Abstract
Background
This systematic literature review examines the current immune checkpoint inhibitors treatment paradigms, treatment gaps and unmet needs for treating SCLC with respect to efficacy, safety, health related quality of life (HRQoL) and cost-effectiveness.
Methods
A search strategy was developed and executed using the National Library of Medicine bibliographic database (PubMed), Cochrane Library, Embase and Google Scholar. Data regarding efficacy, safety, cost-effectiveness and HRQoL were extracted and entered in a data extraction sheet created a priori.
Results
A total of 4961 patients were comprised in all the 12 studies combined. All the studies focus on extensive stage SCLC (ES-SCLC) and not limited stage SCLC (LS-SCLC). All studies used an ICI as the intervention arm and chemotherapy as the control arm. A statistically significant increase in overall survival (OS) and progression free survival (PFS) was observed when ICIs were added to chemotherapy, especially atezolizumab and durvalumab. ICIs in SCLC resulted in immune-related toxicities that have been well-documented in prior immunotherapy trials; their addition to cytotoxic chemotherapy did not worsen chemotherapy-related toxicities. Out of 12 studies, only 3 (25%) included measures to assess the impact of immunotherapy on SCLC patients’ HRQoL. Although domain level scores were limited, the addition of ICIs did not seem to worsen symptoms. Two studies conducted a cost-effectiveness analysis of the combination of atezolizumab plus chemotherapy vs. chemotherapy. The addition of atezolizumab to chemotherapy was not found to be cost-effective in either study.
Conclusion
Combining ICIs with chemotherapy enhanced OS and PFS as well as not worsening HRQoL. Among all ICIs, PD-L1 inhibitors showed better effectiveness. Future studies should focus on real-world settings and more clinical trials using ICIs for not only ES-SCLC but also LS-SCLC.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide with 1,761,007 deaths and 2,093,876 new cases reported in 2020Citation1. Approximately, 10–15% of all lung cancer cases is attributed to small cell lung cancer (SCLC) accounts which has a prevalence of approximately 1–5/10,000 personsCitation2. SCLC is primarily reported in elderly people with a median age of 63 years and a history of long-term tobacco exposureCitation2. Clinically, this highly aggressive malignancy is characterized by rapid growth and early metastasis associated with a variety of non-specific symptoms and signs (i.e. persistent cough, dyspnea, weight loss), resulting in the majority of SCLCs diagnosed at an advanced stageCitation2.
Prognosis and treatment of SCLC are mainly dependent on the extent of disease spread at the time of diagnosis. The most commonly used staging system is the Veterans Administration Lung Study Group (VALG) that classifies patients into two categories: (1) limited stage (LS), which is confined to one hemithorax and one radiation field, or (2) extensive stage (ES), which extends beyond one hemithorax and outside a single radiation fieldCitation3,Citation4. For patients with LS-SCLC, a combination of radiation therapy and platinum-based chemotherapy is commonly used; median overall survival (OS) rates have been reported in the range of 15–20 monthsCitation5. Patients with ES-SCLC are treated with systemic treatment, which for the past four decades, has remained chemotherapy with cisplatin or carboplatin plus etoposide (topoisomerase II inhibitor) for first-line treatment and was associated with a median OS of approximately 10 monthsCitation4. While SCLC is exceptionally sensitive to the tumoricidal mechanisms of action of chemotherapy and radiotherapy, these approaches have significant limitations including emergence of early treatment-resistance and severe systemic toxicities. The significant responses of immunotherapies observed in other solid malignancies, specifically immune checkpoint inhibitors targeting the PD-1/PD-L1 axis, have spurred intense interest in the use of these novel agents as more efficacious and tolerable treatments in SCLC.
