?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To assess how the use of calcipotriol and betamethasone dipropionate (Cal/BDP) cream impacted efficacy, patients’ quality of life (QoL), and treatment satisfaction versus Cal/BDP foam.
Methods
Data from clinical trials of Cal/BDP cream and foam were analyzed, by applying the common anchor Cal/BDP gel. Efficacy was assessed by Physician Global Assessment (PGA) treatment success and ≥75% reduction in Psoriasis Area and Severity Index (PASI75 response); QoL by Dermatology Life Quality Index (DLQI); treatment satisfaction by Psoriasis Treatment Convenience Scale (PTCS) and Topical Product Usability Questionnaire (TPUQ).
Results
Treatment with Cal/BDP cream was on par with foam on PGA treatment success (risk ratio (RR) for Cal/BDP cream versus foam: 0.80; 95%CI: 0.56, 1.14; p = .21) and PASI75 response (RR for Cal/BDP cream vs. foam: 0.85; 95%CI: 0.64, 1.13; p = .27) when assessed at the treatment duration of 8 weeks for Cal/BDP cream and 4 weeks for Cal/BDP foam. Treatment with Cal/BDP cream was associated with significantly greater treatment satisfaction versus foam on the domains: overall treatment satisfaction (p = .01), “ease of application” (p < .001), “lack of greasiness” (p < .001), “moisturizing effect” (p = .01), and almost significantly greater improvement on the domain “easily incorporated into daily routine” (p = .07). Furthermore, there was a trend for greater DLQI improvement with cream versus foam when assessed at recommended treatment duration [mean difference (MD) for Cal/BDP cream vs. foam: −1.00; 95%CI: −2.20, 0.20; p = .10].
Conclusions
Indirect comparison analyses showed that Cal/BDP cream significantly improves treatment satisfaction and tends to improve QoL versus foam. Cal/BDP cream is on par with foam on efficacy.
Introduction
Psoriasis is a chronic immune-mediated inflammatory skin disease characterized by the formation of erythematous, scaly plaques in various regions of the body.Citation1 The prevalence of psoriasis in Western Europe varies from 1.1% to 3.5% with an average of ∼2%Citation2. According to a Global Burden of Disease 2019 study, there were around 40.8 million prevalent cases of psoriasis worldwide in 2019Citation3. The negative impact of psoriasis on patients’ quality of life (QoL) can be considerableCitation1. Psoriasis poses a significant problem for affected subjects in everyday life, and studies have shown that patients with psoriasis are emotionally and physically impaired by their disease compared to what is seen with cancer, heart disease, rheumatoid arthritis, diabetes, or depressionCitation4.
There exists a wide range of therapeutic options for the treatment of psoriasis, e.g. various topical therapies, phototherapies, and systemic treatments. Besides current treatment options, several new molecules and potential new therapeutic options are under investigation and pending approval. The choice of treatment typically depends on several factors, such as the age of the patient, disease severity, location, comorbidities, experience with psoriasis treatment, the cost of treatment, treatment satisfaction and adherence, the administration form, efficacy and safety of the therapeutic options, and impact on health-related quality of lifeCitation5–11.
Topical treatments are an essential part of the therapeutic options for psoriasisCitation7,Citation9,Citation12,Citation13. Fixed combinations of calcipotriol and betamethasone dipropionate (Cal/BDP) are a cornerstone of treatment and are found to be efficacious and safe in the treatment of plaque psoriasis. Cal/BDP cream (WynzoraFootnotei) is a new topical treatment for plaque psoriasis which is based on a new proprietary PAD Technology and is recommended for up to 8 weeks of treatmentCitation14. Cal/BDP cream received US FDA approval in July 2020 and is approved in Europe through the decentralized procedure. The concentrations of Cal and BDP in Cal/BDP cream are identical to the concentrations in other Cal/BDP products (Cal 50 µg/g; BDP 0.64 mg/g).
