Abstract
Objective
To evaluate analgesic efficacy and safety/tolerability of the nonbenzodiazepine antispasmodic pridinol (PRI) in patients with muscle-related pain.
Methods
Systematic review and meta-analysis of randomized placebo-controlled trials (RCTs) according to PRISMA guidelines and Cochrane recommendations. Data sources included Google Scholar, Embase, PubMed, ClinicalTrials.gov, EU Clinical Trials Registry, Chinese Clinical Trial Registry, UMIN Clinical Trials Registry, and product manufacturer archives from inception to 31 January 2022. Eligibility criteria for study selection were randomized, placebo-controlled trials with PRI in adults (≥18 years) with muscle-related pain. Data extraction, synthesis, and analysis carried out by two reviewers independently identified studies, extracted data, and assessed the risk of bias using the Cochrane risk of bias tool. Categorial global response rates (number of patients) based on clinical judgement of study physicians (as primary efficacy endpoint), and response on pain at rest, pain at movement, stiffness, tenderness, and movement restriction (as secondary efficacy endpoints), as well as the number of patients with drug-related adverse events (DRAEs) were meta-analytically evaluated using the Review Manager Software version 5.4.1.
Results
In total, 38 records were identified, but only two placebo-controlled studies (with 342 patients with mild to moderate acute muscle pain [55.3% female, age 50.6 ± 16.6 years], of whom 173 received PRI and 169 placebo, each as monotherapy) proved to be suitable for quantitative and qualitative analysis. Treatment with PRI was (irrespective of its mode of administration as oral tablet or intramuscular injection) associated with a significantly higher global response rate compared to placebo (74.0 vs. 49.7%; OR 2.86, 95%-CI: 1.82–4.51; p < .00001; Cohen´s h: 0.506, NNT: 4.1; Chi2 for heterogeneity 1.41 (p = .24], I2 = 29%), and significantly higher response rates were also found for all secondary efficacy endpoints. The safety of PRI was comparable to that of placebo: DRAEs were only seen in one of the two studies and reported for 13 vs. 10 patients (OR: 0.76 95%-CI: 0.32–18.1; p = .54, NNH: 62.6), and related discontinuations were reported for four vs. one patient (2.3 vs. 0.6%; p = .231).
Conclusions
The results from this meta-analysis as based on two placebo-controlled studies in adult patients with mild to moderate acute muscle pain demonstrate that a 3-week monotherapy with PRI showed a comparable safety profile, but significantly better analgesic effects and improvements of related impairments such as stiffness, tenderness, and movement restrictions compared with placebo – irrespective of its mode of administration.
PLAIN LANGUAGE SUMMARY
Muscle pain is one of the most common pain problems worldwide.
In the majority of cases, muscle pain is temporary, transient, and benign in nature. However, people affected may still experience severe pain and significant pain-related disabilities in daily life activities that may require temporary drug treatment – also in order to be able to undertake the non-drug treatment measures necessary to prevent recurrence.
Current treatment recommendations for muscle pain are largely ´non-specific´ and limited to symptomatic pain-relieving measures (e.g. non-steroidal anti-inflammatories), while muscle relaxants – such as pridinol (PRI), which has been reapproved in Germany in 2017 and first time approved in the United Kingdom, Spain, and Poland in 2020 – are currently not recommended (primarily due to insufficient efficacy data from controlled clinical trials) but nevertheless frequently prescribed.
Due to our systematic literature research of double-blind randomized and placebo-controlled trials, a 3-week monotherapy with PRI vs. placebo proved to be comparably tolerated, but significantly more effective in patients with muscle pain – irrespective of the mode of administration (oral or as intramuscular injection).
These outcomes confirm already available real-world evidence on the beneficial efficacy and tolerability of PRI in daily practice. However, more recent RCTs or numerically larger comparative real-world evidence analyses are needed to evaluate the efficacy of PRI in comparison to currently recommended first-line therapies for patients with muscle pain.
Background
The use of nonbenzodiazepine antispasmodics for the treatment of muscle-related pain is quite popular in industrialized countries of the western world, although published evidence is limited, and advice in evidence-based guidelines are controversial, ranging from positive, open, or even negative recommendationsCitation1–4.
In 2018, over 30 million muscle relaxants were prescribed in the US (∼9.1 per 100,000 residents)Citation5, and in 2020 over 1.3 million prescriptions were made in England (∼2.3 per 100,000)Citation6 and more than 3.6 million (∼4.3 per 100,000) in Germany, whereby the latter prescription frequency corresponds to over 171 million daily defined doses (DDD), around 2.1 per resident and year in GermanyCitation7. In all three countries, muscle relaxants have for some time been among the three most commonly prescribed medicines for the treatment of acute (low) back painCitation5–7 – a health disorder that either has its origin in a malfunction of the muscle-tendon units or is at least associated with increased muscle toneCitation3.