In July 2020, the National Comprehensive Cancer Network (NCCN) recommended the addition of anti-PD-L1 immune checkpoint inhibitor (i.e. atezolizumab) to standard chemotherapy following the reporting of IMPOWER 133Citation7, which demonstrated improved survival and acceptable toxicity with this new regimenCitation6. Since then, several other studies investigating ICIs in SCLC have been reported, including the CASPIAN trial, which showed another anti-PD-L1 immune checkpoint inhibitor (i.e. durvalumab) demonstrating similar results. In addition, other immune checkpoint inhibitors targeting PD-1 (i.e. pembrolizumab, nivolumab) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4 i.e. ipilimumab) have also shown positive results in subsequent treatment linesCitation7–14. As more immunotherapy combination regimens, including those with novel targets such as TIGIT, are emerging at a rapid pace, it will be critical to build off the current experience and understand the differences between each regimen. The primary aim of this study is to review the current ICI treatment paradigms for SCLC, including clinical outcomes, health related quality of life and cost-effectiveness of various ICI treatment regimens, as well as to describe any related unmet needs.
Materials and methods
Search strategy
Both the Systematic Reviews and Meta-Analyses (PRISMA) guidelines and Preferred Reporting Items were followed, and the details are listed below. PubMed, Web of Science, EMBASE and Cochrane Library were searched up to 8 March 2021, using the following terms: (“small cell lung cancer” OR “SCLC” OR “ES-SCLC” OR” LS-SCLC” OR “extensive stage SCLC” OR “limited stage SCLC”) AND (“programmed death ligand 1” OR “programmed cell death protein 1” OR “programmed death ligand 1” OR “PD-1” OR “PD(L)1” OR “CTLA-4 inhibitor” OR “anti-CTLA-4” OR “anti-CTLA-4) AND (“atezolizumab” OR “pembrolizumab” OR “”nivolumab” OR “ipilimumab” OR “durvalumab” OR “cemiplimab” OR “avelumab”) AND (“health related quality of life” OR “HRQOL” OR “HRQoL”) AND (“unmet medical need” OR “unmet need” OR “treatment gap” OR “Cost-Effectiveness Analysis” OR “Cost Utility Analysis” OR “Budget Impact Model” OR “Cost minimization analysis” OR “Cost benefit analysis”). References lists were used to identify additional clinical studies.
Study selection
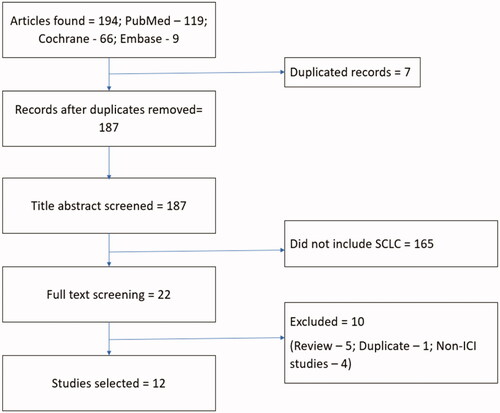
The search strategy resulted in a total of 194 articles, 7 of which were duplicates. The abstracts of the remaining 187 papers were examined to determine if they meet the following inclusion criteria: (1) study extractable information pertaining to efficacy, safety, or quality of life data; (2) patients diagnosed as extensive stage or limited stage SCLC specifically; (3) treatment strategies included ICIs (either in the form of monotherapy, and/or combination treatment). Out of the 187 articles, 165 were excluded because they focused on NSCLC. The full text of the remaining 22 articles was reviewed and a total of 10 additional papers were excluded (1 for duplicate reporting, 4 for lack of ICIs n = 4 and 5 review articles n = 5). This resulted in 12 studies for analysis ().
Data extraction
Details about the first author’s name, publication year, study design, number of patients, control and interventions were extracted. The primary outcomes were OS and PFS, and objective response rate (ORR). The secondary outcome was safety, HRQoL and cost-effectiveness ().
Table 1. Data extraction summary.
Results
Safety and efficacy
Of the 12 eligible studies, eight (67%) studies included data on the safety and efficacy of ICIs in patients with SCLC, two were PRO studies and the other two were cost-effective analysis studies. All the studies were conducted in ES-SCLC patients and originated from the United States and Europe. provides the efficacy outcomes of these ICI studies.
Table 2. Safety and efficacy studies: summary.
