. FDCs of antihypertensive medications, including FDC bisoprolol and amlodipine, have the potential to provide an effective hypertension therapy in a single convenient daily dose.
The utility of FDC bisoprolol and amlodipine in patients with essential hypertension is well acceptedCitation14–17. However, until more recently, there was limited information on its efficacy as a potential second-line therapy in patients whose BP was not controlled with either treatment as monotherapyCitation7. Here we describe the growing evidence base for the use of FDC bisoprolol and amlodipine in replacement or second-line treatment for hypertension.
Methods
We conducted a literature search to identify studies evaluating FDC bisoprolol and amlodipine for hypertension. We used PubMed to search MEDLINE and PubMed Central electronic databases using combinations of the following search terms: amlodipine, bisoprolol, fixed-dose, fixed dose and combination. We identified a prior review of FDC bisoprolol and amlodipine studies published up to 2015Citation7; therefore in our literature search, we selected all studies that were published with the combination of bisoprolol and amlodipine between January 2016 and January 2022 either in replacement or in second-line indication for inclusion in the current review. The quality of studies was assessed using the Jadad scale for randomized controlled trialsCitation18 and the Newcastle–Ottawa scale for observational studiesCitation19. Studies are summarized in .
Table 1. Summary of recent studies involving fixed-dose combinations of bisoprolol and amlodipine.
Results
Improved BP control and adherence with FDC bisoprolol and amlodipine after switching from a free-dose combination in a large cohort study
A large, prospective non-interventional study was carried out in six Eastern European countries (Czech Republic, Hungary, Poland, Romania, Serbia and Slovakia). Prior to recruitment to the study, patients with hypertension (N = 12,424) had been treated with a free-dose combination of bisoprolol and amlodipine prior to switching to FDC bisoprolol and amlodipine for 4 weeks prior to recruitment. Patient data and clinical findings were recorded at study baseline and after 6 months of FDC treatment. Adherence was measured by tablet count (tablets taken divided by tablets prescribed, multiplied by 100) and defined as: excellent >90%; good 76–90%; moderate 50–75%; and poor <50%Citation8.
This study observed good-to-excellent adherence in 99% of patients 6 months after initiating FDC bisoprolol and amlodipine. This adds to previous evidence of a high patient adherence rate, which increased 2.2-fold under FDC bisoprolol and amlodipine relative to the equivalent free-dose combinationCitation14. Patient adherence in this study was also associated with improved control of previously elevated BPCitation8. Substantial reductions in systolic and diastolic BP from baseline to 6 months were seen: mean systolic BP (SBP) from 147.6 to 131.2 mmHg; and mean diastolic BP (DBP) from 88.3 to 78.9 mmHg, decreasing by 16.5 mmHg and 9.5 mmHg, respectivelyCitation8. Notably, 30% of patients (n = 3664) did not have controlled BP at the start of the study; after 6 months of treatment, only 1% of patients (n = 130) had insufficiently controlled BP. This may be due to the high adherence rate with the FDCCitation8. Additionally, the results for BP reductions support previous studies showing that combination therapies are more effective than monotherapies in controlling hypertensionCitation23,Citation24.
Pulse pressure and heart rate, both independent risk factors for cardiovascular diseaseCitation25, improved considerably during this studyCitation8,Citation25. Mean pulse pressure improved from 59.3 mmHg at baseline to 52.3 mmHg after 6 months’ treatment. Similarly, mean heart rate was reduced from 75.8 to 68.4 beats per minute (bpm) after 6 months of FDC treatment. A previous study has similarly shown pulse pressure control with FDC bisoprolol and amlodipine, potentially linked to better patient adherence to the treatmentCitation14.
The high adherence to FDC bisoprolol and amlodipine may lead to improved BP control and reduced risk of cardiovascular events. This is in line with recent studies indicating that a high rate of drug adherence may improve BP control and lower the risk of cardiovascular diseaseCitation11,Citation26, thus reinforcing the clinical benefit of improved adherence to FDC bisoprolol and amlodipine versus the free-dose combinationCitation14.
Furthermore, most patients (90%) preferred the FDC over the free-dose combination, demonstrating a high acceptance of the therapyCitation8.