In Europe, the spectrum of available antispasmodics has been significantly narrowed in recent years due to several risk-benefit assessments published by the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) and consecutive extensions of the official product characteristic and the inclusion of new side effects (tizanidine)Citation8, restrictions on approval (tolperisone)Citation9, the temporary suspension (tetrazepam)Citation10, or even withdrawal of marketing authorization (flupirtine)Citation11. This contrasts to, pridinol (PRI), a nonbenzodiazepine antispasmodic acting via cholinergic antagonism of muscarinic acetylcholine receptors (M1 subtype)Citation12,Citation13, which was originally discovered in the late 1950s and brought to market approval in various European and non-European countries in the 1960s. End of 2017, PRI was reauthorized in Germany to treat central and peripheral muscle spasms, torticollis, lumbago, and general muscle pain in adult patients. Subsequently and for the first time PRI has been approved by the UK, Poland, and Spain in 2020.
At present, PRI is one of only two muscle relaxants approved for the treatment of peripheral muscle spasms associated with (low) back pain in Germany and its prescription patterns raise since its re-approval. In 2020, 5.5 million daily defined doses (DDD) were prescribed in Germany only, an increase of 96.4% as compared to 2019Citation7. A development that, given a lack of positive recommendations for PRI or even blanket negative recommendations for all muscle relaxants in current evidence-based national care guidelines, reveals the large discrepancy between the specific patient needs and the available scientific evidence.
In light of this and due to the observation that the number of publicly available reports and publications on the efficacy and tolerability of PRI are limited, the German Pain Association initiated this systematic literature research and meta-analysis project to gain insight into the available evidence for the use of PRI in patients suffering from muscle-related pain.
Study aim
The purpose of this systematic meta-analysis was to evaluate the analgesic efficacy and safety/tolerability of PRI versus placebo in prospective, double-blind randomized, placebo-controlled trials with patients suffering from muscle pain.
Study design
This review used the PRISMA recommendationsCitation14,Citation15 and those of the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0; 2011Citation16, and is based on the results of a literature search according to the guidelines described below.
Materials and methods
Eligibility criteria
In and exclusion criteria for the literature research were defined according to the modified recommendations of the PICO framework and aimed to identify studies that were as high quality and meaningful as possibleCitation17:
Inclusion criteria
Population (P): adult patients (≥18 years) with muscle pain
Intervention (I): PRI (regardless of the form of application: po and/or im)
Control (C): matching placebo
Outcome (O): (a) primary efficacy endpoint: Global assessment of response to treatment (i.e. improvement vs. no improvement or worsening) as judged by the study physicians; (b) secondary efficacy endpoints: response to treatment with respect to distinct treatment targets (e.g. pain intensity, pain-related movement restrictions, stiffness, etc.), different types of muscle pain, and alternative modes of application; (c) safety endpoint: drug-related adverse events (DRAEs).
Timing (T): (a) at least 7 d treatment duration; (b) all available studies up to initiation of the study detection/identification process.
Study type (S): prospective randomized, double-blind, and placebo-controlled, parallel groups or crossover studies.
Exclusion criteria (see Supplementary material)
Studies with less than 25 patients per treatment cohort.
Studies with high risk of bias and/or an overall insufficient (low) methodological quality.
Studies and/or reports not published/available in English, French, Spanish, Chinese, Japanese, or German.
Literature search
The starting point for the literature search was various biomedical online databases (Google Scholar, Embase, and PubMed) as well as four international trial registries (“clinical-trials.gov”, “EU clinical trials registry,” “Chinese clinical trial registry” and “UMIN clinical trials registry”), which were searched from inception to 31 January 2022 using the keywords “pridinol” and “pridinol mesylate” (as the non-proprietary names of the target medication) as well as “lyseen”, “loxeen”, “myoson”, and “konlax” (different brand names) for references to clinical trials. Research results were limited by restricting them to publications and reports according to the predefined inclusion and exclusion criteria. Finally, electronic database search was complemented by a web-based keyword search performed via three alternative web browsers (Google, Bing, and Safari) according to the above specifications. In addition, a systematic review of the references of all identified studies and publicly available review articles, meta-analyses, and guidelines on the use of muscle relaxants in pain medicine was conducted, as well as a request for suitable studies send to the pharmaceutical company responsible for the production and distribution of PRI in Germany, literature references and/or biometrical reports.
All references identified or received during this screening process were recorded in Endnote X9.1 (Clarivate Analytik) and checked both electronically and manually for duplicate entries.