The effect of a treatment is directly dependent on the patients’ adherence to the therapy, which may be one of the largest barriers to treatment successCitation15. The results of several studies on medication adherence in psoriasis show that 39–73% of patients do not use medication as prescribedCitation16. Important factors for adherence are how practical and pleasant the formulation is, for instance, how easy it is to apply, how quickly it is absorbed into the skin, and whether it is pleasant to use. The treatment vehicle is, therefore, an important consideration in topical therapies. Cosmetic acceptability and ease of use affect treatment preference and adherence, with patients generally preferring less messy treatments that are easy to applyCitation16. In a European survey of non-adherence to topical psoriasis treatment, 73% of patients did not adhere to their topical treatment, with product greasiness as the main reason for non-adherenceCitation17.
Cal/BDP cream has demonstrated statistically significantly greater efficacy and improved QoL in comparison with Cal/BDP gel (DaivobetFootnoteii gel) and the cream vehicle in two randomized phase 3 clinical trials (NCT03802344 and NCT03308799)Citation14,Citation18,Citation19. Efficacy was assessed by the Physician Global Assessment (PGA) scale as a PGA score of 0 (clear) or 1 (almost clear) with a minimum two-point improvement from baseline and by the Psoriasis Area and Severity Index as a reduction of at least 75% from baseline (PASI75 response), while QoL was assessed by improvement on the Dermatology Life Quality Index (DLQI). Moreover, the treatment satisfaction score in Cal/BDP cream-treated patients, using the Psoriasis Treatment Convenience Scale (PTCS), was superior compared with Cal/BDP gel-treated patientsCitation14,Citation18,Citation19.
Similarly, Cal/BDP foam (EnstilarFootnoteiii) has shown significantly greater efficacy and improved QoL in comparison with Cal/BDP gel in the randomized phase 3 clinical trial PSO-ABLECitation20–22. The primary objective was to compare the efficacy of Cal/BD foam at week 4 to that of Cal/BD gel at week 8. Moreover, in the randomized phase 3b clinical trial PSO-INSIGHTFUL, treatment satisfaction scores, assessed by the Topical Product Usability Questionnaire (TPUQ), were high for both Cal/BDP foam and gel with significant differences in favor of Cal/BDP gel for many of the itemsCitation23. Treatment satisfaction with Cal/BDP foam has also been assessed in a real-life settingCitation24.
To date, no head-to-head clinical trials or published analyses have been conducted comparing Cal/BDP cream with Cal/BDP foam. An indirect treatment comparison between Cal/BDP cream and foam is important to real-world decision-making, as new topical treatments, such as Cal/BDP cream have advanced the psoriasis treatment landscape. It is common to undertake indirect comparison analyses, especially when relevant head-to-head data do not exist. Various health technology assessment (HTA) agencies, like in the UK, France, and Germany, accept indirect comparison analysesCitation25–28, and EUNETHTA has also provided clear guidance for using various indirect comparison methodsCitation29.
Thus, the purpose of this analysis was to assess how the use of Cal/BDP cream impacted efficacy, patients’ QoL, and treatment satisfaction compared with Cal/BDP foam in an indirect treatment comparison analysis among patients with psoriasis vulgaris. In accordance with the recommended treatment durations for Cal/BDP cream and foam, analyses comparing Cal/BDP cream at week 8 with Cal/BDP foam at week 4 were conducted as well as separate analyses for each available week (1, 4, 6, and 8), e.g. comparing 4 weeks of Cal/BDP cream treatment with 4 weeks of Cal/BDP foam treatment (with common comparator Cal/BDP gel, also assessed at 4 weeks).
Methods
Selection of studies and outcomes of interest
For Cal/BDP cream, two randomized, controlled clinical phase 3 studies were conducted in Europe (NCT03802344)Citation18 and the US (NCT03308799)Citation14,Citation19 with a Cal/BDP cream and gel arm were includedCitation30.
For Cal/BDP foam, a systematic review of the literature was conducted to identify studies eligible for the indirect comparison analyses. The search was conducted in the MEDLINE database through the PubMed platform in July 2021. The search string was constructed of three clusters including search terms relating to the drug, the outcomes of interest, and the disease (). Title and abstracts of all identified articles were reviewed for eligibility. Eligible articles proceeded to full-text review, and articles failing to meet the inclusion criteria were excluded. Articles were limited to English-language publications. References were also excluded if they did not report results on at least one of the defined outcomes of interest: PGA treatment success, PASI75 response, DLQI improvement, and a treatment satisfaction measure comparable with the PTCS applied in the Cal/BDP cream studies. In addition, references were excluded if they did not compare Cal/BDP foam with Cal/BDP gel or if the comparisons only included subgroups of patients.