A total of 101 adverse events (AEs) were reported, with only eight serious AEs, six of which were considered unrelated to the treatmentCitation8. In an earlier study, AEs were reported in fewer than 10% of patients treated with bisoprolol and amlodipine combinationCitation14. These results are in line with other reports of AEs associated with bisoprolol or amlodipine monotherapyCitation27,Citation28. Furthermore, it is worth noting that elevated transaminases and triglyceride levels have previously been associated with amlodipine and bisoprolol combination therapyCitation29; however they were not reported in this studyCitation8.
This study had a discontinuation rate of only 0.07%Citation8. This mirrors the low discontinuation rates due to AEs during trials of the FDC of bisoprolol and amlodipine; typically, fewer than 1% of participants discontinued treatmentCitation15,Citation16 with no reports of abnormal laboratory valuesCitation16,Citation20, supporting the safety and tolerability of FDC bisoprolol and amlodipine.
This study assessing the use of FDC bisoprolol and amlodipine in daily practice provides further evidence endorsing its good tolerability and high patient adherence to the therapy, which may improve BP control and reduce the risk of cardiovascular events compared with the free-dose combination.
Improved BP control with FDC bisoprolol and amlodipine following monotherapy failure in a phase 3 clinical trial
A Phase 3 clinical trial assessed the second-line effectiveness of FDC bisoprolol and amlodipine in patients who failed bisoprolol or amlodipine monotherapyCitation20.
This 18-week, multicenter, randomized, comparative Phase 3 study (NCT01977794) of 200 patients with BP inadequately controlled by bisoprolol or amlodipine monotherapy (5 mg once daily) was conducted at 10 clinical trial sites in Guatemala. Patients began treatment with FDC bisoprolol and amlodipine 5/5 mg once daily and were grouped according to their previous failed bisoprolol or amlodipine monotherapyCitation20. Patients with controlled BP at week 6 continued the 5/5 mg FDC dose (83.2%); those with uncontrolled BP were randomized to a higher FDC dose (either 5/10 mg or 10/5 mg). At week 12, patients with controlled BP continued their current dose; patients with uncontrolled BP on 5/10 mg or 10 m/5 mg received up-titration to 10/10 mg. The primary endpoint was the change in SBP at week 18 versus baseline (corresponding to SBP under monotherapy).
The efficacy of FDC bisoprolol and amlodipine in reducing BP was demonstrated irrespective of bisoprolol or amlodipine monotherapy failure. Mean ± SD SBP decreased from 151.1 ± 10.04 mmHg at study baseline to 125.8 ± 11.91 mmHg at 18 weeks; mean reduction in SBP was −25.3 mmHg (p < .001). Similar results were observed for DBP; mean ± SD DBP decreased from 91.2 ± 7.95 mmHg at study baseline to 77.7 ± 7.35 mmHg at 18 weeks. Mean reduction in DBP was −13.5 ± 12.25 mmHg (p < .001)Citation20.
Increased heart rate is an independent risk factor for cardiovascular eventsCitation25. In this study, significant mean heart rate reductions from baseline to week 4 were observed for both groups (p < .001): −6.6 ± 9.67 bpm reduction and −11.5 ± 8.65 bpm reduction for the previous bisoprolol and amlodipine monotherapy failure groups, respectivelyCitation20. This is also supported by previous studies in which heart rate was slowed significantly versus baseline by an FDC of bisoprolol and amlodipineCitation14–16.
Among patients who received the lowest dose of FDC bisoprolol and amlodipine, this study observed that BP was well controlled within a short period of time (6 weeks) in most patients (83.2%), without the need for dose up-titrationCitation20. The benefit of a successful response to a low-dose therapy means that dose escalation can be avoided, leading to fewer AEs and potentially lowering the risk of treatment discontinuationCitation20,Citation30.
The rate of AEs related to study therapy was relatively high (52.5%)Citation20, even compared with the prevalence in patients treated with a single antihypertensive drug (5.2%) or with two drugs (7.5%)Citation23; notably, however, most related AEs were mild-to-moderate (52.5%) in severity and the discontinuation rate was low (3%). This is in line with the high tolerability of FDC bisoprolol and amlodipine reported in previous studiesCitation14–16. Moreover, bradycardia and peripheral edema are known AEs of bisoprolol or amlodipine therapyCitation20; however, only six patients (3%) experienced low heart rate (bradycardia of <50 bpm) and 8.5% experienced peripheral edema in this studyCitation20.