Selection and data collection process
Titles, abstracts (and if necessary, also full texts) of the references identified were independently screened by two authors with respect to their eligibility according to the predefined selection criteria. Any disagreements were solved by consensus on the basis of the full-text article and with the inclusion of the third author if necessary.
From those articles classified as eligible, the following parameters were independently extracted by two authors using a standardizes data collection form: application mode (im or po), treatment duration, pain syndrome, and relevant outcome measures (as raw data extracts either mean and SD for continuous variables or frequency of events/study participants for categorial/dichotomous outcomes). Missing data were not imputed and any disagreements were solved by consensus on the basis of the full-text article and with the inclusion of the third author if necessary.
Assessment of methodological study quality and risk of bias
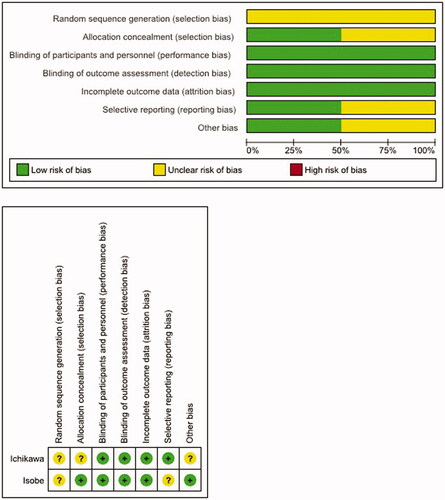
The assessment of the methodological quality of the post-screening studies was done with reference to the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) and according to the recommendations of the Cochrane Handbook version 5.1.0, Chapter 8.5.Citation16,Citation18,Citation19 and based on the evaluation of the full-length records. In total, seven different areas were assessed: (a) random sequence generation, (b) allocation concealment, (c) participant and investigator blinding, (d) blinding of outcome assessment, (e) incomplete outcome data, (f) selective reporting, and (g) other types of bias. For each domain, the risk of bias resulting from this assessment was categorized into three levels (high risk, low risk, or unclear risk) and studies with high risk of bias in at least one of the aforementioned domains and those without any low-risk categorizations were excluded from the meta-analysis.
The assessment of study quality and the final determination of which studies were eligible for this meta-analysis was carried out in a blinded assessment by all authors independently and majority decision in case of any disagreements.
Quality of evidence across pooled studies was assessed by two authors independently on the basis of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approachCitation20,Citation21. Disagreements were cleared by discussion and by consultation of the third author and majority decision if necessary.
Data extraction and statistical analysis
The primary efficacy assessment was dichotomized on the basis of the documented response to treatment with PRI vs. placebo at the end of the respective treatment period for pain or pain-related impairments compared to baseline and with reference to the corresponding clinical global impression (CGI) of the respective treating physician: (a) improvement (either excellent, good, or sufficient) vs. (b) no improvement (i.e. no change or worsening). In the event that efficacy data were available for different application forms of PRI (oral vs. injection), appropriate secondary comparative analyses were planned. The assessment of safety and tolerability was carried out meta-analytically by a quantitative analysis of the DRAEs documented under PRI or placebo as well as by a supplementary qualitative assessment of the DRAE spectrum.
Raw data on the frequency of events (DRAEs) or participants with response were extracted from the full-length reports/publications of eligible trials independently by two authors independently using a standardized data collection form. Any differences were resolved by backward tracing to the original data and consensus or by consultation of a third author, if necessary.
The Cochrane–Mantel–Haenszel test was used for the meta-analytic evaluation of the responder frequencies of effect (improvement) and safety/tolerability (adverse event frequency). Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were used and p scores less than .05 and ORs with a lower margin of the 95% CI above 1 were considered as statistically significant difference. Heterogeneity of effect sizes between eligible studies was assessed using the Cochrane Q-statistic (p scores <.05 were considered as an indicator for a significant heterogeneity) and the I2-statistic (0–30% insignificant, 31–70%: moderate, 1–100%: significant/considerable)Citation16,Citation22,Citation23. If no evidence of significant heterogeneity was found between studies, a fixed-effects model was used for the meta-analysis, otherwise a random-effects model was used.
The results of these meta-analytic evaluations were summarized separately for each evaluated parameter in appropriate tabular and graphical form (Forrest plots) according to Cochrane rulesCitation16,Citation18,Citation19. Publication bias was assessed by visual inspection of the funnel plots for all evaluated endpoints, whereby an asymmetric funnel plot regarding the evaluated efficacy and safety parameters was assessed as an indication of possible publication biasCitation24. All of the above statistical analyses were performed using Review Manager version 5.4.1 software (RevMan version 5.4.1, Cochrane Collaboration, Oxford, UK).
