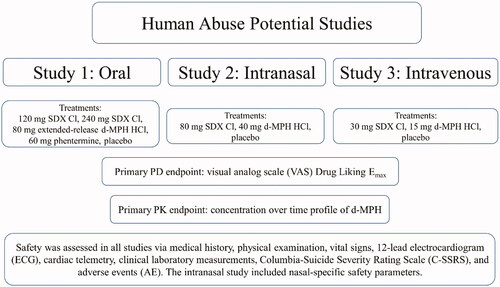
Abstract
Objectives
Serdexmethylphenidate (SDX) chloride (Cl) is a novel prodrug of d-methylphenidate (d-MPH). These studies evaluated the abuse potential of SDX Cl when administered orally, intranasally (IN), and intravenously (IV).
Methods
Three randomized, double-blind, placebo- and active-controlled crossover studies were conducted in recreational drug users to evaluate the abuse-related effects of oral SDX (120 and 240 mg) vs. extended-release (ER) d-MPH (80 mg) and phentermine (60 mg); IN SDX (80 mg) vs. d-MPH (40 mg), and IV SDX (30 mg) vs. d-MPH (15 mg). Abuse-related subjective measures, pharmacokinetics, and safety were assessed.
Results
The primary endpoint of maximum (Emax) Drug Liking (DL) (0–100-point scale) was significantly higher following d-MPH vs. placebo, validating the studies. In the oral study, DL Emax was significantly higher following 80 mg ER d-MPH (Emax = 81.5) than 120 mg SDX (Emax = 62.8, p < .001) and 240 mg SDX (Emax = 63.8, p = .006); and following 60 mg phentermine (Emax = 80.2) than 120 mg SDX (p = .0195), but not 240 mg SDX (p = .0665). DL Emax scores were significantly higher following IN d-MPH vs SDX (Emax = 93.2 vs. 71.0, p < .0001) and following IV d-MPH vs. SDX (Emax = 84.3 vs. 56.6, p = .001). Intravenous SDX was non-inferior to placebo (p = .001) for DL Emax. Secondary endpoints (e.g. Take Drug Again) were generally consistent with the primary endpoint. Maximal and overall d-MPH exposure was lower for SDX than d-MPH for all routes. Adverse events typical of stimulants were more frequent with d-MPH than SDX.
Conclusions
These findings indicate that the novel d-MPH prodrug, SDX, has lower abuse potential than d-MPH and support its classification as a C-IV controlled substance.
Introduction
First-line pharmacologic options for the treatment of attention-deficit/hyperactivity disorder (ADHD) include central nervous system (CNS) stimulants such as methylphenidate (MPH) and amphetamine (AMP)Citation1,Citation2. Due to their high potential for abuse, FDA-approved CNS stimulants are classified as Schedule II controlled substances by the Drug Enforcement Administration (DEA). Despite the regulations and restrictions associated with this control status, approximately 5 million people in the United States (U.S.) used prescription stimulants nonmedically in 2018Citation3,Citation4. Data from the National Survey on Drug Use and Health found that among users of prescription stimulants, 24.2% and 51.3% of respondents aged 12–17 years and 18–25 years, respectively, reported nonmedical useFootnotei in the past yearCitation5. In a prospective, 17-year longitudinal study, nonmedical use of prescription stimulants during adolescence was associated with more substance use disorder symptoms and lower educational attainment compared with strictly medical use of prescription stimulantsCitation6.
Nonmedical use of prescription stimulants is associated with a wide range of dose-dependent, adverse health effects, including psychotic symptoms, increases in heart rate and blood pressure, dangerous elevations in body temperature, cardiac complications such as arrhythmias and heart failure, and seizuresCitation7–11. The health risks vary according to route of administration, with non-oral routes conferring greater risk. For example, an analysis of data from the U.S. National Poison Data System (2012–2016) showed that the average number of adverse medical outcomes associated with nonmedical use of amphetamines was higher for intravenous (IV; 2.95) and intranasal (IN; 2.46) routes relative to nonmedical oral use (2.17) and unintentional oral exposure (1.57)Citation12. Similarly, the odds of dying were found to be 13 and 22 times greater among IN and IV amphetamine abusers, respectively, relative to non-abusersCitation12.
According to data from the public health surveillance system, NAVIPPRO, non-oral use of prescription stimulants by adolescents and adults occurs most commonly via IN (snorting) and IV routes of administrationCitation13. Among abusers of prescription stimulants, including methylphenidate products, 30–40% reported snorting, and up to 10% reported injectingCitation13–17. A survey of adults with a history of non-oral prescription stimulant misuse found that a majority reported oral use prior to non-oral use, and most (70%) transitioned from oral to IN (snorting) administrationCitation18. These findings suggest a progression of abuse-related behaviors over time than can result in more risky routes of administration.
Nonmedical users of prescription stimulants report a range of non-mutually exclusive motives for such use, including the desire to get “high,” increase alertness, help with concentration/performance, and experimentation/curiosityCitation14,Citation17. Nonmedical users of stimulants whose motive is to get “high” tend to seek a rapid onset of effect, which requires plasma drug concentrations to rise quickly to high levels following administration. Accordingly, drug formulations and routes of administration (IN, IV) that produce a higher peak plasma concentration (Cmax) and/or shorter time to Cmax (Tmax) engender higher reports of drug likingCitation19–26. For stimulants such as methylphenidate that have low oral bioavailability, non-oral routes of administration which avoid first-pass metabolism allow a greater fraction of the dose to reach the CNS compared to an equivalent dose administered orallyCitation27. While the clinical utility of prescription stimulants in the treatment of ADHD and other CNS disorders is well-established, there remains a need for stimulant-like therapies with a lower propensity for nonmedical use and diversion.
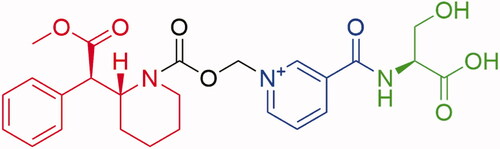
Serdexmethylphenidate (SDX) (see for chemical structure) is an extended-duration prodrug of d-MPH that was developed, in part, to produce lower abuse-related effects than d-MPH hydrochloride (HCl) when administered via oral and non-oral routes. SDX is approved as a combination product, SDX/d-MPH (70/30 molar ratio) capsules (AZSTARYS [Schedule II]), for the treatment of ADHD; the IR component was included to achieve faster d-MPH exposures following oral administration and thus efficacy earlier in the dosing interval. SDX is currently under investigation as a single-entity product for the treatment of various CNS-related conditions. Intact SDX chloride (Cl) is pharmacologically inactive until converted to active d-MPH, a process likely occurring primarily in the lower intestinal tractCitation28. Preclinical studies in several animal species were suggestive of low relative abuse potential insofar as: (1) oral administration of SDX Cl yielded a slow onset and relatively long duration of d-MPH exposure, and (2) IV administration of SDX Cl resulted in very low plasma concentrations of d-MPH relative to d-MPH HCl administrationCitation29. The objective of the 3 studies described here was to evaluate the human abuse potential of SDX Cl following oral, IN, and IV administration. These studies were conducted as a part of the overall abuse potential assessment to inform a scheduling decision for SDX under the Controlled Substances Act.
Figure 1. Serdexmethylphenidate chemical structure. SDX consists of a single d-MPH molecule covalently attached via a carbamate bond to a methylene oxide linker, which in turn is connected to a nicotinoyl-serine moiety. Molecular components: red = d-methylphenidate, black = carboxymethylene linker, blue = niacin, green = l-serine.