The outcomes of this study add to existing evidence demonstrating the efficacy and tolerability of FDC in second line. FDC bisoprolol and amlodipine was associated with significant BP improvement, affirming its suitability to manage essential hypertension, and allow patients who have previously failed to achieve BP control with either monotherapy to achieve their therapeutic goals.
Improved BP control with bisoprolol and amlodipine versus a monotherapy of amlodipine in a phase 3 multicenter, randomized, double-blind, clinical trial (AMCOR)
This was a Phase 3 multicenter, randomized, double-blind, clinical trial. The study included a total of 367 patients with uncontrolled BP following treatment with 5 mg amlodipine who were randomly assigned to 5/5 mg of bisoprolol and amlodipine (n = 181) or placebo and 5 mg amlodipine (n = 186) for 8 weeks (EudraCT Number: 2019-000751-13Citation21). This study aimed to demonstrate the superiority of bisoprolol and amlodipine combination over amlodipine monotherapy; the primary endpoint was the difference in SBP change from baseline to Week 8 between the two treatment groups. Secondary endpoints included the differences in SBP after 4 weeks of treatment, DBP and percentage of patients with BP control after 4 and 8 weeks of treatment, and frequency and severity of AEs in each treatment group.
After both 4 and 8 weeks of treatment, there was a significant difference in the decrease of SBP and DBP between the bisoprolol-treated (combination therapy) and the placebo group (amlodipine monotherapy). Differences between the two treatment groups were −7.2 ± 12.74 mmHg and −6.10 ± 12.91 mmHg for SBP after 4 and 8 weeks, −3.95 ± 8.85 mmHg and −3.84 ± 9.46 mmHg for DBP, all differences being in favor of the combination group and highly statistically significant (p < .0001). These results are in line with previous studies demonstrating the efficacy of FDC bisoprolol and amlodipine versus monotherapy. A study observed that the FDC bisoprolol and amlodipine significantly decreased SBP and DBP values after 2 weeks of treatment in patients with essential hypertension who had failed to respond to either amlodipine or bisoprolol monotherapyCitation17.
Patients treated with bisoprolol and amlodipine also had a lower heart rate than the placebo and amlodipine group, and the difference after 4 and 8 weeks being −7.23 ± 9.84 (p < .0001) and −6.25 ± 9.26 (p < .0001), respectively. This is in line with previous studies in which heart rate was slowed significantly versus baseline by an FDC bisoprolol and amlodipineCitation14–16.
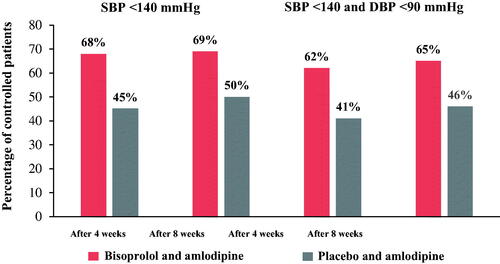
After 4 and 8 weeks respectively, SBP <140 mmHg was achieved in 68% and 69% of patients treated with bisoprolol and amlodipine versus 45% and 50% in those receiving placebo and amlodipine (). After 4 weeks of treatment with bisoprolol and amlodipine, 62% of patients achieved both target SBP and DBP compared with 41% in the placebo and amlodipine group (p = .0002). Moreover, after 8 weeks of treatment with bisoprolol and amlodipine versus placebo and amlodipine, 65% and 46% patients were SBP-and-DBP-controlled, respectively (p = .004) ().
Figure 1. Percentage of SBP and DBP controlled patients in both the bisoprolol and amlodipine versus placebo and amlodipine groups.
No deaths or serious AEs (defined as AE grade 4) were reported in the trial. AEs of mild or moderate severity occurred in 22 patients in the placebo group, but without the need for treatment discontinuation during the study period. In the bisoprolol and amlodipine-treated group, 23 patients experienced an AE of mild or moderate severity. AEs reported in previous studies with FDC bisoprolol and amlodipine, and also with bisoprolol or amlodipine monotherapy, were generally experienced by <10% of patientsCitation14,Citation15; in this study, a slightly higher percentage of patients (∼13%) experienced an AE with the combination of bisoprolol and amlodipine therapy. However, it should be noted that these events were of mild or moderate severity and treatment discontinuation was low (3.9%); treatment was stopped in a total of 7 patients due to suspected bradycardia (4 patients), leg paresthesia (1 patient), weakness and fatigue (1 patient) and transient elevation of ALT activity (1 patient). These results reflect the low treatment discontinuation rates observed in other studies (typically, <1% of patientsCitation15,Citation16; offering further evidence of the tolerability of the combination of FDC bisoprolol and amlodipine in patients with hypertension.