ICIs were given as first-line treatment in five trialsCitation7–11 and as second-line treatment in the remaining three trialsCitation12–14. ICIs were given in combination with chemotherapy (i.e. platinum plus etoposide) in five of the six trialsCitation7–11 and as monotherapy in two trialsCitation13,Citation14. One trial randomized ipilimumab concurrently with paclitaxel/carboplatin vs. induction paclitaxel/carboplatin followed by ipilimumab/paclitaxel/carboplatinCitation8. Finally, another study utilized nivolumab and ipilimumab combination therapyCitation12.
Overall survival and progression free survival
The primary endpoint(s) of all trials was OS and/or PFS. Median PFS with ICIs among the first-line trials () ranged from 4.5 to 6.4 months, while median OS ranged from 9.1 to 13 months. For second-line trials, study arms with ICIs demonstrated much lower survival outcomes with a median PFS ranging between 1.4 and 1.7 months and a median OS ranging from 7.5 to 9.5 months.
In both the IMPOWER 133 and the CASPIAN trials, survival (OS and PFS) was significantly improved in patients receiving atezolizumab and durvalumab, respectively, in combination with chemotherapy (platinum plus etoposide) in the first-line compared to chemotherapy aloneCitation7,Citation10. In the phase 3 trials, CASPIAN allowed the use of either carboplatin or cisplatin backbone and up to six cycles of chemotherapy while IMPOWER 133 allowed 4 cycles of carboplatin-based treatment. Additionally, the CASPIAN trial allowed patients to receive prophylactic cranial irradiation (PCI) while IMPOWER 133 did not. The OS and PFS outcomes were very similar between both trials and the benefit was consistent in the most updated data – however, it is worth noting that the median follow-up reported is longer for the CASPIAN trial vs. IMPOWER 133 trial (39.4 vs. 23.1 months, respectively). The efficacy of an anti-PD-1 inhibitor in first-line has demonstrated mixed results. In the KEYNOTE-604 phase 3 trial, pembrolizumab in combination with chemotherapy showed significant improvements in PFS but not for OS compared to chemotherapy.
Anti-CTLA-4 inhibitors have also demonstrated mixed results in the first-line setting. The combination of the anti-CTLA-4 antibody ipilimumab plus carboplatin and paclitaxel was first evaluated in a randomized phase 2 study in patients with a new diagnosis of ES-SCLC. Patients received paclitaxel plus carboplatin along with placebo or ipilimumab concurrently administered with chemotherapy (concurrent ipilimumab) or after two doses of chemotherapy (phased ipilimumab). The phased regimen was used to induce antigen release with chemotherapy before ipilimumab exposure so as to increase the effectiveness of immunotherapy. Improvement in PFS was observed when ipilimumab was administered in a phased form and not in concurrence. Building on these results, a randomized, double-blind phase 3 trial evaluated the efficacy and safety of ipilimumab or placebo plus platinum-etoposide in patients with ES-SCLC. No advantage was found in terms of OS (median months: 11 vs. 10) or PFS (median months: 4.6 vs. 4.4) in the intervention group. A possible explanation is that ipilimumab, which stimulates early stage T-cell activation, may not generate an effective antitumor response in local tumor environment without corresponding effector T-cell activation.
In the second-line and subsequent-line setting, CheckMate 451, a double-blind phase 3 study, of patients who did not progress after platinum-based chemotherapy were randomized to receive nivolumab plus ipilimumab, nivolumab alone, or placebo. Overall survival was not prolonged with nivolumab plus ipilimumab vs. placebo. Overall survival was prolonged with nivolumab vs. placebo and a modest improvement in PFS was observed. The ipilimumab plus nivolumab experienced a modest improvement in PFS relative to placebo. CheckMate 032Citation19, a phase 1/2 basket trial examined the effectiveness of nivolumab vs. or nivolumab plus ipilimumab in different tumor types, including SCLC. In the SCLC cohort, patients with limited and extensive stage disease who had progressed after at least one line of chemotherapy with cisplatin were included. Median OS was 4.4 months in the nivolumab alone group, 7.7 months in the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg group and 6 months in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg group. Median PFS was 1.4 months in the nivolumab alone group, 2.6 months in the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg group and 1.4 months in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg group. In an updated analysis, a median OS of 4.1 months and 7.9 months was observed in the nivolumab monotherapy and nivolumab 1 mg/kg plus ipilimumab 3 mg/kg group, respectively. The phase 2 KEYNOTE-158Citation20 study investigated the activity of pembrolizumab in 11 different types of solid tumors including SCLC patients. Primary endpoint was ORR. ORR was 18.7%, with an observed median PFS of 2 months and median OS of 9.1 months. In the IFCT-1603 trial, atezolizumab monotherapy did not show improvement in PFS or in OSCitation14. The two arms did not differ in OS.