Methods
Study designs
The overall study designs, including the selection of pharmacodynamic (PD) endpoints and subject population, were developed in accordance with 2017 Food and Drug Administration (FDA) Guidance for Industry, Assessment of Abuse Potential of DrugsCitation30. Briefly, all 3 studies included a Screening Period, an in-clinic Drug Discrimination Phase, an in-clinic Treatment Phase, and a Follow-up Visit. In the Drug Discrimination Phase, the ability of subjects to discriminate the abuse-related effects of d-MPH (administered as Focalin XR for the oral study and d-MPH HCl for the IN and IV studies) vs. placebo was evaluated. Drug Liking scores (see Pharmacodynamic Endpoints section) were used to determine if subjects could discriminate between d-MPH HCl and placebo, and in combination with safety and tolerability data, whether subjects would be eligible for the Treatment Phase according to the following criteria: (1) maximum score (Emax) of at least 65 points for d-MPH HCl on the Drug Liking Visual Analog Scale (VAS) and at least 15 points higher in response to d-MPH HCl vs. placebo (so that subjects have a minimum increase in response to d-MPH HCl above the placebo response); (2) placebo response in the range of 40–60 points (inclusive) on the Drug Liking VAS (so that subjects neither endorsed liking nor disliking of placebo); (3) acceptable overall responses to d-MPH HCl and placebo on all other PD measures, as judged by the Investigator or designee; (4) acceptable safety and tolerability profile following oral (Study 1), IN (Study 2), or IV (Study 3) administration of d-MPH HCl. Subjects meeting these criteria were randomized to receive SDX Cl, active comparator(s), and placebo in the Treatment Phase of each respective study, during which PD, pharmacokinetic (PK), and safety data were collected as described below and depicted in .
Study 1: oral abuse potential of SDX Cl
This was a Phase 1, randomized, double‑blind, single-dose, 5-treatment, crossover study evaluating oral doses of SDX Cl, extended-release (ER) d-MPH HCl (Focalin XR, C-II product), phentermine (C-IV product), and placebo in recreational stimulant users aged 18–50 years. Successfully screened subjects who were able to discriminate a dose of 80 mg ER d-MPH HCl from placebo entered the Treatment Phase. For each of the 5 treatment periods, subjects were randomized to receive one of the following 5 treatments: 120 mg SDX Cl (equimolar to 60 mg d-MPH HCl), 240 mg SDX Cl (equimolar to 120 mg d-MPH HCl), 80 mg ER d-MPH HCl (primary positive control), 60 mg phentermine (secondary positive control), or placebo. Treatment periods were separated by a minimum 96-h washout period. Per FDA guidance, oral doses of SDX Cl were selected to be approximately 2- to 4-fold higher than the highest therapeutic dose contained in 52.3/10.4 mg SDX/d-MPH (equivalent to 56/12 mg SDX Cl/d-MPH HCl) capsules (Azstarys)Citation30.
Study 2: intranasal abuse potential of SDX Cl
This was a Phase 1, randomized, double‑blind, single-dose, 3-treatment, crossover study evaluating IN doses of SDX Cl compared with d-MPH HCl and placebo in recreational stimulant users 18–55 years of age who had used CNS stimulants by nasal insufflation more than once within 12 weeks prior to the Screening Visit. Subjects who were able to discriminate a dose of 40 mg IN d-MPH HCl from placebo were randomized to receive the following IN treatments: 80 mg SDX Cl (equimolar to 40 mg d-MPH HCl), 40 mg d-MPH HCl + 40 mg microcrystalline cellulose (MCC; added to match volumes) (active control), or matching placebo (80 mg MCC). The 3 treatment periods were separated by a minimum 96-h washout period. The dose of the active comparator, d-MPH HCl, was selected based on results of a prior dose-ranging PD study in recreational stimulant usersCitation31. The study found that 40 mg IN d-MPH HCl produced robust abuse-related effects with tolerable adverse effects. The SDX Cl dose, in turn, was selected to be equimolar to the d-MPH HCl dose.
Study 3: intravenous abuse potential of SDX Cl
This was a Phase 1, randomized, double-blind, single-dose, 3-treatment, crossover study evaluating IV doses of SDX Cl compared with d-MPH HCl and placebo in recreational stimulant users 18–50 years of age who had used stimulants via the non-oral route and who had used cocaine within 6 months prior to the Screening Visit. Part A of the study was a dose-escalation phase that determined, based on PD endpoints (VAS for Drug Liking, Good Effects, Bad Effects) and safety, the optimal IV d-MPH HCl dose to be used in assessing the abuse potential in Part B of the study. The IV SDX Cl dose, in turn, was selected to be equimolar to the IV d-MPH HCl dose. In Part B, subjects who were able to discriminate the optimal dose of IV d-MPH HCl (15 mg) from placebo were randomized to receive the following IV treatments: 30 mg SDX Cl (equimolar to 15 mg d-MPH HCl), 15 mg d-MPH HCl, or matching placebo (saline solution).
Subjects
All three studies enrolled male and non-pregnant, non-breastfeeding female subjects who were not currently dependent (in the opinion of the investigator) on CNS stimulants and had a body mass index (BMI) between 18 and 34 kg/m2 (inclusive). Subjects must have had ≥10 lifetime experiences with any stimulant (e.g. amphetamines, cocaine, and/or MPH) and used any stimulant for non-therapeutic purposes at least 5 times within the last 6 months prior to the Screening Visit. Subjects were excluded if they were currently seeking treatment for substance use disorder or had a history of drug or alcohol dependence. Major exclusions also included medical or psychiatric conditions or abnormalities that could be impacted by study participation in regard to subject safety; allergic or adverse responses to any stimulant in the past; participation in another clinical trial in the past 30 days; or unapproved use of over-the-counter or prescription medications, vitamins, herbal products, or dietary supplements prior to the Drug Discrimination Phase or Treatment Phase of the study. Written informed consent was obtained for all studies, and the study protocols were approved by an Institutional Review Board.
Pharmacodynamic assessments
Visual Analog Scale (VAS) assessments recommended for use in human abuse potential studies were scored on a 0–100-point scaleCitation30. These assessments included both “at-the-moment” effects (Drug Liking, Feeling High, Good Effects, Bad Effects, Any Effects, and Drowsiness/Alertness) and retrospectively assessed endpoints that measure the overall balance of drug effects (Take Drug Again and Overall Drug Liking), assessed on bipolar or unipolar scales. For the primary efficacy assessment, Drug Liking VAS, subjects responded to the question, “At this moment, my liking for the drug is?,” with 0 = strong disliking, 50 = neither like nor dislike, and 100 = strong liking. The Addiction Research Center Inventory measures (ARCI-Amphetamine [ARCI-A] and ARCI-Benzedrine Group [ARCI-BG]) scales were also assessedCitation32. The ARCI-A questionnaire assesses stimulant effects, and the ARCI-BG questionnaire assesses energy and intellectual efficiency. Subjects answered True or False to a series of 19 questions that assess stimulant-like effects. Scores from several questions were used in both the ARCI-A and ARCI-BG subscales (11 questions for ARCI-A; 13 questions for ARCI-BG). The maximum score is 11 for ARCI-A and 13 for ARCI-BG, with higher scores indicating higher subjective effects. For the study of IN administration (Study 2), subject-reported ease of nasal insufflation was assessed within 5 min after IN drug administration during the Treatment Phase. The question was scored using a 0–100-point unipolar VAS anchored with “Very Easy” (score of 0) to “Very Difficult” (score of 100).
For the oral administration study, at-the-moment PD assessments were conducted at pre-dose (non-drug-specific measures only) and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, and 24 h post-dose. For the IN administration study, at-the-moment PD assessments were conducted at pre-dose and 0.083 (5 min), 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, 16, and 24 h post-dose. For the IV administration study, at-the-moment PD assessments were conducted at pre-dose and 0.083 (5 min), 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, and 24 h post-dose. Drug Liking was also assessed at 2 min post-dose in the IV study. Overall PD assessments (Take Drug Again and Overall Drug Liking) were assessed 12 and 24 h post-dose in all 3 studies. The ARCI-A and ARCI-BG subscales were assessed at pre-dose and 0.5, 1, 2, 4, and 8 h post-dose in all 3 studies.