Table 1. Literature search string in PubMed (July 2021).
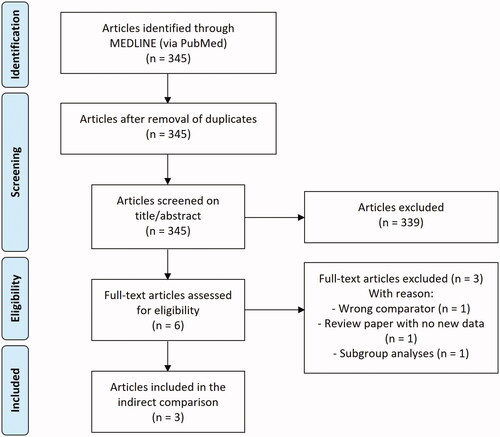
Out of 345 screened articles, two studies (published in three articles) met the inclusion criteria, referred to as the PSO-ABLE study and the PSO-INSIGHTFUL studyCitation20,Citation22,Citation23. The PSO-ABLE study reported PGA treatment success, PASI75 response, and DLQI improvement, and was designed as a randomized, vehicle-controlled, investigator-blinded clinical phase 3 study with a Cal/BDP foam and gel armCitation20,Citation22. Patients were randomized to Cal/BDP foam, Cal/BDP gel, foam vehicle, or gel vehicle for up to 12 weeks. The PSO-INSIGHTFUL study was a randomized, open-label, two-arm crossover phase 3b study with a Cal/BDP foam and gel arm and reported results on treatment satisfaction measured by the TPUQCitation23. Patients were randomized to Cal/BDP foam treatment for 1 week, followed by Cal/BDP gel treatment for 1 week, or vice versaCitation23. provides a PRISMA flow diagram showing the number of articles identified and the number of included and excluded articles.
The outcomes of interest for this analysis were PGA treatment success, PASI75 response, DLQI improvement, and treatment satisfaction. PGA treatment success, PASI75 response, and DLQI improvement were reported for Cal/BDP cream versus gel in the Cal/BDP cream studiesCitation14,Citation18,Citation19 and for Cal/BDP foam versus gel in the PSO-ABLE studyCitation22. Treatment satisfaction was measured with the PTCS for Cal/BDP cream versus gel in the Cal/BDP cream studiesCitation14,Citation18,Citation19 and with the TPUQ for Cal/BDP foam versus gel in the PSO-INSIGHTFUL studyCitation23. PTCS questions were matched with relevant TPUQ questions covering five treatment satisfaction domains: ease of application, not greasy (measured with two questions on the PTCS), felt moisturizing, easily incorporated into daily routine, and overall treatment satisfaction ().
Table 2. PTCS questions matched with TPUQ questions covering five treatment satisfaction domains.
Statistical analyses
Indirect comparison analyses between Cal/BDP cream and foam were conducted by applying the common comparator Cal/BDP gel for all available timepoints. Where possible, indirect comparisons were also performed at 8 weeks, as the recommended treatment duration is up to 8 weeks for Cal/BDP cream and 4 weeks for Cal/BDP foam.
For PGA treatment success and PASI75 response, which are binary outcomes, the risk ratios (RRs) for Cal/BDP cream versus gel were estimated. Likewise, the RRs for Cal/BDP foam versus gel was estimated. Bucher’s adjusted indirect comparison method was used to estimate RRs and absolute differences between Cal/BDP cream and foam. Bucher and co-authors suggested a simple method of adjusted indirect comparison. The indirect comparison of treatment A (Cal/BDP cream) and treatment B (Cal/BDP foam) is adjusted according to the results of their direct comparisons with a common intervention, treatment C (Cal/BDP gel)Citation31. The application of multiple treatment comparison has become increasingly common since first being adopted in the late 1990s, and Bucher’s method of adjusted indirect comparison is the most appropriate for the clinical start pattern network data we have available from this systematic literature reviewCitation29.