In addition, the clinical significance of the meta-analytically evaluated event frequencies was assessed using Cohen’s h (0.2–0.49: meaningful; 0.5–0.79: clinically meaningful, and ≥0.8: practically significant)Citation25 and the calculation of the associated number-needed to treat (NNT)Citation26.
Patient involvement
In the context of this study, only already available data from studies and reports were analyzed in depersonalized form (i.e. without concrete reference to previously involved patients and/or study centers).
Results
Literature search, quality assessment, and risk of bias evaluation
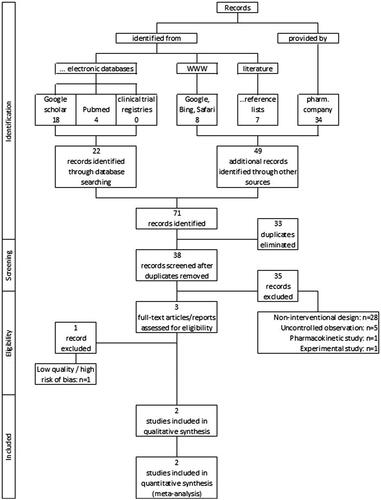
As a result of the literature search described above (for search strings see Box 1), 23 records were identified in electronical databases, and a further 49 through other sources (of which 34 were provided by the pharmaceutical company we have contacted; see ). Of the total of 71 references,33 duplicates were identified and removed, and the remaining 38 were screened with regard to their usability in the context of the present meta-analysis (see ). As a result of this evaluation process, a total of 35 studies had to be excluded: 28 because of a non-interventional study design, five because of an uncontrolled single-cohort study design and one each because of a purely pharmacokinetic study concept or an experimental study approach in healthy subjects.
Box 1 Search strings/syntax for electronic database/literature research.
Table 1. Summary overview of the studies found in the literature search and their fulfillment of the specified inclusion and exclusion criteria.
As a consequence of the methodological study evaluation and the risk of bias analysis, one of the remaining three studies had to be excluded due to its insufficient study quality (high risks of bias in all six domains evaluated), so that ultimately only two studies could be included in the qualitative and quantitative analysis (see summary on the evaluation of bias risk and study quality in ). Both studies were only available as biometrical reports including the tabular analyses and were provided by the pharmaceutical company upon our requestCitation27,Citation28. Quality of evidence for both studies included in the final meta-analysis was rated as moderate for all comparison.
Figure 2. Evaluation of bias risk and quality of clinical studies included in the meta-analysis. Risk of bias graph (upper panel): review authors´ judgments about each risk of bias item presented as percentage across all included studies. Risk of bias summary (lower panel) based on review authors´ judgment about each risk of bias item for each study included.
Study characteristics
Main characteristics of the two studies which qualified for this meta-analysis can be found in . Both studies evaluated the effects of PRI (n = 37/136) – either given as intramuscular injection (3 mg every other day) or as tablet (3 mg TID) – vs. placebo (n = 35/134) in adult patients (55.3% female; age: 50.6 ± 16.6, range 19–86 years) with mild to moderate acute muscle pain (spondylosis deformans n = 162, humeroscapular periarthritis n = 87; or cervico-omo-brachial syndrome n = 93) as part of prospective, double-blind randomized, and placebo-controlled parallel group multicenter trials (n = 7/10 study sites) and were performed in 1973 in Japan. In both studies, medications were given as monotherapy and compared to matching placebo with a treatment effect evaluation period of three weeks each.
Table 2. Main characteristics of studies included in this meta-analysis.
Qualitative analysis
Drop-out rates were comparable in both studies (n = 29/8, corresponding to 9.7/10%). For the remaining patients (n = 270/72), both reports described a favorable response of PRI vs. placebo (see ). Irrespective of the underlying type of muscle pain, percentages of patients who presented with a global improvement vs. baseline were significantly larger for those who received PRI compared to those with placebo. DRAEs were only reported by patients who received oral treatment but with only minor between cohort differences (n = 13/136 with PRI (9.6%) vs. n = 10/134 with placebo [7.5%]; p = .537).