Pharmacokinetic assessments
Blood samples were collected at different times after the administration of study drug to evaluate the oral, IN, and IV PK profile of SDX and SDX-derived d-MPH relative to d-MPH HCl controls. In the oral study, samples were collected pre-dose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 16, 24, 36, and 48 h post-dose. In the IN and IV studies, samples were collected pre-dose and at 0.83 (5 min), 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 16 (IN study only), 24, 36, and 48 (IN study only) hours post-dose.
Bioanalytical assay
Quantitation of SDX and d-MPH in plasma samples was performed using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) assay.
Serdexmethylphenidate
SDX was extracted from 100 μL of plasma using solid phase extraction (SPE) with an Oasis HLB 96-well plate and SDX-d6 as internal standard. Monitored mass transitions for SDX and SDX-d6 were m/z 500.2→142.1 and m/z 506.2→266.1, respectively. The main chromatographic conditions were Phenomenex Kinetex C18 2.6-µm, 50 × 2.1 mm HPLC column, 1 mM ammonium trifluoroacetate in water as aqueous mobile phase, acetonitrile/formic acid (1000:1) as organic phase, and gradient elution. Detection was performed with a Sciex API 5000 Triple Quad LC-MS/MS. The validation range of the method was 0.100–100 ng/mL with a lower limit of quantitation (LLOQ) of 0.100 ng/mL. The intra- and inter-run precisions (coefficient of variance [CV]) were <7.4% and <6.2%, respectively. The intra- and inter-run accuracies were 96.4% to 107.5% and 99.0% to 102.8%, respectively.
Methylphenidate
SPE with an Oasis HLB 96-well plate and racemic d,l-MPH-d3 as internal standard was used to extract d-MPH and l-MPH from 50 μL of plasma. The analysis was performed by LC-MS/MS. Monitored mass transitions for MPH and MPH-d3 were m/z 234.3→84.1 and m/z 237.3→84.1, respectively. The main chromatographic conditions were Supelco CHIROBIOTIC 5-μm, 2.1 × 150 mm HPLC column, methanol/ethanol/ammonium trifloroacetate (600:400:0.1, vol/vol/wt) as mobile phase, and isocratic elution. Detection was performed with a Sciex API 5000 Triple Quad LC-MS/MS. The validation range of the method was 0.200–200 ng/mL with an LLOQ of 0.200 ng/mL. The intra- and inter-run precisions were <4.1% and <6.5%, respectively. The intra- and inter-run accuracies were 100.3% to 117.1% and 94.7% to 108.5%, respectively.
Safety assessments
Safety assessments were performed throughout all phases of the studies and included adverse events (AEs) (monitored continuously and solicited using a non-leading question at predefined times), vital signs (blood pressure, heart rate, and respiratory rate), 12-lead electrocardiograms (ECGs), continuous cardiac telemetry (from at least 15 min pre-dose to 4 h post-dose), clinical laboratory tests, physical examinations, and the Columbia-Suicide Severity Rating Scale (C-SSRS) questionnaire.
Statistical analyses
PD and PK analyses were conducted in the Completers population, defined as subjects who received all treatments and completed all treatment periods in the Treatment Phase and had at least one response on the VAS for Drug Liking within 2 h of Tmax for each treatment. The primary PD endpoint, the maximum score (Emax) on Drug Liking VAS, was analyzed using a one-sided hypothesis test at a significance level of α = 0.05 and reported with 95% confidence intervals (CIs) and pre-specified margins (δ)Citation30. Selection of δ1 = 15 (extended-release d-MPH vs. placebo, study validity) was based on prior Drug Liking data of an extended-release methylphenidate formulation relative to placeboCitation23. Selection of δ2 =10 (extended-release d-MPH vs. SDX, relative abuse potential) was based on findings that a 10-point difference was determined to be clinically meaningful for Drug LikingCitation33. Selection of δ3 =11 (SDX vs. placebo, absolute abuse potential) was based on a meta-analysis of 8 human abuse potential studies evaluating the Emax of Drug Liking VAS of test drugs from 2 drug classes (stimulants and sedatives) vs. placeboCitation34. In the IV study, Take Drug Again VAS was designated a key secondary endpoint that was evaluated with the same margins as Drug Liking. Selection of δ4 = 10 (phentermine vs. placebo, secondary study validity) was lower than for the primary study validity comparison because phentermine is a C-IV product with lower abuse potential than d-methylphenidate (C-II product). Other secondary and exploratory endpoints were performed as 2-sided, confirmatory hypothesis tests at a significance level of α = 0.05 and reported with 95% CIs, with the exception of SDX Cl vs. placebo, which were performed as 2-sided hypothesis tests at a significance level of α = 0.10 and reported with 90% CI. The significance level for SDX Cl vs placebo was increased as a conservative strategy to minimize Type I error and thus, to reduce the probability of falsely concluding that SDX Cl is different from placebo.
All PD endpoints were initially analyzed using a mixed-effect Analysis of Covariance (ANCOVA) model with treatment, period, treatment sequence as fixed effects, baseline (pre-dose) measurement as a covariate (where applicable), and subject as random effect. The residuals from this mixed-effect model were investigated for normality using the Shapiro-Wilk test. If the test indicated normal distribution, it was determined if carryover effects should be included. This approach addressed the possibility that a previous treatment could alter responses to a subsequent treatment. The carryover effect was defined as the treatment administered in the previous treatment period. If the carryover effect was found to be non-significant at α = 0.25, the term was dropped from the analysis model. If the normality assumption of the model was not satisfied, the distribution of the paired treatment differences for each endpoint was evaluated for symmetry with a skewness test. In case the distribution was found to be symmetric, a paired t-test was performed. Otherwise, the Sign test was used to compare the pairwise treatment differences. The median differences and associated confidence intervals were estimated using the method of Hodges-Lehman. The derived parameters TEmax and TEmin parameters were analyzed using a non-parametric method.
PK parameters (Cmax, Tmax, AUC0-last, AUC0-inf, AUC0-t [partial areas], and T1/2) were estimated from plasma concentration-time profiles of SDX and d-MPH using standard, non-compartmental methods with Phoenix WinNonlin (Version 6.4 or higher, Certara, L.P., Princeton, NJ, USA). Log-transformed PK parameters were statistically analyzed using a mixed-effect ANOVA model, with treatment, period, and treatment sequence as fixed effects and subject (nested within sequence) as a random effect. Relative bioavailability of d-MPH for SDX vs. d-MPH comparators was assessed using least squares geometric means (LSGM) of the PK parameters for each treatment. Similar bioavailability of d-MPH was concluded if the 90% CIs of the LSGMs was within the range of 0.80–1.25 for Cmax, AUC0-last, and AUC0-inf. The 90% CIs for partial AUCs as compared to the 0.80–1.25 range were used to assess the change in relative bioavailability from earlier to later time points after dosing. Values of Tmax and T½ (without log-transformation) were compared between treatments using the Wilcoxon Signed-Rank Test, and median difference and associated 2-sided 90% CIs were estimated using the method of Hodges–Lehmann.
Safety analyses (descriptive) were conducted in the Safety Population, defined as all randomized subjects who received at least one dose of study drug in the Treatment Phase and had at least one post-dose safety assessment.
Results
Subject demographics
shows demographic characteristics for subjects enrolled in studies of oral, IN, and IV administration. Of the 50 subjects who entered the Treatment Phase of Study 1 (oral administration), 45 subjects (90%) completed the study, and 5 subjects (10%) discontinued. Reasons for discontinuation were withdrawal by subject (two subjects), AE (one subject), and other (two subjects). The majority of subjects were white, non-Hispanic males with a mean age of 30.2 years. Of the 49 subjects entering the Treatment Phase of Study 2 (IN administration study), 45 (91.8%) subjects completed the study and four (8.2%) subjects discontinued. Reasons for discontinuation were AEs of anxiety and claustrophobia in one subject and withdrawal of consent in three subjects. The majority of subjects were white, non-Hispanic males with a mean age of 36.0 years. Of the 31 subjects entering the Treatment Phase of Study 3 (IV administration study), 30 (96.8%) subjects completed the study. One subject discontinued due to a family emergency. The majority of subjects were black, non-Hispanic males with a mean age of 32.0 years.