The RR of the adjusted indirect comparison of treatments A and B can be estimated as:
where
and
denote log RR of the study that compares treatment A with treatment C, and treatment B with treatment C, respectively. The absolute difference between treatments A and B can be estimated as:
For the continuous outcomes of DLQI improvement and treatment satisfaction, the mean difference (MD) between Cal/BDP cream and foam was estimated by the difference-in-differences method.
The Cal/BDP cream studies and the PSO-INSIGHTFUL study for Cal/BDP foam applied different scales for treatment satisfaction, PTCS and TPUQ, respectively. PTCS ranges from 1 to 10, and TPUQ ranges from −2 (very dissatisfied) to 2 (very satisfied), with higher scores indicating higher satisfaction. TPUQ mean and standard deviation values from the PSO-INSIGHTFUL study were converted to PTCS values, and PTCS mean and standard deviation values from the Cal/BDP cream studies were converted to TPUQ values. Both conversions were conducted by multiplying with a factor taking the different scale ranges into account.
In the indirect comparison between treatment A and treatment B, the MD can then be estimated as:
where
denotes the MD of the outcome of treatment A in the study that compares treatment A and treatment C, and
denotes the MD of the outcome of treatment B, in the study that compares treatment B and treatment C.
Results
Baseline demographics
A total of 1,093 patients from the two studies with Cal/BDP cream (pooled Cal/BDP cream: n = 551; pooled Cal/BDP gel: n = 542), 373 patients from the Cal/BDP foam PSO-ABLE study (Cal/BDP foam: n = 185; Cal/BDP gel: n = 188) and 212 patients from the Cal/BDP foam and gel PSO-INSIGHTFUL study were included in the indirect comparison analyses. Baseline demographics are presented in . Most of the characteristics were considered similar across the studies. The distribution of PGA in terms of mild, moderate, and severe disease varied between the studies; in the Cal/BDP cream studies, patients with “severe disease” were excluded, but fewer patients had a “mild disease” than in the PSO-ABLE and PSO-INSIGHTFUL studies. Duration of psoriasis, body surface area (BSA), and PASI at baseline also varied between the studies.
Table 3. Patient demographics and baseline characteristics.
Efficacy
In , we have provided the efficacy outcomes on PGA treatment success and PASI75 response from the two studies with Cal/BDP cream and from the PSO-ABLE study on Cal/BDP foamCitation20,Citation30. The percentage of subjects achieving PGA treatment success as well as PASI75 response at weeks 1, 4, 6, and 8 are provided. PGA treatment success and PASI75 response from the respective studies were used to undertake the indirect comparison of Cal/BDP cream versus Cal/BDP foam.
Table 4. PGA treatment success and PASI75 response per week.
Treatment with Cal/BDP cream was on par with Cal/BDP foam on PGA treatment success (RR for Cal/BDP cream vs. foam: 0.80; 95%CI: 0.56, 1.14; p = .21) and PASI75 response (RR for Cal/BDP cream vs. foam: 0.85; 95%CI: 0.64, 1.13; p = .27) when assessed at the treatment duration of 8 weeks for Cal/BDP cream and 4 weeks for Cal/BDP foam (). Likewise, treatment with Cal/BDP cream was on par with Cal/BDP foam on PGA treatment success and PASI75 response at weeks 1, 4, and 6. At week 8, Cal/BDP foam was significantly in favor compared with Cal/BDP cream on both PGA treatment success (RR for Cal/BDP cream vs. foam: 0.68; 95%CI: 0.48, 0.97; p = .03) and PASI75 response (RR for Cal/BDP cream vs. foam: 0.75; 95%CI: 0.57, 0.99; p = .04).
Table 5. Indirect comparison of PGA treatment success and PASI75 response between Cal/BDP cream and foam with the common comparator Cal/BDP gel.
Quality of life
In , we have provided the quality of life outcomes on DLQI improvement from the two studies with Cal/BDP cream and from the Cal/BDP foam PSO-ABLE studyCitation22,Citation30. Changes in DLQI from baseline at weeks 4 and 8 are provided. DLQI improvement from the respective studies was used to undertake the indirect comparison of Cal/BDP cream versus Cal/BDP foam.
Table 6. Change in DLQI from baseline—DLQI improvement.