This study demonstrated that the combination of bisoprolol and amlodipine was superior to amlodipine monotherapy in lowering BP and heart rate. The significant BP reductions and positive AE profile shown in this study further add to the evidence pool on the efficacy and safety of a combination of bisoprolol and amlodipine, supporting its use to manage hypertension.
Meaningful reductions in diastolic BP with bisoprolol and amlodipine in an indirect treatment comparison (COMBIPRESS)
This analysis was a population-adjusted indirect comparison, more specifically, an anchored, simulated treatment comparison, comparing changes in SBP and DBP from baseline to 8 weeks between amlodipine and bisoprolol (5/5 mg) and dose
up-titration of amlodipine to 10 mg, in patients with uncontrolled hypertension receiving amlodipine monotherapy (5 mg)Citation22. This was a two-step process; first, a systematic literature review of clinical trials published between 2001 and 30 April 2021 was performed in May 2021 and run on the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), ClinicalTrials.gov registers, MEDLINE and EMBASE. The database searches aimed to identify RCTs that evaluated the difference in BP decrease at 8 weeks following up-titration of amlodipine from 5 mg to 10 mg, compared with maintaining an amlodipine dose of 5 mg. The second step was a population-adjusted indirect comparison using individual patient data from an investigator-sponsored study (AMCOR; the same study as section 3, above) of bisoprolol and amlodipine versus each identified RCT.
From the systematic review search and screening process, only one study was selected (NCT00558428)Citation31. This was an 8-week, randomized, double-blind, parallel-group study investigating single-pill combinations of telmisartan 40 mg or 80 mg/amlodipine 5 mg versus monotherapy with amlodipine 5 mg or 10 mg in patients with uncontrolled hypertension (N = 1097)Citation31. Patients were recruited from 129 centers in Belgium, Canada, Denmark, Finland, France, Korea, Netherlands, Norway, Philippines, South Africa, Sweden and Taiwan between October 2007 and October 2008Citation31.
The observed mean decrease of SBP from baseline to 8 weeks was −6.18 mmHg (standard error [SE] 0.73) in the amlodipine 5 mg arm (N = 255), and −11.11 mmHg (SE, 0.72) in the amlodipine 10 mg arm (N = 261). The mean decrease of DBP was −5.71 mmHg (SE, 0.49) and −7.95 mmHg (SE, 0.49) in the amlodipine 5 and 10 mg arms, respectively.
Two regression models were derived for the individual patient data from AMCOR to match to the patient population from Neldam et al.Citation31; one model to predict systolic BP at week 8, and one to predict diastolic BP at week 8Citation22. These two models were then used to predict BP in a population comparable to the Neldam et al. trialCitation31.
The simulated treatment comparison identified reductions in SBP and DBP from baseline to 8 weeks with bisoprolol and amlodipine versus amlodipine 10 mg monotherapy (−1.6 mmHg [SE, 1.9; 95% CI, −5.3 to 2.2] and −3.3 mmHg [SE, 1.3; 95% CI, −5.9 to −0.7] for SBP and DBP), respectively. Although no meaningful difference was observed for SBP in the simulated treatment comparison, an important decrease in DBP was observed with the combination of bisoprolol and amlodipine versus the up-titration to amlodipine 10 mgCitation22. This confirms previous studies showing that the use of two-dose therapies with distinct mechanisms of action, such as bisoprolol and amlodipine, can be more effective than high-dose monotherapy for controlling BPCitation23,Citation24. Additionally, clinically meaningful BP reduction can be achieved with a low-dose combination of bisoprolol and amlodipine, thus avoiding the need for a dose increase of amlodipine as monotherapy, which has been associated with an increased incidence of AEs in comparison to the lower 5 mg doseCitation31.