Table 1. Study participant demographics (Completers population).
Pharmacodynamics and pharmacokinetics
Study 1: oral SDX Cl
Mean Drug Liking VAS scores over time for the 120 mg and 240 mg doses of SDX Cl were lower than for the 80 mg ER d-MPH HCl and 60 mg phentermine treatments, with particularly noticeable differences at the 1.5- to 3-h post-dose timepoints (). At 2 h post-dose, for example, mean Drug Liking VAS scores were 56.0 and 53.6 points for 120 mg and 240 mg SDX Cl, respectively, compared with 76.4 and 72.6 points for 80 mg ER d-MPH HCl and 60 mg phentermine, respectively. Drug Liking VAS scores following 80 mg ER d-MPH HCl dropped below 50 from 12–24 h post-dose, indicative of at least some disliking. Mean Drug Liking VAS scores over time for the 120 mg and 240 mg doses of SDX Cl were not distinct from those observed following placebo administration (∼50). Mean (SD) Drug Liking Emax values for 120 mg and 240 mg SDX Cl were 62.8 (16.4) and 63.8 (15.7) points, respectively, notably lower than for 80 mg ER d-MPH HCl and 60 mg phentermine (81.5 [12.5] and 80.2 [14.3] points, respectively), but higher than for placebo (55.8 [11.8] points) ().
Figure 3. Mean Drug Liking VAS over time (top left) and Emax values for primary endpoint (Drug Liking, top right) and secondary endpoints (Take Drug Again, Overall Drug Liking, bottom) following oral (po) administration of SDX Cl (120 and 240 mg), extended-release d-MPH (80 mg), phentermine (60 mg), and placebo. Raw means are shown for Emax scores. Treatment comparisons were performed with a mixed-effect ANCOVA model as described in the “Statistical analyses” section.
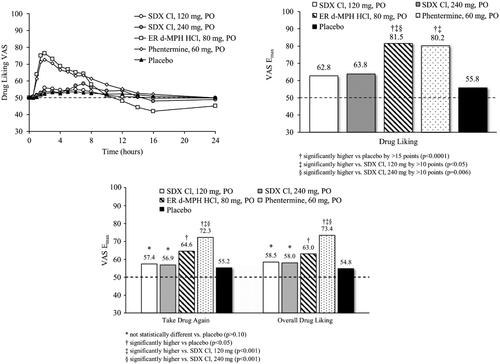
Study validity was assessed by comparing Drug Liking for active controls (ER d-MPH HCl and phentermine) relative to placebo. ER d-MPH HCl (80 mg) produced a mean difference in Drug Liking Emax of 25.01 points relative to placebo, which was statistically significantly greater than the 15-point superiority margin (95% CI: [20.69, inf]; p < .0001). Phentermine (60 mg) also demonstrated a significant mean difference in Drug Liking Emax of 22.27 points compared with placebo, which was statistically significantly greater than the 10-point superiority margin (95% CI: [17.91, inf]; p < .0001). Therefore, the null hypotheses for each comparison were rejected, indicating that the study was valid.
Relative abuse potential was assessed via comparison of Drug Liking Emax for both SDX Cl doses to ER d-MPH HCl and phentermine. The mean differences in Drug Liking Emax of 18.22 and 16.74 between 80 mg ER d-MPH HCl and 120 mg and 240 mg SDX Cl, respectively, were statistically superior by a margin of more than 10 points (120 mg SDX CI: 95% CI: [13.87, inf]; p = .0011; 240 mg SDX Cl: 95% CI: [12.37, inf]; p = .0058). Therefore, the null hypothesis was rejected, indicating that oral SDX Cl has statistically significantly lower abuse potential than ER d-MPH HCl even when administered at doses that are higher on a molar basis (e.g. 240 mg SDX Cl) relative to ER d-MPH HCl. Analysis of the partial AUE curve parameters indicated significantly more cumulative Drug Liking for 80 mg ER d-MPH HCl than for 120 mg or 240 mg SDX Cl at 1 h post-dose and later (Drug Liking VAS at 0.5 h post-dose was low and similar across all treatments).
The mean difference in Drug Liking Emax responses between 60 mg phentermine and 120 mg SDX Cl was 15.48 points, which was statistically significantly greater than the 10-point superiority margin (95% CI: [11.12, inf]; p = .0195). The mean difference of 14.00 points between 60 mg phentermine and 240 mg SDX Cl was not statistically significantly greater than 10 points, although the lower bound of its 95% CI was only marginally below the 10-point superiority margin (95% CI: [9.62, inf]; p = .0664).
Absolute abuse potential was assessed via comparison of Drug Liking Emax for both SDX Cl doses to placebo. The respective mean differences of 6.79 points (120 mg SDX Cl) and 8.27 points (240 mg SDX Cl) were not found to be statistically significantly smaller than the prespecified margin of 11 points, indicating that both doses of SDX Cl showed oral abuse potential that was relatively close to, but not non-inferior to, placebo (120 mg SDX Cl: 95% CI: [-inf, 11.17]; p = .0567; 240 mg SDX Cl: 95% CI: [-inf, 12.62]; p = .1502).
The Emax values of the secondary endpoints, Take Drug Again and Overall Drug Liking VAS, were statistically higher for ER d-MPH HCl and phentermine relative to placebo (p < .05). Although 120 mg and 240 mg SDX Cl did not statistically differentiate from placebo on either measure (p ≥ .10), there was also no statistical difference between ER d-MPH HCl and either dose of SDX Cl (p ≥ .05) (). This result may be attributable to the negative effects experienced with ER d-MPH HCl by most subjects as indicated by Drug Liking after the 12-h timepoint and with Bad Effects that were significantly higher than for SDX Cl and placebo. However, the Emax values for phentermine were statistically higher compared with both doses of SDX Cl (p ≤ .0005). As shown in , all other endpoints also showed reduced abuse potential of both doses of SDX Cl relative to 60 mg phentermine and 80 mg ER d-MPH HCl.
Table 2. Summary results for mean Emax of secondary VAS endpoints for study 1 (oral administration), study 2 (IN administration), and study 3 (IV administration).
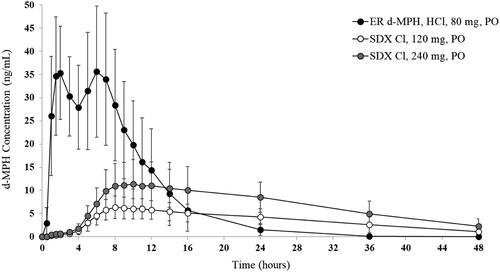
The mean d-MPH plasma concentration-time curves for both doses of SDX Cl and ER d-MPH HCl are depicted in . The median d-MPH Tmax was significantly longer (p < .0001) for both 120 mg and 240 mg SDX Cl (9 and 11 h, respectively) compared with the median d-MPH Tmax for ER d-MPH HCl (1.5 h). The median T1/2 of d-MPH was also longer for 120 mg and 240 mg SDX Cl (10.0 and 10.8 h, respectively) compared to the T1/2 of d-MPH derived from ER d-MPH HCl (3.7 h) (Supplemental Table 1). Relative bioavailability of d-MPH based on peak and total systemic exposure was significantly lower for 120 mg SDX Cl compared to 80 mg ER d-MPH HCl (geometric least squares mean [GLSM] ratios of Cmax, AUC0-last, and AUC0-inf were approximately 18%, 42%, and 46%, respectively). The relative bioavailability of d-MPH based on peak and cumulative systemic exposure (up to about 36 h post-dose) was also lower for 240 mg SDX Cl relative to 80 mg ER d-MPH HCl (GLSM ratios of Cmax and AUC0–36 were approximately 33% and 69%, respectively). However, AUC0-last and AUC0-inf were comparable for 240 mg SDX Cl and 80 mg ER d-MPH HCl (GLSM ratios of AUC0-last, and AUC0-inf were approximately 81% and 90%, respectively).