Treatment with Cal/BDP cream was associated with a trend for greater DLQI improvement than Cal/BDP foam when assessed at the treatment duration of 8 weeks for Cal/BDP cream and 4 weeks for Cal/BDP foam (MD for Cal/BDP cream vs. foam: −1.00; 95%CI: −2.20, 0.20; p = .10) (). Cal/BDP cream was on par with Cal/BDP foam on DLQI improvement at week 4 (MD for Cal/BDP cream vs. foam: −0.20; 95%CI: −1.37, 0.97 p = .74) and week 8 (MD for Cal/BDP cream vs. foam: −0.80; 95%CI: −2.04, 0.44; p = .21).
Table 7. Indirect comparison of DLQI improvement between Cal/BDP cream and foam with the common comparator Cal/BDP gel.
Treatment satisfaction
Treatment satisfaction analyses at week 1 showed significant differences in favor of Cal/BDP cream compared with Cal/BDP foam on the treatment satisfaction domains “ease of application” (MD on PTCS for Cal/BDP cream vs. foam: 1.10; 95%CI: 0.61, 1.59; p < .001), “not greasy” (MD on PTCS for Cal/BDP cream vs. foam: 1.58; 95%CI: 0.88, 2.27; p < .001) and “felt moisturizing” (MD on PTCS for Cal/BDP cream vs. foam: 0.62; 95%CI: 0.14, 1.11; p = .01), and an almost significant difference in favor of Cal/BDP cream compared with Cal/BDP foam on the domain “easily incorporated into daily routine” (MD on PTCS for Cal/BDP cream vs. foam: 0.43; 95%CI: −0.03, 0.88; p = .07) (). For overall treatment satisfaction, Cal/BDP cream showed significantly greater improvement compared with Cal/BDP foam (MD on PTCS for Cal/BDP cream vs. foam: 0.62; 95%CI: 0.13, 1.12; p = .01).
Table 8. Indirect comparison of treatment satisfactiona in week 1 between Cal/BDP cream and foam with the common comparator Cal/BDP gel.
Discussion
Based upon a systematic literature review, efficacy, QoL, and treatment satisfaction were compared in indirect comparison analyses of Cal/BD cream and Cal/BD foam with the common comparator Cal/BDP gel via Bucher’s method of adjusted indirect comparison and the difference-in-differences method. This analysis showed that treatment with Cal/BDP cream was on par with Cal/BDP foam on PGA treatment success and PASI75 response when assessed at the treatment duration of 8 weeks for Cal/BDP cream and 4 weeks for Cal/BDP foam. Treatment with Cal/BDP cream was associated with significantly greater overall treatment satisfaction versus Cal/BDP foam. Moreover, treatment satisfaction analyses at week 1 showed significant differences in favor of Cal/BDP cream compared with Cal/BDP foam on the treatment satisfaction domains “ease of application”, “not greasy”, and “felt moisturizing”, and an almost significant difference in favor of Cal/BDP cream compared with Cal/BDP foam on the domain “easily incorporated into daily routine”. Furthermore, treatment with Cal/BDP cream showed a trend for greater improvement in DLQI compared with Cal/BDP foam when assessed at the recommended treatment duration.
Overall, the indirect comparison analyses showed that Cal/BDP cream tends to improve QoL and significantly improves overall treatment satisfaction compared with Cal/BDP foam. Moreover, Cal/BDP cream is on par with Cal/BDP foam on efficacy measured by PGA treatment success and PASI75 response. Considering that a major European survey found that greasiness was the main reason for non-adherenceCitation17, and that we found a significant difference in favor of Cal/BDP cream compared with foam on the treatment satisfaction domain “not greasy” in addition to improved overall treatment satisfaction, it is likely that we will see a greater treatment adherence to Cal/BDP cream in real life. The efficacy of a Cal/BDP treatment is directly dependent on the patient’s adherence to the therapy; therefore, we will probably see greater real-life efficacy with Cal/BDP cream compared to foam. This highlights the importance of taking the patient’s preferences into account when choosing the right treatment for the specific patient to ensure adherence and, as a result of this, efficacy.