All hazard ratios were calculated using Cox proportional hazards model around the outcomes. The covariates used were ethnicity, sex, age, smoking status, region of enrollment, etc. However, the hazard ratios were inconsistent across the studies and the statistical significance was variable.
Safety
All trials reported immune-related toxicities that have been well-documented in prior immunotherapy trials in other disease groups. Those trials that included combinations with cytotoxic chemotherapy observed chemotherapy-related toxicities (e.g. cytopenia, nausea/anorexia) – notably, the addition of immunotherapy did not seem to worsen the chemotherapy-related toxicities compared to the non-immunotherapy arm. Between IMPOWER 133 and CASPIAN trials, there were higher reported immune-related toxicities with the atezolizumab vs. durvalumab. However, the differences in the toxicities between the two anti-PDL1-containing regimens may be related to the design of the trials rather than the differences in tolerability between the drugs. IMPOWER 133 was a double-blinded trial while CASPIAN was an open-label trial, potentially affecting adverse event reporting. In the KEYNOTE-604 study, pembrolizumab with chemotherapy reported a higher incidence of adverse events in first-line SCLC patients compared to other first-line trials reviewed in this studyCitation11. Ipilimumab administered in phased fashion showed higher hematological adverse events than the concurrent regimenCitation8,Citation9. Reports of hypothyroidism and hyperthyroidism were less frequent for CTLA-4 inhibitors relative to PD-1/PD-L1 inhibitors.
Similarly, second-line SCLC patients treated with nivolumab and ipilimumab combination therapy experienced skin (47.5%) and gastrointestinal (27.3%) adverse events like pruritis, rash, low appetite and diarrheaCitation12. Adverse events in nivolumab monotherapy were mostly asthenia (8.9%), fatigue (8.9%) and decreased appetite (7.4%) while in atezolizumab monotherapy were mostly fatigue (16.7%), nausea (10.4%) and anemia (10.4%)Citation13,Citation14. In contrast, PD-1 monotherapy showed fewer adverse events for second-line SCLC therapy as compared to first-line. However, the percentage increased when combined with CTLA-4 inhibitors. Overall, it was observed that patients on second-line ICI treatment showed a higher number of skin-related and gastrointestinal-related adverse events than first-line treatment.
Health related quality of life
Out of 12 studies, only 3 (25%) included PRO measures to assess the impact of immunotherapy on SCLC patients’ HRQoLCitation14–16. The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire – Core 30 (QLQ-C30)Citation21, Quality of Life Questionnaire – Lung Cancer 13 (QLQ-LC13)Citation22 and the Lung Cancer Symptom Scale (LCSS) were used to assess HRQoL and symptoms. The PROs included EORTC QLQ-C30 measuring symptoms, functioning (physical, cognitive, role, social, emotional) and global health status/QoL. Additional symptoms were assessed using QLQ-LC13. Changes from the baseline in treatment-related and disease-related symptoms were observed for approximately 52 weeks in both studiesCitation15,Citation16. For symptom items higher scores reflect greater symptom severity, while in the global health status/QoL item higher scores suggest better functioning and health status/QoL.
Both the IMPOWER 133 trialCitation15 and CASPIAN trial had high uptake of the surveys. In IMPOWER 133 at baseline, 87% of patients in the intervention arm and 89% in the control arm completed the QLQ-C30, while 88% and 83%, respectively, completed the QLQ-LC13. In the CASPIAN trialCitation16, the completion rate of the QLQ-C30 at baseline was 94% in each arm, while the QLQ-LC13 had a completion rate of 93% in the intervention arm and 94% in the control arm. However, neither study reported a clinically meaningful change in mean baseline scores for symptoms, functioning and HRQoL over the study duration.