This study demonstrated a similar benefit regarding SBP and a meaningful decrease in DBP under the low-dose combination of bisoprolol and amlodipine versus amlodipine up-titration to 10 mgCitation22, adding to previous evidence on the effectiveness of the combination of bisoprolol and amlodipine to manage hypertension.
Discussion
Building on previous studies, this review collates additional evidence supporting the efficacy and safety of FDC bisoprolol and amlodipine to manage hypertension. The additional evidence was generated using diverse methods: a prospective observational study; two RCTs; and an indirect comparison.
FDC bisoprolol and amlodipine was developed to simplify the treatment regimen of patients with hypertension. Prospective data from a large cohort showed a high level of patient adherence with FDC bisoprolol and amlodipine in daily practice, accompanied by striking improvements in BP control versus baseline and an expected reduction in cardiovascular risk factors. The tolerability of the therapy further supports its use to manage hypertension. Results from the Phase 3 clinical trials demonstrated the favorable efficacy and tolerability of the therapy in second line, supporting its use to manage hypertension following monotherapy failure. Lastly, in an indirect treatment comparison, the combination of bisoprolol and amlodipine treatment resulted in a similar influence on SBP and a meaningful reduction in diastolic BP compared with up-titration of amlodipine to 10 mg, further supporting the efficacy of the therapy.
Limitations of this literature review include the small number of studies published since January 2016 and therefore assessed in this review. Only two of the included studies were randomized, controlled trialsCitation20, which contained limited numbers of patients, questioning the generalizability of their results. Only the AMCORCitation21 and non-interventional study by Hostalek et al. 2016Citation8 were assessed as high-quality studies using the JadadCitation18 and Newcastle–Ottawa scalesCitation19, respectively.
Due to keen interest in FDCs in hypertension, additional clinical data on FDC bisoprolol and amlodipine are expected in the coming years. The recent multicenter, double-blind, randomized Phase 3 QUARTET study of a FDC of ultra-low-dose irbesartan 37.5 mg, amlodipine 1.25 mg, indapamide 0.625 mg, and bisoprolol 2.5 mg demonstrated that a single pill containing four different antihypertensive drug classes is a more effective hypertension management strategy than starting standard monotherapy, providing further evidence of the utility of FDCs containing amlodipine and bisoprololCitation32.
Conclusion
The studies in this updated review of FDC bisoprolol and amlodipine add to the growing body of evidence for the use of this FDC treatment to help patients manage their hypertension and achieve their therapeutic BP goals.
Transparency
Declaration of funding
Medical writing support was funded by Merck Healthcare KGaA, Darmstadt, Germany.
Declaration of financial/other relationships
Ulrike Hostalek: Employee of Merck Healthcare KGaA. Zbigniew Gaciong: Payments for lectures and consultancy from Merck Healthcare KGaA. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Both authors contributed to the conception, drafting and reviewing of the manuscript, and approved the final version. Both authors agree to be accountable for all aspects of the work.
Acknowledgements
The authors thank Priyanka Bose, PhD, of Scientific Pathways Ltd, a Nucleus Global company, for providing medical writing support, which was funded by Merck Healthcare KGaA, Darmstadt, Germany in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Data availability statement
The data on which this review is based are available from the corresponding author upon reasonable request.
References
- WHO. Hypertension: key facts. WHO. 2021. [cited August 2021]; Available from: https://www.who.int/news-room/fact-sheets/detail/hypertension.
- Williams B, Mancia G, Spiering W, et al. ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104.
- Gradman AH, Basile JN, Carter BL, et al. Combination therapy in hypertension. J Clin Hypertens (Greenwich). 2011;13(3):146–154.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA. 2014;311(5):507–520.
- Unger T, Borghi C, Charchar F, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357.
- Fung V, Huang J, Brand R, et al. Hypertension treatment in a medicare population: adherence and systolic blood pressure control. Clin Ther. 2007;29(5):972–984.
- Gottwald‐Hostalek U, Sun N, Barho C, et al. Management of hypertension with a fixed-dose (single-pill) combination of bisoprolol and amlodipine. Clin Pharmacol Drug Dev. 2017;6(1):9–18.
- Hostalek U. Treatment of hypertension with a fixed-dose combination of bisoprolol and amlodipine in daily practice: results of a multinational non-investigational study. Cardiovasc Disord Med. 2016;1(3):1–5.