Indirect treatment comparisons are important to real-world decision-making, as new topical treatments, such as Cal/BDP cream have advanced the psoriasis treatment landscape. Undertaking indirect treatment comparisons is an opportunity to compare treatment options that have not been studied in head-to-head clinical trials. In the absence of direct evidence, indirect treatment comparisons can be performed to evaluate the difference in, for example, efficacy, QoL, and treatment satisfaction, and are often used in health technology assessments to authorities in various countries—e.g. in the UK, Germany, France, Norway and DenmarkCitation25,Citation26,Citation28,Citation29,Citation32–34.
Limitations of the study
There are several limitations to this study that should be considered when interpreting the results. One limitation relates to the indication of heterogeneity across study populations. For example, patients with “severe disease” were excluded in the Cal/BDP cream studies, but not in the PSO-ABLE and PSO-INSIGHTFUL studies. However, fewer patients in the Cal/BDP cream studies had a “mild disease” than in the PSO-ABLE and PSO-INSIGHTFUL studies, and the study population in the Cal/BDP cream studies also seemed to have higher baseline BSA and PASI than the study populations in both the PSO-ABLE and PSO-INSIGHTFUL studies. These differences could be potential effect modifiers and add bias to the results.
Regarding the treatment duration, the recommended treatment duration of Cal/BDP cream is up to 8 weeks, but we compare 8 weeks of Cal/BDP cream and gel with 4 weeks of Cal/BDP foam according to what was measured in the clinical trials. While the compared treatment durations are in accordance with the recommended treatment duration for each Cal/BDP treatment and their respective regulatory approved regimens in the US and the EUCitation35–37, and thus their comparisons appear to align with current real-world clinical practice (for Cal/BDP gel and foam), there is inherent variability associated with different treatment durations that can influence the analyses. Therefore, separate analyses for each available week were also conducted, e.g. comparing 4 weeks of Cal/BDP cream treatment with 4 weeks of Cal/BDP foam treatment (with common comparator Cal/BDP gel, also assessed at 4 weeks).
The treatment satisfaction analysis is associated with several uncertainties. The PSO-INSIGHTFUL study was designed as a crossover study with 1 week of Cal/BDP foam treatment followed by 1 week of Cal/BDP gel treatment, or vice versa, while the Cal/BDP cream studies were randomized clinical trials with 8 weeks of follow-up. This implied that it was only possible to assess treatment satisfaction between Cal/BDP cream and foam after 1 week of treatment, which may be too short given the longer recommended treatment duration of both Cal/BDP cream and foam. Moreover, an obvious limitation is the comparison of two different questionnaires to assess treatment satisfaction: the PTCS and the TPUQ. The PTCS questionnaire was developed by MC2 Therapeutics, while the TPUQ was developed by LEO Pharma. Both questionnaires were used for the first time in the respective studies, and there is thus a lack of experience with the questionnaires. Furthermore, the six questions from the PTCS questionnaire were matched with five similar items from the 26-item TPUQ questionnaire. Others might argue for another matching of questions, which could have an impact on the results of the analysis. Lastly, the PTCS values were converted to TPUQ values, and vice versa, by multiplying with a factor taking the different scale ranges into account. There might be issues with this approach, and the results of the treatment satisfaction analysis should therefore be interpreted with caution.
Nevertheless, while this indirect comparison analysis cannot replace a head-to-head randomized clinical trial, it may be the best option to provide additional insights into the comparative effect, QoL, and treatment satisfaction of Cal/BDP cream versus foam.
Conclusions
The indirect comparison analyses showed that Cal/BDP cream significantly improves most domains and overall treatment satisfaction and tends to improve QoL compared with foam. Moreover, Cal/BDP cream is on par with Cal/BDP foam with regards to efficacy. Treatment satisfaction seems to depend on the Cal/BDP formulation, and the patient’s satisfaction with treatment might be an important factor for adherence and, as a result of this, efficacy. It is therefore important to take patients′ preferences into account when choosing the right treatment for the specific patient. Future direct and indirect comparisons, including population-adjusted indirect comparisons, will be important to provide additional evidence on the relative treatment effects of Cal/BDP cream versus foam.
Transparency
Declaration of funding
Sponsorship for this study was funded by Almirall, Barcelona, Spain and MC2 Therapeutics, Hørsholm, Denmark.