Time to deterioration
Time to Deterioration (TTD) was another measure used to assess treatment impact on HRQoL. TTD was determined using Kaplan-Meier curves and the key HRQoL determinants were calculated using Cox proportional hazards model or log rank test. In IMPOWER 133, TTD of treatment-related symptoms were similar between the atezolizumab plus chemotherapy arm vs. placebo plus chemotherapy arm. Key symptoms such as cough (HR = 0.96; 95% CI [0.59‒1.57], p = .89); chest pain (HR = 0.86; 95% CI [0.56‒1.31], p = .48); and peripheral neuropathy (HR = 0.91; 95% CI [0.60‒1.38], p = .65) were similar while dyspnea (HR = 0.62; 95% CI [0.42‒0.87], p = .005) was significantly improved in the intervention armCitation15. Patients in the intervention arm achieved meaningful improvements in HRQoL that persisted in most visits through the end of the study. In the CASPIAN trial, patients receiving durvalumab plus chemotherapy experienced longer TTD relative to chemotherapy for all symptoms. Key symptoms were cough (HR = 0.78; 95% CI [0.600‒1.026], p = .07); dyspnea (HR = 0.79; 95% CI [0.625‒1.006], p = .057); chest pain (HR = 0.76; 95% CI [0.575‒0.996], p = .046); fatigue (HR = 0.82; 95% CI [0.653‒1.027], p = .083); appetite loss (HR = 0.70; 95% CI [0.542‒0.899], p = .005)Citation16.
The IFCT-1603 trialCitation14 analyzed the proportion of second-line SCLC patients experiencing at least a one point decrease in each symptom assessed by the LCSS in the intervention arm (atezolizumab monotherapy) vs. chemotherapy. Impairment is assessed using a 100-mm visual analogue scale, with scores reported from 0 to 100 with 0 representing the best score. Most scores had improved at evaluation; notably a higher percentage was observed in fatigue and symptom distress domains for chemotherapy. On the other hand, cough, pain and hemoptysis domains were high for atezolizumab. The study failed to report granular PRO results and its statistical outcomes making it difficult to draw any robust conclusions.
Cost-effectiveness analysis
Out of the 12 studies, two (16.67%) conducted a cost-effectiveness analysis of first-line atezolizumab plus chemotherapy in comparison to chemotherapy in the treatment of extensive stage SCLC. In both studies the disease process of ES-SCLC, including PFS, progressive disease (PD) and death were simulated using a Markov model. The study by LiCitation17 et al. was conducted to analyze the cost-effectiveness from a China perspective, while the study by ZhouCitation18 et al. was conducted from a US perspective. Both studies used utility values for metastatic NSCLC which were available in the literature due to unavailability of SCLC utility scores. In addition, Li et al. also included supportive care and disease management costs.
Quality-adjusted life years (QALY) gained, and an estimate of overall costs were used to evaluate the incremental cost-effectiveness ratio (ICER). In the Chinese study, the ICER between atezolizumab-containing arm and chemotherapy alone was $489,013/QALY, making it not cost-effective at a willingness to pay (WTP) threshold of $25,929/QALYCitation17. From the US perspective, the ICER was $528,810/QALY, which was also beyond the WTP of $100,000/QALYCitation18.
Discussion
In this systematic review of ICI trials in extended stage SCLC, we evaluated 12 studies to characterize the efficacy, safety, impact on HRQoL and cost-effectiveness of different ICI regimens in the first-line and second-line settings. Key findings of efficacy included: (1) significant improvement in PFS and OS with the addition of anti-PDL1 inhibitors to chemotherapy, but no OS benefit with the addition of an anti-PD1 inhibitor; (2) CTLA-4 inhibitors did not show significant improvement in survival; (3) ICIs showed acceptable safety profile in the second-line setting, though no significant improvement was observed in survival outcomes.