Study 2: intranasal SDX Cl
Mean Drug Liking VAS scores for IN d-MPH HCl increased rapidly, with a score of 79.8 at 0.25 h post-dose and peak scores of >85 occurring 0.75 to 1 h post-dose (). In contrast, mean Drug Liking VAS scores for IN SDX Cl remained below 60 for the entire assessment interval (). Mean (SD) Drug Liking VAS Emax values were 93.2 (10.5), 71.0 (18.5), and 51.1 (1.4) after IN administration of d-MPH HCl, SDX Cl, and placebo, respectively ().
Figure 5. Mean Drug Liking VAS over time (top left) and Emax values for primary endpoint (Drug Liking, top right) and secondary endpoints (Take Drug Again, Overall Drug Liking, bottom) following intranasal (IN) administration of SDX Cl (80 mg), d-MPH HCl (40 mg), and placebo. Raw means are shown for Emax scores. Pairwise comparison of d-MPH HCl and placebo was performed using the Sign test. The remaining treatment comparisons were conducted with a matched-pairs t-test. The detailed test methodologies are described in the “Statistical Analyses” section.
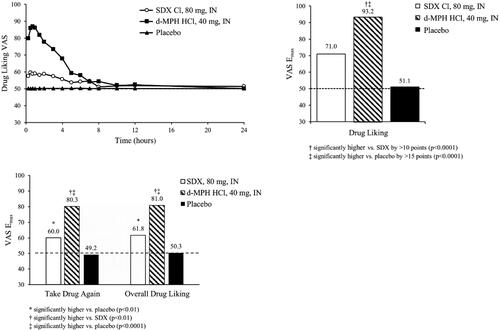
Study validity was confirmed by comparing Drug Liking Emax of IN d-MPH HCl to placebo, which resulted in a median difference of 45.0 points that was statistically significantly greater than the 15-point superiority margin (95% CI: [41.0, inf]; p < .0001). Relative abuse potential was assessed by comparing Drug Liking Emax of IN d-MPH HCl to IN SDX Cl, which resulted in a mean difference of 22.3 points that was statistically significantly greater than the 10-point superiority margin (95% CI: [17.3, inf]; p < .0001). Therefore, the null hypothesis was rejected, indicating that SDX Cl, when administered IN at an equimolar dose, has statistically significantly lower abuse potential than d-MPH HCl. Absolute abuse potential was assessed by comparing Drug Liking Emax of IN SDX Cl to placebo, which resulted in a median difference of 19.9 points, and thus was not statistically significantly lower than the prespecified 11-point, non-inferiority margin (95% CI: [-inf, 24.6]; p = .999).
The Emax scores for the secondary endpoints, Take Drug Again and Overall Drug Liking VAS, were found to be significantly higher for IN d-MPH HCl compared to IN SDX Cl (p < .01) and placebo (p < .0001) (). Both endpoints were significantly higher for IN SDX Cl (p < .01) compared with placebo, which produced neutral responses (). All other endpoints demonstrated a similar profile of differences between treatments ().
Ease of Insufflation VAS was scored 5 min following the insufflation of each treatment. The mean (SD) score for SDX Cl (65.8 [4.8]) was significantly higher than for d-MPH HCl (18.1 [3.5], p < .0001) and placebo (6.9 [2.8], p < .0001), indicative of greater difficulty in snorting SDX Cl.
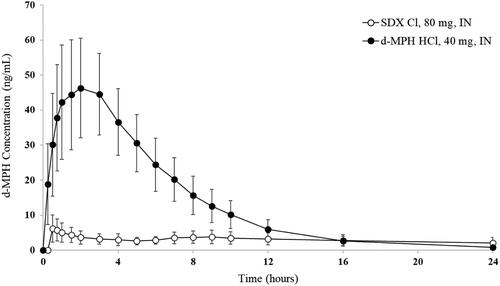
Plasma d-MPH concentrations rose rapidly after IN administration of d-MPH HCl (40 mg), reaching 18.4 ng/mL within 5 min and mean peak of 45.7 ng/mL at 2 h post-dose followed by a decline to less than 0.5 ng/mL by 36 h post-dose in most subjects. In contrast, the d-MPH plasma concentration-time curve was markedly lower and flatter following IN administration of SDX Cl (80 mg) (). Relative bioavailability of d-MPH based on peak and total systemic exposure was significantly lower for IN SDX Cl compared to IN d-MPH HCl (the GLSM ratios of Cmax, AUC0-last, and AUC0-inf were approximately 13%, 22%, and 25%, respectively). The median T1/2 was significantly shorter for d-MPH HCl (3.7 h) than for d-MPH derived from SDX Cl (8.8 h).
Study 3: intravenous SDX Cl
Mean Drug Liking VAS scores for 15 mg IV d-MPH HCl increased rapidly, with scores in the “liking range” (i.e. > 60) from 5 min to 2 h post-dose, and mean peak score (76.9) occurring 0.25 h post-dose. In contrast, mean Drug Liking VAS scores for 30 mg IV SDX Cl and placebo remained close to 50 (i.e. 50, “neither like nor dislike”) throughout the entire assessment period. Mean (SD) Drug Liking VAS Emax values were 84.3 (13.9), 56.6 (11.8), and 53.8 (8.0) after IV administration of d-MPH HCl, SDX Cl, and placebo, respectively ().
Figure 7. Mean Drug Liking VAS over time (top left) and Emax values for primary endpoint (Drug Liking, top right) and secondary endpoints (Take Drug Again, Overall Drug Liking, bottom) following intravenous (IV) administration of SDX Cl (30 mg), d-MPH HCl (15 mg), and placebo. Raw means are shown for Emax scores. Matched-pairs t-tests were performed for the comparisons of Drug Liking Emax and Overall Drug Liking Emax between d-MPH HCl and placebo, and Overall Drug Liking Emax between SDX Cl and placebo. Pairwise comparisons of Drug Liking Emax and Overall Drug Liking Emax between d-MPH HCl and SDX Cl, and Drug Liking Emax between SDX Cl and placebo were conducted using the Sign test. All treatment comparisons of Take Drug Again Emax were performed with a mixed-effect ANCOVA model. The detailed test methodologies are described in the “Statistical Analyses” section. Note that Take Drug Again was assessed on a unipolar scale.
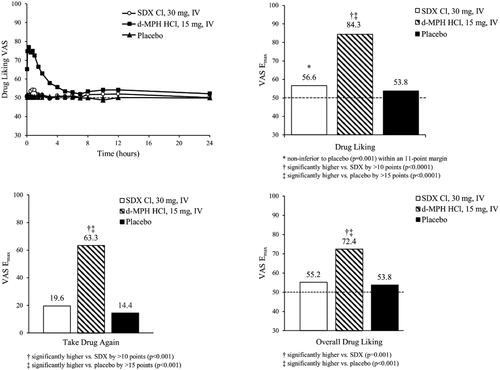
Study validity was confirmed by comparing Drug Liking Emax of IV d-MPH HCl to placebo, which resulted in a median difference of 30.5 points that was statistically significantly greater than the 15-point superiority margin (95% CI: [25.9, inf]; p < .001). Relative abuse potential was assessed by comparing Drug Liking Emax of IV d-MPH HCl to IV SDX Cl, which resulted in a median difference of 29.0 points that was statistically significantly greater than the 10-point superiority margin (95% CI: [22.5, inf]; p = .001). Therefore, the null hypothesis was rejected, indicating that SDX Cl, when administered IV at an equimolar dose, has significantly lower abuse potential than d-MPH HCl. Absolute abuse potential was assessed by comparing Drug Liking Emax of IV SDX Cl to placebo, which resulted in a median difference of 0.5 points, and thus was statistically significantly lower than the prespecified 11-point, non-inferiority margin (95% CI: [-inf, 5.5]; p = .001). Consequently, the null hypothesis was rejected, indicating that SDX Cl was not different from placebo.