Declaration of financial/other relationships
Sandra Elkjaer Stallknecht and Anne Danø are employees of Incentive, a paid vendor to Almirall and MC2 Therapeutics. Johan Selmer and Paw Trebbien are employees of MC2 Therapeutics. Jordi Galván and Aina Pi-Blanque are employees of Almirall S.A. Adam Reich is an employee at the Department of Dermatology, University of Rzeszow, Poland and Anthony Bewley is an employee at the Whipps Cross University Hospital and The Royal London Hospital (Barts Health) NHS Trust, London, UK.
Adam Reich has worked as a consultant or speaker for AbbVie, Bioderma, Celgene, Chema Elektromet, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, LEO Pharma, Medac, Menlo Therapeutics, Novartis, Pierre-Fabre, Sandoz, Sanofi Aventis, and Trevi Therapeutics; participated as principal investigator or sub-investigator in clinical trials sponsored by AbbVie, AnaptysBio, Argenx, Corbus, Drug Delivery Solutions Ltd, Galderma, Genentech, Janssen, Kymab Limited, LEO Pharma, Menlo Therapeutics, MetrioPharm AG, MSD, Novartis, Pfizer, and Trevi Therapeutics.
Anthony Bewley has served as a consultant for Abbvie, Almirall, Celgene, Galderma, Janssen, LEO Pharma, Lilly, Novartis, Sanofi, and UCB; has participated in advisory boards for the Psoriasis Association, Changing Faces, ISG and NES; has received grants from EADV and travel grants from Janssen, LEO Pharma, and Almirall; has participated in guidelines committees of BAD; is working as an editor for the journal Practical Psychodermatology, as a treasurer for BAD, as a secretary for ESDaP and as a chair for APPGOS.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
All authors contributed to the study concept and manuscript design. Material preparation, data collection, and analysis were performed by Sandra Elkjaer Stallknecht and Anne Danø. All authors interpreted the data and provided critical feedback on the analyses and the manuscript. All authors read and approved the final manuscript for submission and are accountable for the accuracy and integrity of the manuscript.
Ethical approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Acknowledgements
No assistance in the preparation of this article is to be declared. This manuscript is based on work that has been previously presented as a poster at the European Academy of Dermatology and Venereology (EADV’s) 30th congress from 29 September until 2 October 2021 (poster number: P1382).
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available due to privacy reasons.
Notes
i Wynzora is a registered trademark of MC2 Therapeutics.
ii Daivobet is a registered trademark of LEO Pharma A/S.
iii Enstilar is a registered trademark of LEO Pharma A/S.
References
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. 2021;8:743180.
- Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 Pt 1):401–407.
- Caputo V, Strafella C, Cosio T, et al. Pharmacogenomics: an update on biologics and small-molecule drugs in the treatment of psoriasis. Genes. 2021;12(9):1398.
- Campione E, Cosio T, Di Prete M, et al. Experimental pharmacological management of psoriasis. J Exp Pharmacol. 2021;13:725–737.
- National Institute for Health and Care Excellence (NICE). Psoriasis: assessment and management. Clinical Guideline; 2017.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445–1486.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137–174.
- Nast A, Boehncke WH, Mrowietz U, et al. German S3-guidelines on the treatment of psoriasis vulgaris (short version). Arch Dermatol Res. 2012;304(2):87–113.
- Stein Gold L, Green LJ, Dhawan S, et al. A phase 3, randomized trial demonstrating the improved efficacy and patient acceptability of fixed dose calcipotriene and betamethasone dipropionate cream. J Drugs Dermatol. 2021;20(4):420–425.
- Carroll CL, Feldman SR, Camacho FT, et al. Better medication adherence results in greater improvement in severity of psoriasis. Br J Dermatol. 2004;151(4):895–897.
- Bewley A, Page B. Maximizing patient adherence for optimal outcomes in psoriasis. J Eur Acad Dermatol Venereol. 2011;25(Suppl 4):9–14.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19 Suppl 3:2–6.
- NCT03802344 – a randomised, multicentre, investigator-blind, parallel-group trial to evaluate the efficacy and safety of MC2-01 cream compared to vehicle and active comparator in subjects with mild-to-moderate psoriasis vulgaris [Internet]. [cited 2021 Jul 6]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03802344?term=NCT03802344&draw=2&rank=1
- NCT03308799 – a randomised, multicentre, investigator-blind, parallel-group trial to evaluate the efficacy and safety of MC2-01 cream compared to vehicle and active comparator in subjects with mild-to-moderate psoriasis vulgaris [Internet]. [cited 2021 Jul 6]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03308799?term=NCT03308799&draw=2&rank=1
- Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31(1):119–126.