The key observation made which has shifted the treatment paradigm of ES-SCLC is that the addition of ICIs in combination with chemotherapy showed significant improvement in survival outcomes as compared to chemotherapy alone. Notably, statistically significant improvement in OS and PFS was observed when PD-L1 inhibitors (i.e. atezolizumab, durvalumab) were added to chemotherapy; however, the addition of a PD-1 inhibitor (i.e. pembrolizumab) improved PFS but did not confer an OS benefit. Pembrolizumab has demonstrated activity in ES-SCLC in third-line settings, and several studies have demonstrated improved outcomes of PD-1 inhibitors compared to PD-L1 inhibitors across disease types. A potential explanation for differences in OS between PD-1 and PD-L1 plus chemotherapy trials is the amount of crossover, where KEYNOTE-604 (pembrolizumab) had the greatest amount of crossover from placebo to ICI arm compared to both IMPOWER 133 (atezolizumab) and CASPIAN (durvalumab). Due to the lack of OS benefit, pembrolizumab is not FDA approved for the first-line treatment of ES-SCLC, while the two anti-PDL1 inhibitors in combination with chemotherapy have become the standard of care.
The survival outcomes of atezolizumab and durvalumab in combination with chemotherapy were very similar in the IMPOWER 133 and CASPIAN trials, respectively. Atezolizumab in combination with chemotherapy received FDA approval nearly 1 year before the approval of durvalumab plus chemotherapy, potentially leading to increased use among providers based on familiarity. However, there are key differences in trial design to highlight that may guide clinician preference. The dosing schedule of the durvalumab regimen (every 4 weeks) may be more appealing to patients when compared to the atezolizumab regimen (typically every 3 weeks). While the CASPIAN trial also allowed for the choice between 4 and 6 cycles of cisplatin or carboplatin-based chemotherapy, several previous studies predating ICIs found no difference in efficacy between cisplatin vs. carboplatin containing chemotherapy, while carboplatin was generally found to be more tolerableCitation23,Citation24. Furthermore, while the optimal number of chemotherapy cycles is unclear, 4 cycles may be sufficient given the similar outcomes seen in CASPIAN vs. IMPOWER 133 trials. The clinical efficacy of ICIs for the second-line treatment of ES-SCLC, following progression on conventional chemotherapy, was significantly less compared to ICIs in first-line regimens.
Most immune-related AEs (irAEs) observed across all trials evaluated were mild to moderate in severity, with severe or life-threatening toxicities reported in up to 2% of patients in clinical trials. The studies by PuzanovCitation25 and KennedyCitation26 discussed the general toxicities observed with ICIs, as well as management. Pembrolizumab had the greatest number of patients with AEs for the first-line. None of the studies reported extreme or idiosyncratic toxicity, though, it is noteworthy that atezolizumab noticeably demonstrated peripheral neuropathy as a treatment-related AE. This can serve as a good reference point for further comparative analyses. Among all trials, PD-tolerance was better for 1/PD-L1 inhibitors than CTLA-4 inhibitors. Generally, the rate of irAEs by PD-L1 inhibitors was lower than that of PD-1 inhibitors. Furthermore, a combination of ipilimumab and nivolumab had brought higher toxicities.
Another key objective of this review was to identify and examine the PROs used to measure HRQoL for SCLC patients. All studies assessed symptoms, functioning (physical, role, emotional, social and cognitive) and global health status using the EORTC QLQ-C30 core cancer instrument, the lung cancer specific module the EORTC QLQ-LC13, and LCSS. Although addition of atezolizumab or durvalumab did not radically improve baseline scores, they did not worsen overall symptoms from treatment or disease. There were minor clinical improvements from baseline in observed global health status, physical functioning, appetite loss, nausea and constipation. Mean scores showed worsening for alopecia, pain and peripheral neuropathy. Durvalumab combination therapy showed a longer TTD as compared to chemotherapy alone. The most impacted domains were physical functioning, HRQoL, fatigue, appetite loss, dyspnea and cough while emotional, cognitive and social functioning domains were not as affected. The LCSS questionnaire did not show any statistical evidence for the results. The study did not provide in-depth information about changes in mean scores or TTD, which is a genuine caveat. However, there is lack of PRO usage in SCLC trials. In addition, domain level scores were seldom reported which would have allowed for a better understanding to draw more robust conclusions. Therefore, future research should report all domain level results as well as overall global HRQoL.