Quantitative analysis
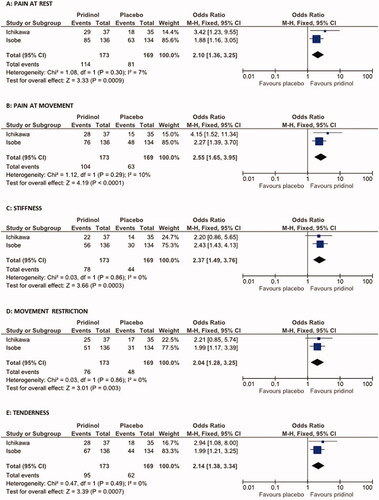
Both studies shared several similarities (especially with respect to treatment duration and reported efficacy data) which supported our meta-analytical evaluation approach. Beyond information on the global response of patients to the study treatments evaluated (the primary efficacy endpoint of our analysis) and the number/spectrum of DRAEs (our safety endpoint), both reports contained detailed information on the effects of the study medications on five further outcomes – post-hoc defined and evaluated as additional secondary efficacy endpoints: a) pain intensity at rest, b) pain intensity at movement, c) stiffness, d) movement restrictions, and e) tenderness.
Primary efficacy endpoint
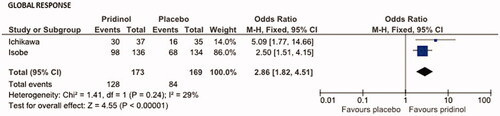
Based on the number of patients for whom study physicians reported a global improvement in response to PRI (n = 128/173, 74.0%) vs. placebo (n = 84/169, 49.7%), the overall effect (OR, Mantel–Haenszel test, fixed model) has been calculated to be 2.86 (95% CI: 1.82–4.51; p < .00001; ). Corresponding Cohen´s h was 0.506 (indicating not only a significant, but even a clinically meaningful effect in favor of PRI) and the NNT has been calculated to be 4.1.
Secondary efficacy endpoints
In addition to the global response also all secondary efficacy endpoints showed a better response for PRI vs. placebo (see ). Among all parameters, pain at movement was the one with the highest effects (OR: 2.55, 95%CI: 1.65–3.95; p < .00001, Cohen’s h: 0.461, NNT: 4.4), followed by stiffness (OR: 2.37, 95%CI: 1.49–3.76; p = .0003; Cohen’s h: 0.401; NNT: 5.3). Response rates reported for tenderness (OR: 2.14; p = .0007; Cohen´s H: 0.368; NNT: 5.5) and pain at rest (OR: 2.10; p = .0009; Cohen´s h: 0.365; NNT: 5.6) were comparable and lowest response rates were those seen for movement restrictions (OR: 2.04; p = .003; Cohen’s h: 0.325; NNT: 6.4).
Figure 4. Effects of pridinol compared with placebo on secondary efficacy endpoints. Meta-analytical assessment of the number of patients reporting an overall improvement at end of the treatment vs. baseline with respect to pain at rest (A), pain at movement (B), stiffness (C), movement restrictions (D), and tenderness (E).
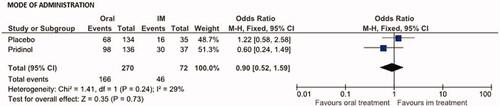
A comparison of the global effect rates in response to PRI vs. placebo given either as oral tablet (n = 98/136 vs. 68/134; 72.1 vs. 50.7%) or as im-injection (n = 30/37 vs. 16/35; 81.1 vs. 45.7%) showed only minor and statistically insignificant differences (). OR for PRI was 0.60 (95% CI: 0.24–1.49; p = .267; NNT: 11.1) in favor of the oral treatment, for placebo 1.22 (95% CI: 0.58–2.58; p = .596; NNT: 19.9) in favor of the im-injection, and for the combined assessment 0.90 (95% CI: 0.52–1.59; p = .73; NNT: 41.5).
Figure 5. Effects of the mode of administration (oral vs. im-injection) on the primary efficacy endpoint (global response) for pridinol and placebo. Meta-analytical assessment of the number of patients reporting an overall improvement at end of the treatment vs. baseline.
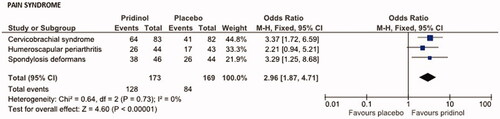
Response to PRI (and placebo) differed among the pain syndromes evaluated (see ). Highest global response rates were seen for PRI /placebo in patients suffering from spondylosis deformans (82.6/59.1%), followed by those with cervicobrachial syndrome (77.1/50.0%), and humeroscapular periarthritis (59.1/39.5%). Significant treatment effects in favor of PRI vs. placebo were seen in patients with cervicobrachial syndrome (OR: 3.37, 95% CI: 1.72–6.59; p < .001, NNT: 3.7), and spondylosis deformans (OR: 3.29, p = .014, NNT: 4.3), whereas effects reported for patients with humeroscapular periarthritis were insignificant (OR: 2.21, 95% CI: 0.94–5.21; p = .068, NNT: 5.1).