For the key secondary endpoint, Emax of Take Drug Again VAS, the LS mean difference between IV d-MPH HCl and placebo was 49.1 (95% CI: 35.2, inf; p < .001), indicating that subjects were more willing to take IV d-MPH HCl again relative to placebo (). Similarly, the LS mean difference between d-MPH HCl and SDX Cl was 43.1 (95% CI: 29.2, inf; p < .001), demonstrating a greater willingness to take IV d-MPH HCl again compared with SDX Cl. While the LS mean difference between IV SDX Cl and placebo was 6 (95% CI: inf, 19.9; p = .275), the upper limit of the 95% CI exceeded the 11-point non-inferiority margin. For Overall Drug Liking VAS, Emax for IV d-MPH was statistically higher compared with those of placebo (p < .001) and IV SDX Cl (p = .001), which were not different from each other (p = .658). All other endpoints demonstrated a similar profile of differences between treatments ().
Figure 8. Plasma d-MPH concentrations after intravenous (IV) administration of SDX Cl and d-MPH HCl. Bars are standard deviations.
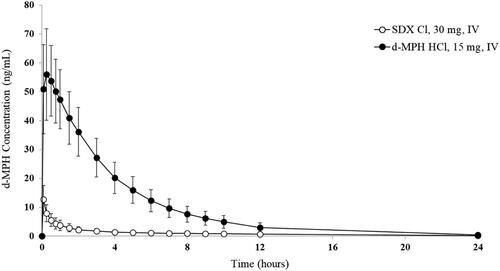
Plasma d-MPH concentrations rose rapidly after IV administration of 15 mg d-MPH HCl, reaching a mean peak of 56.0 ng/mL at 15 min post-dose and declining in a monophasic manner thereafter, with plasma concentrations less than 0.5 ng/mL by 36 h post-dose in most subjects (). In contrast, plasma d-MPH concentrations following IV administration of 30 mg SDX Cl reached a mean peak concentration of 12.8 ng/mL at 5 min post-dose, then declined steadily over time, with plasma concentrations below 0.5 ng/mL by 24 h post-dose in most subjects (). Relative bioavailability of d-MPH based on peak and total systemic exposure was significantly lower for IV SDX Cl compared to IV d-MPH HCl (the GLSM ratios of Cmax, AUC0-last, and AUC0-inf were approximately 21%, 10%, and 12%, respectively). The median T1/2 was significantly shorter for d-MPH HCl (4.0 h) than for d-MPH derived from SDX Cl (7.8 h).
Safety and tolerability
Across studies, SDX was well-tolerated and generally had a lower incidence of treatment-emergent AEs (TEAEs) relative to the positive controls. In the study of oral administration, 18 (38.3%) and 22 (45.8%) subjects had TEAEs after receiving 120 mg and 240 mg SDX Cl prodrug, respectively, compared with 39 (86.7%) and 34 (72.3%) after receiving 80 mg ER d-MPH HCl and 60 mg phentermine, respectively. All TEAEs were mild or moderate in severity, and no subject experienced a serious AE. Across all treatments, the most commonly reported TEAEs (e.g. euphoric mood, palpitations, hypervigilance) were typical of stimulant administration (). Notably, the TEAE incidences of euphoric mood, palpitations, dry mouth, and hyperhidrosis following ER d-MPH HCl were 3.2-fold, 2.5-fold, 5.8-fold, and 4.8-fold the respective incidences for 240 mg SDX Cl. The incidence of euphoric mood following phentermine was also 3.6 times the incidence for 240 mg SDX Cl.
Table 3. Treatment-emergent adverse events reported by >20% of subjects (for any treatment).
Similar to the oral study, IN administration of SDX Cl and d-MPH HCl produced a profile of TEAEs that was consistent with stimulant administration, although the incidence rate was markedly greater for d-MPH HCl (). For example, TEAEs incidences of euphoric mood, hypervigilance, and palpitations following IN d-MPH HCl were 3.2-fold, 3.5-fold, and 5.6-fold the respective rates for IN SDX Cl. Of note, nasal discomfort and nasal congestion were more common following insufflation of SDX Cl vs. d-MPH HCl. One subject discontinued the study due to anxiety and claustrophobia after IN administration of SDX Cl. Mean values for blood pressure (systolic and diastolic) and heart rate were elevated following dosing with d-MPH HCl. Additionally, marked increases in mean heart rate (67.5 bpm) from mean baseline were observed from 0.5 h (96.1 bpm) to 6 h (95.0 bpm), followed by a gradual decline toward baseline values over the next 30 h. In contrast, for IN SDX Cl, there were only modest, transient increases from mean baseline pulse rate (from 66.8 to 76.2 bpm), which occurred 0.25 h after insufflation, followed by a rapid return to baseline values.
For the IV administration study, the incidences of stimulant-like TEAEs were again higher following d-MPH HCl compared to SDX Cl (). For example, TEAE incidence rates of euphoric mood and hypervigilance following IV d-MPH HCl were 4.4-fold and 2.6-fold the respective incidences for IV SDX Cl. One subject reported a serious AE of multiple traumatic injuries secondary to a motor vehicle accident while driving under the influence of alcohol approximately 4 days following administration of SDX Cl, though the event was considered unrelated to study drug. Heart rate and mean systolic and diastolic blood pressure increased following administration of d-MPH HCl, typically peaking at 15 min post-dose. Mean heart rate values were above the normal range (40–100 bpm) at 15 min (118.3 bpm) and 0.5 h (104.1 bpm) post-dose for d-MPH HCl. In contrast, mean heart rate values for SDX Cl commonly decreased or showed only small elevations at 15 min post-dose.
There were no clinically significant clinical laboratory values, ECG results, or out-of-range vital sign values following oral, IN, or IV SDX Cl administration.
Discussion
The objective of these studies was to evaluate the human abuse potential of SDX, a novel prodrug of d-MPH, by routes of administration (oral, IN, and IV) commonly reported by nonmedical users of prescription stimulants. For all 3 routes of administration, SDX Cl was associated with lower abuse potential than d-MPH HCl comparators as evidenced by statistically significantly lower abuse-related effects in PD assessments (including the primary endpoint, Drug Liking Emax) and fewer stimulant-like AEs (e.g. euphoric mood, hypervigilance, palpitations). PK data were concordant with PD findings in that d-MPH exposure was markedly lower following oral, IN, and IV administration of SDX Cl relative to d-MPH HCl.
In the oral administration study, Drug Liking Emax was statistically significantly higher for 80 mg ER d-MPH HCl than 120 mg and 240 mg SDX Cl by a margin of more than 10 points. Other abuse-related endpoints (including the retrospective assessments, Take Drug Again and Overall Drug Liking) were consistent with the reported Drug Liking Emax differences, demonstrating statistically significantly higher scores for ER d-MPH HCl. SDX Cl was not non-inferior to placebo although Drug Liking Emax scores did not exceed 65 for either dose. From a PK perspective, the blunted d-MPH Cmax and longer Tmax for SDX Cl relative to ER d-MPH HCl are also supportive of a lower potential for abuse, as previously demonstrated in the literatureCitation21–26. In addition, Drug Liking Emax for the secondary positive control, phentermine (C-IV drug, DEA), was significantly higher than for the 120-mg dose of SDX Cl by a margin of no less than 10 points, but not the 240-mg dose (p = .066) as tested with the same 10-point margin. Other secondary endpoints, including Take Drug Again, Overall Drug Liking, and Feeling High, were all markedly and statistically significantly higher for phentermine vs. both doses of SDX Cl. These findings indicate that orally administered SDX Cl produced abuse-related effects that are equivalent to or lower than for a C-IV stimulant. Overall, the abuse-related effects of SDX Cl appear to be limited, most likely due to lower and delayed exposure to d-MPH resulting from slower absorption in the digestive tract (the d-MPH absorption rate is limited by the release rate from SDX).