- Düsing R. Optimizing blood pressure control through the use of fixed combinations. Vasc Health Risk Manag. 2010;6:321–325.
- Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713–719.
- Parati G, Kjeldsen SE, Coca A, et al. Adherence to single-pill versus free-equivalent combination therapy in hypertension: a systematic review and meta-analysis. Hypertension. 2021;77(2):692–705.
- Gradman AH, Basile JN, Carter BL, et al. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4(2):90–98.
- Merck. Summary of Product Characteristics Concor AM. KGaA, 2020. [cited August 2021]; Available from: https://hcp.merckgroup.com/en/cmc/cardiovascular/products/concor-am/indications-and-dosing.html.
- Chesnikova AC, Safronenko VS, Kolomakskaya OK. [Evaluating the effectiveness of a fixed combination of amlodipine and bisoprolol in ambulatory patients with arterial hypertension and ischemic heart disease]. Kardiologiia. 2014;2014(9):17–23.
- Rana R, Patil A. Efficacy and safety of bisoprolol plus amlodipine fixed dose combination in essential hypertension. Indian Pract. 2008;61(4):225–234.
- Mehta S, Shah M, Shah A. Efficacy and tolerability of a fixed dose combination of amlodipine and bisoprolol in essential hypertension. Indian Pract. 2005;58(12):751–759.
- Shirure PA, Tadvi NA, Bajait CS, et al. Comparative effect of fixed dose combination of amlodipine + bisoprolol versus amlodipine and bisoprolol alone on blood pressure in stage-2 essential hypertensive patients. Int J Med Res Health Sci. 2012;1(1):13–19.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
- Khan S, Memon B, Memon MA. Meta-analysis: a critical appraisal of the methodology, benefits and drawbacks. Br J Hosp Med (Lond). 2019;80(11):636–641.
- Gottwald-Hostalek U, Li L, Montenegro P. Bisoprolol/amlodipine combination therapy improves blood pressure control in patients with essential hypertension following monotherapy failure. Curr Med Res Opin. 2016;32(10):1735–1743.
- EMA. EU clincal trials register: clinical trials for 2019-000751-13. [cited April 2022]; Available from: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2019-000751-13
- Foch C, Feifel J, Gottwald-Hostalek U. An anchored simulated treatment comparison of uptitration of amlodipine compared with a low-dose combination treatment with amlodipine 5 mg/bisoprolol 5 mg for patients with hypertension suboptimally controlled by amlodipine 5 mg monotherapy. Curr Med Res Opin. 2022;38(4):587–593.
- Law MR, Wald NJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427.
- Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122(3):290–300.
- Lonn EM, Rambihar S, Gao P, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol. 2014;103(2):149–159.
- Lee H, Yano Y, Cho SMJ, et al. Adherence to antihypertensive medication and incident cardiovascular events in young adults with hypertension. Hypertension. 2021;77(4):1341–1349.
- Channaraya V, Marya RK, Somasundaram M, et al. Efficacy and tolerability of a β-1 selective β blocker, bisoprolol, as a first-line antihypertensive in indian patients diagnosed with essential hypertension (BRIGHT): an open-label, multicentric observational study. BMJ Open. 2012;2(3):e000683.
- Milpharm. Summary of product characteristics amlodipine. 2020. [cited October 2021]; Available from: https://www.medicines.org.uk/emc/product/4717/smpc#companyDetails
- Liu J, Li X, Zhang H, et al. S-amlodipine-bisoprolol combination therapy caused elevated transaminases and triglyceride levels in healthy Chinese subjects: a randomized controlled, open-label, multiple-dose pharmacokinetic interaction study. Expert Opin Drug Metab Toxicol. 2019;15(9):687–695.
- Zanchetti A. Contribution of fixed low-dose combinations to initial therapy in hypertension. Eur Heart J Supplements. 1999;1(L):L5–L9.
- Neldam S, Lang M, Jones R. Telmisartan and amlodipine single‐pill combinations vs amlodipine monotherapy for superior blood pressure lowering and improved tolerability in patients with uncontrolled hypertension: results of the TEAMSTA‐5 study. J Clin Hypertens (Greenwich). 2011;13(7):459–466.
- Chow CK, Atkins ER, Hillis GS, et al. Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines versus standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomised, double-blind, active-controlled trial. Lancet. 2021;398(10305):1043–1052.