Safety/tolerability endpoint
Global safety analysis on the number of patients who reported DRAEs in response to their study medication showed no differences between PRI and placebo (see ). Overall, 13 patients treated with PRI reported at least one DRAE vs. 10 treated with placebo. Corresponding OR calculation was 0.76 (95% CI: 0.32–1.81; p = .54; NNH: 62.6).
Figure 7. Effects of pridinol compared with placebo on the safety endpoint: drug-related adverse events (DRAEs). Meta-analytical assessment of the number of DRAEs until end of treatment.
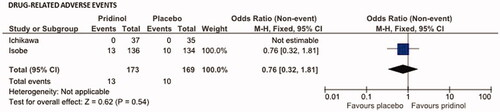
DRAEs reported for PRI vs. placebo affected predominantly the GI-tract (n = 9 for both) and the central nervous system (n = 5 vs. n = 1). Premature discontinuation of study treatment as a consequence of these DRAEs was reported for four patients with PRI (2.3%) and for one patient with placebo (0.6%; p = .231).
Discussion
This is the first systematic review and meta-analysis evaluating the nonbenzodiazepine antispasmodic PRI and its effects in adult patients with acute muscle pain in comparison to placebo. Based on two clinical trials with 342 evaluable patients who received study medications either as oral tablets (n = 270) or im-injection (n = 72) for 21 d, PRI showed a comparable safety, but significantly better efficacy than placebo – especially for pain during movement and muscular stiffness – independent of its mode of administration.
These data are in contrast to the results of earlier studies on the effects of muscle, whose general statements are often transferred to individual active substances in particular – regardless of whether these were subject of the original studies or not. A recently published comprehensive meta-analysis found only minor/small and clinically irrelevant effects of muscle relaxants given for up to two weeks to patients with acute low back pain, but an increased risk for minor adverse eventsCitation27. However, in this review, only data on few non-benzodiazepine antispasmodics (carisoprodol, cyclobenzaprine, metaxalone, methocarbamol, orphenadrine, tizanidine, and thiocolchicoside) were reported and no data on PRI evaluated – simple due to the fact that the search strategy applied to identify appropriate trials in this review failed to detect relevant data.
To avoid this problem for our analysis, we did – in contrast to previous reviews and meta-analyses on muscle relaxants – not only review usual electronic databases and clinical trial registries for relevant studies but additionally asked the responsible pharmaceutical companies to review their archives and to provide information on unpublished trials or those studies published, but not referenced in the electronic databases and registries which are usually searched for the purpose of a meta-analysis. This additional search option provided us with valuable data for our planned meta-analysis, which we were also able to use due to the formats supplied (full-text reports incl. tabular evaluations) – after rigorous assessment of both their methodological quality and their bias risk.
Our meta-analysis supports results from a recent real-world evidence analysis of data from the German Pain e-Registry on the use of oral PRI in patients with acute muscle pain given add-on for up to nine weeksCitation29. In this non-interventional study, 58.8% of patients were classified as global responders to PRI (i.e. they reported a significant and clinically relevant improvement of pain intensity and pain-related disability in daily life, without worsening of their overall wellbeing and without any DRAEs) and only 6.4% as non-responders – either due to lack of efficacy (0.2%) or tolerability problems/DRAEs (6.2%). Furthermore, 4 of 10 patients (41.7%) in this real-world study recorded the termination of at least one, 3 of 10 (30.8%) those of all pain medications beyond PRI. Spectrum of DRAEs reported with routine use of PRI were slightly different from those reported in the controlled trials evaluated during this review and affected predominantly the nervous system (3.2%), the GI-tract (1.7%), and the cardiovascular system (1.2%). However, DRAE-related discontinuations in patients who received PRI in daily practice were less than those found in our review (0.8 vs. 2.3%).
Minor efficacy differences seen between patients with different myofascial syndromes and the fact that PRI failed to reach the primary endpoint in patients with humeroscapular periarthritis (p = .068) while it reached this endpoint in patients with cervicobrachial syndrome (p < .001) and spondylosis deformans (p = .014) do not raise any concerns about the efficacy of this antispasmodic in general, but more on the underlying mechanisms in these pain conditions. While an increase in muscle tone is the predominant pathomechanism in patients suffering from cervicobrachial syndrome and spondylosis deformans, it is “only” one of several factors in patients with periathritis humeroscapularis, which is primarily a tissue inflammation of the shoulder joints and the surrounding soft tissues like muscles, tendons, etcCitation30. However, our meta-analysis on the primary endpoint shows that the between group differences between all three myofascial pain syndromes are insignificant (heterogeneity p = .73), indicating a clinically relevant effect in those patients with periarthritis as well, despite its complex pathomechanisms.