In the IN and IV studies, Drug Liking Emax and all other endpoints (except Bad Effects after IN administration) were statistically significantly higher for d-MPH HCl relative to SDX Cl. When compared to placebo, SDX Cl measures were statistically significantly non-inferior (Drug Liking Emax) or similar (other endpoints) following IV but not IN administration. In addition, SDX Cl was rated as statistically significantly more difficult to snort than d-MPH HCl and placebo in the IN study, a factor that may also diminish the reinforcing effects of IN-administered SDX Cl. PK data indicated limited release of d-MPH from SDX, with overall exposure to d-MPH following IN and IV administration of SDX Cl being reduced to <25% and <12%, respectively, when compared to d-MPH HCl. These findings are comparable to PK data in rats and dogs and are consistent with in vitro studies demonstrating the stability of SDX in blood, plasma, and liver S9 fractionsCitation29. Mechanistically, conversion of inactive SDX to active d-MPH occurs primarily in the lower intestinal tract, whereupon d-MPH is absorbed into systemic circulationCitation28,Citation35. The requirement for SDX to reach the lower intestinal tract to achieve efficient conversion to d-MPH remains a critical feature that, based on the present findings, may render non-oral routes of administration less appealing for abusers.
In all three studies, incidences of AEs typical of high doses of stimulants (e.g. euphoric mood, hypervigilance, palpitations) were higher for d-MPH HCl comparators relative to SDX Cl. Importantly, no new or unusual AEs were identified for SDX Cl. The lack of any unique AEs is supported by the findings that SDX is pharmacologically inactive and that no novel systemically available metabolites are formed following SDX Cl administration when compared to d-MPH HClCitation28,Citation29. Indeed, any pharmacological effects of SDX, including abuse-related effects, are due to its gradual conversion to active d-MPH. Sustained increases in heart rate were observed following IN and IV administration of d-MPH HCl, at times above the normal range (40–100 bpm), whereas only modest, transient elevations in heart rate were observed following IN and IV SDX Cl. Thus, even at doses considered to be “high,” the cardiovascular effects after SDX Cl administration were minimal.
Based primarily on the findings from the current series of studies, SDX has been classified as a Schedule IV controlled substance by the DEA, demonstrating an abuse potential lower than that of d-MPH but similar to that of phentermine, also a Schedule IV drugCitation36. Experiments evaluating the stability of SDX under various hydrolytic conditions were also pertinent to the scheduling status. For example, in vitro tampering studies showed that SDX was stable at pH 1–8 and hydrolyzed nearly quantitatively to ritalinic acid at higher pH without significant amounts of d-MPH remaining (<10%) under any conditionCitation37. In contrast, 96% of the label claim of crushed, extended-release d-MPH comparator product could be extracted from its formulation with tap water in 5 min, resulting in immediate-release d-MPH freely available for abuse.
While the abuse potential of d-MPH has been well-documented in epidemiological studies, very little data exist on its IN and IV abuse potential in the human clinical laboratory. In the only other study of IN administration, racemic d,l-MPH HCl administered up to 30 mg intranasally (containing 15 mg d-MPH HCl) produced dose-dependent reinforcing effects, positive subjective effects, and other characteristic stimulant effectsCitation38. To our knowledge, the current study is the first to examine the abuse-related PD effects of IV d-MPH HCl (or d,l-MPH ) in the clinical laboratory setting. The robust abuse-related effects of IV d-MPH HCl in this study are consonant with the non-negligible rates of IV abuse of prescription stimulants noted in the literatureCitation15,Citation16.
A strength of these studies is the rigorous experimental design, including evaluation of three routes of administration, collection of extensive PD, PK, and safety data in the same subjects, and the incorporation of pre-specified margins in the statistical analyses that were selected to ensure that statistically significant differences would also be clinically meaningful. These studies also have limitations. First, although the objective of these studies was to evaluate the human abuse potential of SDX, the findings cannot be generalized to products such as SDX/d-MPH that contain another drug substance that is controlled under a higher schedule (i.e. Schedule III or II). As noted above, however, SDX as a single-entity product is under investigation for treating other CNS-related disorders. Second, because the real-world abuse potential of SDX relative to d-MPH cannot be definitively ascertained in a controlled clinical setting, these findings may not be generalizable to other settings. Furthermore, assessments consisted only of subjective measures and not direct measures of reinforcing efficacy (e.g. drug self-administration), endpoints that under some experimental conditions can diverge (see, e.g. Comer et al.Citation39). However, the overall design, endpoints, and data analysis were consistent with regulatory guidance for assessing abuse potential of drugsCitation30.
Conclusion
Taken together, these findings suggest that SDX serves as a prodrug of d-MPH that has lower potential for abuse than d-MPH when administered via the most common routes of stimulant abuse. All routes of administration tested with SDX Cl yielded plasma d-MPH concentrations, abuse-related PD effects, and stimulant-like adverse effects that were statistically significantly lower than for d-MPH HCl itself. Under some conditions (IV administration), SDX Cl was non-inferior to placebo for Drug Liking Emax and statistically similar to placebo for most other abuse-related endpoints. When compared to oral administration of the Schedule IV drug, phentermine, oral SDX Cl demonstrated similar or lower levels of Drug Liking. The results of these studies support the designation of SDX as a C-IV controlled substance.
Transparency
Declaration of funding
This work was funded by KemPharm, Inc. Funding for editorial assistance in the form of proofreading, copyediting, and fact-checking was provided by Corium, Inc., and performed by Simpson Healthcare and Ashfield MedComms.
Declaration of financial/other relationships
Dr Shram is an employee of Altreos Research Partners, Inc. Dr Setnik is an employee of Altasciences; she was at INC Research Toronto, Inc. (part of Syneos Health) at the time the study was conducted. Dr Webster has received consultation, advisory board, and travel fees from Charleston Laboratories, Depomed, Egalet, Insys Therapeutics, Mallinckrodt Pharmaceuticals, Pfizer, Teva, and Trevena; consultation and travel fees from Alcobra, Bonti, Daiichi Sankyo, Elysium Health, Indivior, KemPharm, Pain Therapeutics, Pernix Therapeutics, and Shionogi; advisory board and travel fees from BioDelivery Sciences International, Inc., Ensysce Biosciences, and Inspirion Pharmaceuticals; travel fees from Cara Therapeutics; and consultation fees from Jefferies, Merck, Trevi Therapeutics, Vallon Pharmaceuticals, and Vector Pharma. Dr Guenther is a full-time employee and shareholder of KemPharm, Inc. Dr Mickle is a full-time employee and shareholder of KemPharm, Inc. Dr Braeckman is a full-time employee and shareholder of KemPharm, Inc. Dr Kanski is a full-time employee and shareholder of KemPharm, Inc. Ms Martin is a full-time employee and shareholder of KemPharm, Inc. Dr Kelsh is an employee of Altasciences. Dr Vince is an employee of Dr. Vince Clinical Research. Dr Vince was an employee of Altasciences at the time the study was conducted. Dr Barrett is a full-time employee and shareholder of KemPharm, Inc. A reviewer on this manuscript has disclosed that they are a consultant and speaker for Corium; consultant, speaker and researcher for Supernus; consultant, speaker and researcher for Takeda; consultant and speaker for Ironshore; researcher for Akili, researcher for LUMOS and researcher for Otsuka. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors listed below were actively involved in data interpretation, manuscript writing, and editing, and approved the final version of the manuscript. In addition, Drs. Shram, Setnik, Webster, Guenther, Mickle, Braeckman, and Barrett were involved in study concept and design.
Supplemental Material
Download MS Word (14.1 KB)Acknowledgements
The authors acknowledge Karin Keller (Worldwide Clinical Trials, San Antonio, TX) for assistance in developing bioanalytical assays for SDX. Editorial assistance was provided by Simpson Healthcare and Ashfield MedComms, funded by Corium, Inc.