The studies by LiCitation17 et al. and ZhouCitation18 et al. did not demonstrate cost-effectiveness of atezolizumab when compared to chemotherapy in either the US or the Chinese context, respectively. The two main limitations of these studies were – lack of patient level data and unavailability of SCLC utilities. Therefore, the authors relied on published NSCLC literature to capture this information, which may not be consistent for SCLC. Moreover, with atezolizumab as the new standard of care, cost-effective analysis studies comparing atezolizumab to other treatment options are needed.
There are only a few randomized controlled trials evaluating the clinical outcomes of ICIs. Furthermore, the existing trials have not matured, so comprehensive conclusions to guide treatment decisions are still lacking. One way to supplement clinical trial data is the use of real-world data, which also provides increased generalizability. However, to our knowledge, there are not many database analyses studying real-world effectiveness of ICIs. A study by VedadiCitation27 et al. found that patients with SCLC showed worse physical, mental and social functioning associated with the disease as compared to NSCLC patients. They also established a link between higher comorbidities in SCLC and increased symptom severity resulting in poor quality of life. Other studies by PovsicCitation28 et al. and Cramer-van der WelleCitation29 et al., predating the advent of contemporary ICIs, observed that patient outcomes in the real world may be worse than those seen in clinical trials, raising concerns of generalizability in SCLC trials. The studies have demonstrated that the median OS is 21% shorter in real-word studies of patients with SCLC receiving first-line systemic therapy compared to clinical trials. Potential contributing factors to this could be differences in age and high comorbidities in patients diagnosed in the real world compared to the trial populations. In addition, patients in clinical trials may receive different care and monitoring than patients in real-world clinics and which may result in better outcomes in trials than in the real world.
As SCLC typically occurs in older patients with long smoking histories, age-related and smoking-related comorbidities (e.g. chronic obstructive pulmonary disease, cardiovascular disorders, diabetes mellitus) may impact treatment tolerability and efficacy. Furthermore, SCLC is very aggressive and is often diagnosed with distant spread resulting in increased symptoms and rapid deterioration of performance status. However, the clinical trials of ICI in SCLC included in this systematic review were mainly done in patients with Eastern Cooperative Oncology Group (ECOG) 0 or 1 performance status and comorbidity information is not provided. Therefore, without these important clinical variables, the findings of ICI clinical trials may be limited when extrapolating to real-world SCLC patient populations. Real-world studies assessing ICI in NSCLC patients have demonstrated that indeed comorbidities and performance status impact outcomesCitation30–32.
Conclusion
Across the current ICIs, PD-L1 inhibitors are the preferred treatment option for patients with ES-SCLC with respect to survival outcomes and quality of life. Further research is required to understand the underlying biology of treatment response differences between ICIs in SCLC. Additionally, there is limited research regarding treatment of LS-SCLC with ICIs. As more immunotherapy trials are undertaken, other important clinical endpoints such as PROs are warranted. Finally, it is critical to show the effects of the current treatments in the real-world setting outside of and within clinical trials.
Transparency
Declaration of funding
This work was supported by BeiGene, Ltd.
Declaration of financial/other relationships
Rasika Korde has nothing to declare. Rajwanth Veluswamy has served on advisory boards for Bristol-Myers Squibb, AstraZeneca, Merck, Novocure, on unbranded speaker’s bureau of AstraZeneca, received consulting honorarium from Beigene. Jason Allaire has received consulting fees from BeiGene. Gisoo Barnes is an employee of and owns stock in BeiGene. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics statement
Institutional review board approval was not required.
Acknowledgements
None.
References
- International Agency for Research on Cancer: Global Cancer Observatory [accessed 2021 Dec 1]. Available from: https://gco.iarc.fr/
- Orphanet: The portal for rare diseases and orphan drugs [accessed 2021 Dec 1]. Available from: https://www.orpha.net/consor/cgi-bin/index.php?lng=EN
- Pakkala S, Owonikoko TK. Immune checkpoint inhibitors in small cell lung cancer. J Thorac Dis. 2018;10(Suppl 3):S460–S467.