Interestingly, we were not able to find any practically relevant differences for PRI given either as an IM or as an oral formulation. Based on the available pharmacological data, there is clearly no medical need for the IM injection except for patients unable to swallow the medication. However even in those, the oral route might be the better alternative if the gastrointestinal absorption of the drug is possible, but patients cannot take the oral formulation. Due to the fact that the po formulation contains PRI as PRI mesylate without specific release modifications, tablets can be easily resolved with water and given over a nasogastric tube without any changes of the pharmacokinetics. Taken this into account the IM preparation remains as an alternative for patients with structural GI-problems or severe disturbances of GI-absorption.
Strengths and limitations of this review
This review followed the recommendations of PRISMA and the Cochrane Collaboration and reviewed the reports of prospective double-blind, randomized, placebo-controlled trials only, as they provide the best scientific evidence on the efficacy and safety of PRI in muscle pain. We did not only focus our literature search on electronic databases and clinical trial registries, but also on data from so far unpublished trials (if they were available as biometrical reports with tabular analyses) or studies published without reference in current databases. Methodological quality and risk of bias were assessed with the Cochrane risk of bias tool, and efficacy as well as tolerability analyses performed and reported with the recommended strategies of the Cochrane Collaboration and supplemental analyses on their clinical significance. In addition, studies included in the final review evaluated patients only, who received PRI (or placebo) as monotherapy, minimizing the risk of misinterpretation or effect overestimation.
Major limitations of our review result from the small number of double-blind randomized placebo-controlled trials with PRI, the quite considerable study age (49 years) and the fact that the only two studies which were ultimately included in the analysis were not published in a comprehensible way (for whatever reason) but were made available to us by the responsible German pharmaceutical company in form of biometrical reports upon request.
Minor limitations are that both studies were done within one country (Japan) which raise either some concerns on ethical issues and the differential effects of PRI in populations with a different ethnical background and the representativity of the data reported. However, extensive use of PRI in Germany with a predominantly Caucasian population gives no signal for clinically relevant safety or efficacy differences.
Conclusion
This systematic review found evidence that the nonbenzodiazepine antispasmodic PRI – either given orally (3 mg TID) or as im-injection (3 mg every other day) for 3 weeks – provides a biometrically significant and clinically meaningful relief of pain intensity, stiffness, and muscle tension in adult patients with different types of acute muscle pain. Risk and spectrum of DRAEs and related premature treatment discontinuations were comparable without signs for specific or critical side effects. However, due to the advanced age of the available study data and the fact that none of the two studies finally included into our meta-analysis has ever been published in a peer-reviewed journal, the quality of evidence was rated as only moderate for all comparisons. Therefore, either high-quality, placebo- or active-controlled, double-blind randomized up-to-date trials or larger propensity-score matched parallel cohort evaluations of real-world evidence on PRI vs. guideline recommended 1st line treatments are urgently necessary – not only to confirm the results of this meta-analysis, but especially to justify the frequent prescription of PRI in daily practice.
Transparency
Declaration of funding
The concept for this systematic review and meta-analysis was developed by M.A.U. at the Institute of Neurological Sciences (IFNAP) on behalf of the German Pain Association (Deutsche Gesellschaft für Schmerzmedizin, DGS) and the German Pain League (Deutsche Schmerzliga, DSL). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of financial/other relationships
M.A.U. and G.H.H.M.-S. are physicians and independent of any significant/relevant financial or other relationship to the manufacturer of the product under evaluation, except for minor reimbursements for occasional lecture or consulting fees. U.E. is a medical research specialist working as consultant for various pharmaceutical companies and several nonprofit organizations. M.A.U. is honorary member of the management board of the German Pain Association and the German Pain League. G.H.H. M.-S. is past-president of the German Pain Association. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Author contributions
All authors were involved in literature research, screening and eligibility evaluation as well as drafting the article or revising it critically for important intellectual content, and all authors read and approved the final manuscript to be published. M.A.U. takes responsibility for the integrity of the work as a whole, from inception to the finished article and affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the
Supplemental_table.docx
Download MS Word (17.1 KB)Acknowledgements
Preliminary results of this analysis have been presented at the annual Congress of the German Chapter of the International Association for the Study of Pain (IASP), 21–23 October 2021, Mannheim, Germany.
References
- Di Iorio D, Henley E, Doughty A. A survey of primary care physician practice patterns and adherence to acute low back problem guidelines. Arch Fam Med. 2000;9(10):1015–1021.
- Schers H, Braspenning J, Drijver R, et al. Low back pain in general practice: Reported management and reasons for not adhering to the guidelines in The Netherlands. Br J Gen Pract. 2000;50(457):640–644.
- Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–2803.
- Ivanova JI, Birnbaum HG, Schiller M, et al. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J. 2011;11(7):622–632.
- Soprano SE, Hennessy S, Bilker WB, et al. Assessment of physician prescribing of muscle relaxants in the United States, 2005–2016. JAMA Netw Open. 2020;3(6):e207664.
- Schreijenberg M, Koes BW, Lin CC. Guideline recommendations on the pharmacological management of non-specific low back pain in primary care - is there a need to change? Expert Rev Clin Pharmacol. 2019;12(2):145–157.
- Ludwig WD, Seifert R, Mühlbauer B. Arzneiverordnungs report 2021. Berlin, Germany: Springer.
- Position of the co-ordination group for mutual recognition and decentralised procedures for human use on periodic safety update reports fortizanidine. [accessed March 6, 2022]. Available from: https://www.bfarm.de/SharedDocs/Downloads/DE/Arzneimittel/Pharmakovigilanz/PSUSAS/s-z/tizanidin_beschluss_cmdh.pdf?__blob=publicationFile&v=2
- European Medicines Agency recommends restricting use of tolperisone medicines. [accessed March 6, 2022]. Available from: https://www.ema.europa.eu/en/documents/press-release/european-medicines-agency-recommends-restricting-use-tolperisone-medicines_en.pdf
- Recommendation to suspend tetrazepam-containing medicines endorsed by CMDh. [accessed March 6, 2022]. Available from: https://www.ema.europa.eu/en/documents/press-release/recommendation-suspend-tetrazepam-containing-medicines-endorsed-cmdh_en.pdf.
- PRAC recommends that the marketing authorisation of the painkiller flupirtine be withdrawn. [accessed March 6, 2022]. Available from: https://www.ema.europa.eu/en/documents/referral/prac-recommends-marketing-authorisation-painkiller-flupirtine-be-withdrawn_en.pdf
- Pridinol. Myopridin® 3 mg tablets (Strathmann) summary of product characteristics, last updated Aug 2019. [accessed March 6, 2022]. Available from: https://s3.eu-central-1.amazonaws.com/prod-cerebro-ifap/media_all/81560.pdf
- Pridinol – central and peripheral muscle spasms, lumbalgia, torticollis and general muscle pain. Article in German. [accessed on March 6, 2022]. Available from: https://www.kbv.de/media/sp/WirkstoffAktuell_4-21_Pridinol.pdf
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and Meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94.
- Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Hoboken (NJ): Wiley; 2019.
- Leonardo R. PICO: model for clinical questions. Evid Based Med Pract. 2018;3:2.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Furlan AD, Malmivaara A, Chou R, et al. 2015 Updated method guideline for systematic reviews in the cochrane back and neck group. Spine (Phila PA 1976). 2015;40(21):1660–1673.
- Schünemann HJ, Mustafa R, Brozek J, et al. Grade guidelines: 16. grade evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol. 2016;76:89–98.
- Moberg J, Oxman AD, Rosenbaum S, et al. The grade evidence to decision (ETD) framework for health system and public health decisions. Health Res Policy Syst. 2018;16(1):45.
- Whitley E, Ball J. Statistics review 3: hypothesis testing and P values. Crit Care. 2002;6(3):222–225.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in Meta-analyses. BMJ. 2003;327(7414):557–560.
- Egger M, Smith GD, Schneider M, et al. Bias in Meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
- Cohen J. Statistical power analysis for the behavioral sciences. New York (NY): Lawrence Erlbaum Associates; 1988.
- Vancak V, Goldberg Y, Levine SZ. Systematic analysis of the number needed to treat. Stat Methods Med Res. 2020;29(9):2393–2410.
- Ichikawa N, Hirose K, Nakada A, et al. Clinical evaluation of loxeen by double blind method. Japan: Tobishi Pharmaceuticals Co., Ltd.; 1973.
- Isobe R, Umehara T, Ikenuma S, et al. Therapeutic effect of methanesulfonate pridinol upon spondylosis deformans, periarthritis humeroscapularis and cervico-omo-brachial – clinical data by double blind method. Japan: Tobishi Pharmaceuticals Co., Ltd.; 1973.
- Überall MA, Müller-Schwefe GHH, Horlemann J. Efficacy and tolerability of the antispasmodic pridinol in patients with muscle pain – results of a retrospective analysis of open-label real-world data provided by the german pain e-Registry. Cur Med Rev Opin. 2022. DOI:https://doi.org/10.1080/03007995.2022.2072089
- Refior HJ. Clarification of the concept of humeroscapular periarthritis. Orthopade. 1995;24(6):509–511.