Notes
i Nonmedical use is defined as the use of a prescription stimulant without a prescription, or in a way other than prescribed by a physician.
References
- Wolraich ML, Hagan JF, Jr, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4):e20192528. Erratum in: Pediatrics. 2020;145(3).
- Cortese S, D'Acunto G, Konofal E, et al. New formulations of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: pharmacokinetics, efficacy, and tolerability. CNS Drugs. 2017;31(2):149–160.
- Shearer J. Stimulant use and nonmedical use [Internet]. Silver Spring (MD): FDA; 2020. [cited 2021 Apr 12]. Available from: https://www.fda.gov/media/142732/download.
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 national survey on drug use and health (HHS publication no. PEP19-5068, NSDUH series H-54) [Internet]. Rockville (MD); US Department of Health and Human Services; 2019. [cited 2021 Jun 7]. Available from: https://www.samhsa.gov/data
- Agaku I, Odani S, Nelson J. Medical use and misuse of psychoactive prescription medications among US youth and young adults. Fam Med Com Health. 2021;9(1):e000374.
- McCabe SE, Veliz P, Wilens TE, et al. Adolescents' prescription stimulant use and adult functional outcomes: a national prospective study. J Am Acad Child Adolesc Psychiatry. 2017;56(3):226–233.
- Morton WA, Stockton GG. Methylphenidate abuse and psychiatric side effects. Prim Care Companion J Clin Psychiatry. 2000;2(5):159–164.
- Trenque T, Herlem E, Taam MA, et al. Methylphenidate off-label use and safety. Springerplus. 2014;3:286.
- Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202–207.
- Faraone SV, Rostain AL, Montano CB, et al. Systematic review: nonmedical use of prescription stimulants: risk factors, outcomes, and risk reduction strategies. J Am Acad Child Adolesc Psychiatry. 2020;59(1):100–112.
- National Institute on Drug Abuse. Prescription stimulants drugfacts [Internet]. Bethesda (MD): National Institutes of Health; 2018. [cited 2021 Nov 18]. Available from: https://www.drugabuse.gov/publications/drugfacts/prescription-stimulants.
- Faraone SV, Hess J, Wilens T. Prevalence and consequences of the nonmedical use of amphetamine among persons calling poison control centers. J Atten Disord. 2019;23(11):1219–1228.
- Cassidy TA, McNaughton EC, Varughese S, et al. Nonmedical use of prescription ADHD stimulant medications among adults in a substance abuse treatment population: early findings from the NAVIPPRO surveillance system. J Atten Disord. 2015;19(4):275–283.
- Cassidy TA, Varughese S, Russo L, et al. Nonmedical use and diversion of ADHD stimulants among U.S. adults ages 18-49: a national internet survey. J Atten Disord. 2015;19(7):630–640.
- Vosburg SK, Faraone SV, Newcorn JH, et al. Prescription stimulant nonmedical use among adolescents evaluated for substance use disorder treatment (CHAT™). J Atten Disord. 2021;25(13):1859–1870.
- Burtner J, Behling M, Cassidy T, et al. Prevalence of nonmedical use and routes of administration for prescription stimulant medications among adults in a substance abuse treatment population. J Addict Dis. 2018;37(1-2):34–45.
- Teter CJ, McCabe SE, LaGrange K, et al. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26(10):1501–1510.
- Vosburg SK, Robbins RS, Antshel KM, et al. Characterizing pathways of non-oral prescription stimulant non-medical use among adults recruited from reddit. Front Psychiatry. 2020;11:631792.
- Abreu ME, Bigelow GE, Fleisher L, et al. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology. 2001;154(1):76–84.
- Farré M, Camí J. Pharmacokinetic considerations in abuse liability evaluation. Br J Addict. 1991;86(12):1601–1606.
- Lile JA, Babalonis S, Emurian C, et al. Comparison of the behavioral and cardiovascular effects of intranasal and oral d-amphetamine in healthy human subjects. J Clin Pharmacol. 2011;51(6):888–898.
- Parasrampuria DA, Schoedel KA, Schuller R, et al. Do formulation differences alter abuse liability of methylphenidate? A placebo-controlled, randomized, double-blind, crossover study in recreational drug users. J Clin Psychopharmacol. 2007;27(5):459–467.
- Parasrampuria DA, Schoedel KA, Schuller R, et al. Assessment of pharmacokinetics and pharmacodynamic effects related to abuse potential of a unique oral osmotic-controlled extended-release methylphenidate formulation in humans. J Clin Pharmacol. 2007;47(12):1476–1488.
- Spencer TJ, Biederman J, Ciccone PE, et al. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry. 2006;163(3):387–395.
- Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 2003;27(7):615–621.
- Volkow ND, Fowler JS, Wang GJ, et al. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9(6):557–569.
- Srinivas NR, Hubbard JW, Quinn D, et al. Extensive and enantioselective presystemic metabolism of DL-threo-methylphenidate in humans. Prog Neuro Psychopharmacol Biol. 1991;15(2):213–220.
- Center for Drug Evaluation and Research. Drug approval package: AZSTARYS (serdexmethylphenidate and dexmethylphenidate). Silver Spring (MD): FDA; 2021. [cited 2021 Nov 18]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/212994Orig1s000TOC.cfm.
- Shram MJ, Guenther S, Mickle TC, et al. Assessment of the intravenous abuse potential of serdexmethylphenidate (SDX), a novel, investigational prodrug of D-methylphenidate: evidence from nonclinical and clinical studies. Poster presented at: 57th annual meeting of the american college of neuropsychopharmacology (ACNP); 2018 Dec 9–13; Hollywood, FL.
- Guidance document: assessment of abuse potential of drugs, January 2017 [Internet]. Silver Spring (MD): FDA; 2020. [cited 2021 Nov 18]. Available from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessment-abuse-potential-drugs.
- Braeckman R, Guenther S, Mickle TC, et al. Dose-finding study of abuse-related effects of intranasal d-methylphenidate in recreational stimulant abusers. Poster presented at: Annual meeting of children and adults with ADHD (CHADD); 2018 Nov 8–11; St. Louis, MO.
- Martin WR, Sloan JW, Sapira JD, et al. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12(2):245–258.
- Schoedel KA, Addy C, Chakraborty B, et al. Human abuse potential and cognitive effects of taranabant, a cannabinoid 1 receptor inverse agonist: a randomized, double-blind, placebo- and active-controlled, crossover study in recreational polydrug users. J Clin Psychopharmacol. 2012;32(4):492–502.
- Chen L, Bonson KR. An equivalence test for the comparison between a test drug and placebo in human abuse potential studies. J Biopharm Stat. 2013;23(2):294–306.
- Center for Drug Evaluation and Research. Drug approval package: CONCERTA (methylphenidate HCl) extended-release tablets. Silver Spring (MD): FDA; 2000. [updated 2001 June 18; cited 2021 Nov 18]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21-121_Concerta.cfm.
- Drug Enforcement Administration. 21 CFR Part 1308, schedules of controlled substances: placement of serdexmethylphenidate in schedule IV. Washington (DC): US Department of Justice; 2021. [cited 2021 Nov 18]. Available from: https://www.deadiversion.usdoj.gov/fed_regs/rules/2021/fr0507_3.htm.
- Guenther S, Bera B, Lauderback C, et al. In vitro tampering assessment of serdexmethylphenidate, a novel prodrug of d-methylphenidate. Poster presented at: The American Professional Society of ADHD and Related Disorders (APSARD) Annual Conference; 2021 Jan 15–17.
- Stoops WW, Glaser PE, Rush CR. Reinforcing, subject-rated, and physiological effects of intranasal methylphenidate in humans: a dose-response analysis. Drug Alcohol Depend. 2003;71(2):179–186.
- Comer SD, Metz VE, Cooper ZD, et al. Comparison of a drug versus money and drug versus drug self-administration choice procedure with oxycodone and morphine in opioid addicts. Behav Pharmacol. 2013;24(5–6):504–516.