- Anbazhagan R, Tihan T, Bornman DM, et al. Classification of small cell lung cancer and pulmonary carcinoid by gene expression profiles. Cancer Res. 1999;59(20):5119–5122.
- Chen J, Jiang R, Garces YI, et al. Prognostic factors for limited-stage small cell lung cancer: a study of 284 patients. Lung Cancer. 2010;67(2):221–226.
- TECENTRIQ Prescribing Information. Genentech, Inc. 2022.
- Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229.
- Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer [published correction appears in J Clin Oncol. 2019 Dec 1;37(34):3327]. J Clin Oncol. 2016;34(31):3740–3748.
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83.
- Paz-Ares L, Dvorkin M, Chen Y, CASPIAN investigators, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939.
- Rudin CM, Salgia R, Wang X, et al. Randomized phase II study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol. 2008;26(6):870–876.
- Owonikoko TK, Park K, Govindan R, et al. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J Clin Oncol. 2021;39(12):1349–1359.
- Spigel DR, Vicente D, Ciuleanu TE, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331. Ann Oncol. 2021;32(5):631–641.
- Pujol JL, Greillier L, Audigier-Valette C, et al. A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer: Results from the IFCT-1603 trial. J Thorac Oncol. 2019;14(5):903–913.
- Mansfield AS, Każarnowicz A, Karaseva N, et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol. 2020;31(2):310–317.
- Goldman JW, Garassino MC, Chen Y, et al. Patient-reported outcomes with first-line durvalumab plus platinum-etoposide versus platinum-etoposide in extensive-stage small-cell lung cancer (CASPIAN): a randomized, controlled, open-label, phase III study. Lung Cancer. 2020;149:46–52.
- Li LY, Wang H, Chen X, et al. First-line atezolizumab plus chemotherapy in treatment of extensive small cell lung cancer: a cost-effectiveness analysis from China. Chin Med J (Engl). 2019;132(23):2790–2794.
- Zhou K, Zhou J, Huang J, et al. Cost-effectiveness analysis of atezolizumab plus chemotherapy in the first-line treatment of extensive-stage small-cell lung cancer. Lung Cancer. 2019;130:1–4.
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial [published correction appears in Lancet Oncol. 2016 Jul;17 (7):e270] [published correction appears in lancet oncol. 2019 feb;20(2):e70]. Lancet Oncol. 2016;17(7):883–895.
- Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: Results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol. 2020;15(4):618–627.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The european organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQLC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC study group on quality of life. Eur J Cancer. 1994;30(5):635–642.
- Duan J, Cui L, Zhao X, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and Meta-analysis. JAMA Oncol. 2020;6(3):375–384.
- Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS Meta-analysis of individual patient data. J Clin Oncol. 2012;30(14):1692–1698.
- Puzanov I, Diab A, Abdallah K, Society for Immunotherapy of Cancer Toxicity Management Working Group, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5(1):95.
- Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104.
- Vedadi A, Shakik S, Brown MC, et al. The impact of symptoms and comorbidity on health utility scores and health-related quality of life in small cell lung cancer using real world data. Qual Life Res. 2021;30(2):445–454.
- Povsic M, Enstone A, Wyn R, et al. Real-world effectiveness and tolerability of small-cell lung cancer (SCLC) treatments: a systematic literature review (SLR). PLoS One. 2019;14(7):e0219622.
- Cramer-van der Welle CM, Schramel FMNH, Peters BJM, et al. Systematic evaluation of the efficacy-effectiveness gap of systemic treatments in extensive disease small cell lung cancer [published online ahead of print, 2020 Dec 9]. Pharmacoepidemiol Drug Saf. 2021;30(4):445–450.
- Waterhouse D, Lam J, Betts KA, et al. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer. 2021;156:41–49.
- Schwartzberg L, Korytowsky B, Penrod JR, et al. Real-world clinical impact of immune checkpoint inhibitors in patients with advanced/metastatic Non-Small cell lung cancer after platinum chemotherapy. Clin Lung Cancer. 2019;20(4):287–296.e284.
- Miao K, Zhang X, Wang H, et al. Real-world data of different immune checkpoint inhibitors for non-small cell lung cancer in China. Front Oncol. 2022;12:859938.