- Paul C, Leonardi C, Menter A, et al. Calcipotriol plus betamethasone dipropionate aerosol foam in patients with moderate-to-severe psoriasis: sub-group analysis of the PSO-ABLE study. Am J Clin Dermatol. 2017;18(3):405–411.
- Griffiths CE, Stein Gold L, Cambazard F, et al. Greater improvement in quality of life outcomes in patients using fixed-combination calcipotriol plus betamethasone dipropionate aerosol foam versus gel: results from the PSO-ABLE study. Eur J Dermatol. 2018;28(3):356–363.
- Hong C-H, Papp KA, Lophaven KW, et al. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31(11):1876–1883.
- Campanati A, Atzori L, Potenza C, et al. Patient satisfaction with calcipotriol/betamethasone dipropionate cutaneous foam for the treatment of plaque psoriasis: the LION real‐life multicenter prospective observational cohort study. Dermatol Ther. 2021;34(5):34.
- Lebioda A, Gasche D, Dippel F-W, et al. Relevance of indirect comparisons in the German early benefit assessment and in comparison to HTA processes in England, France and Scotland. Health Econ Rev. 2014;4(1):31.
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. General Methods [Internet]; 2020 [cited 2021 Jul 8]. Available from: https://www.iqwig.de/methoden/general-methods_version-6-0.pdf?rev=194070
- Haute Autorité de Santé. Indirect comparisons – methods and validity [Internet]. Saint-Denis La Plaine CEDEX; 2009. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2011-02/summary_report__indirect_comparisons_methods_and_validity_january_2011_2.pdf
- National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal 2013. Process and methods [Internet]; 2013 [cited 2021 Jul 8]. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781
- European Network for Health Technology Assessments (EUnetHTA). Guideline Comparators & comparisons: direct and indirect comparisons [Internet]; 2015 [cited 2021 Jul 8]. Available from: https://www.eunethta.eu/wp-content/uploads/2018/01/Comparators-Comparisons-Direct-and-indirect-comparisons_Amended-JA1-Guideline_Final-Nov-2015.pdf
- Pinter A, Green L, Selmer J, et al. A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis. J Eur Acad Dermatol Venereol. 2022;36(2):228–236.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
- Phillippo D, Ades T, Dias S, et al. NICE DSU Technical Support Document 18: methods for population-adjusted indirect comparisons in submissions to NICE; 2016. [cited 2021 Jul 8]. Available from: https://research-information.bris.ac.uk/en/publications/nice-dsu-technical-support-document-18-methods-for-population-adj
- Danish Medicines Council. The Danish Medicines Council methods guide for assessing new pharmaceuticals [Internet]; 2021 [cited 2021 Jul 8]. Available from: https://medicinraadet.dk/media/wq0dxny2/the_danish_medicines_council_methods_guide_for_assessing_new_pharmaceuticals_version_1-2_adlegacy.pdf
- Statens Legemiddelverk. Guidelines for the submission of documentation for single technology assessment (STA) of pharmaceuticals [Internet]; 2020 [cited 2021 Jul 8]. Available from: https://legemiddelverket.no/Documents/English/Public%20funding%20and%20pricing/Documentation%20for%20STA/Guidelines%2020.05.2020.pdf
- U.S. Food and Drug Administration. Full prescribing information for Wynzora® (calcipotriene and betamethasone dipropionate cream); 2020.
- U.S. Food and Drug Administration. Full prescribing information for Enstilar® (calcipotriene and betamethasone dipropionate foam); 2006.
- U.S. Food and Drug Administration. Full prescribing information for Taclonex® (calcipotriene and betamethasone dipropionate topical suspension); 2006.
- NCT02310646 – a clinical trial gathering insight of patient reported factors that influence preference following once daily topical treatment with LEO 90100 aerosol foam and Daivobet® gel in subjects with psoriasis vulgaris [Internet] [cited 2021 Jul 8]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02310646?term=NCT02310646&draw=2&rank=1