Abstract
Recent increases in the practice of parallel publication, during which a peer-reviewed manuscript is published concurrently with the first dissemination of the same key data at a medical congress as a late-breaking abstract, have highlighted substantial value for this method of publication. Parallel publication can increase access to new clinical information for healthcare providers and patients, as well as promote engagement and reach of the publication and presentation. As the practice becomes more common, there is a need for strategies to address the multiple challenges involved in the development process, such as shortened timelines, journal and congress policies, and stakeholder alignment. We surveyed journals, congresses, and publication professionals on the challenges of parallel publication and recommendations for success. Recommendations from journal editors and congress officials included the importance of adhering to timelines and early communication. Insights from a community of publication professionals showed that timelines and the author review process were among the key challenges of parallel publication development and stressed the importance of clear roles and expectations for authors. To provide real-world insights, we present 3 case studies of successful parallel publication development, highlighting the crucial role of journal selection, planning around data availability, and adapting to unpredictable circumstances. The recommendations described here may provide publication professionals with strategies to successfully plan, execute, and carry out parallel publication.
Introduction
In recent years, there has been a considerable increase in the number of peer-reviewed manuscripts being published concurrently with the first dissemination of the same key clinical data at a medical congress in a late-breaking abstract. This strategy, referred to as parallel publication, can bring value to multiple stakeholders, including healthcare professionals, authors, study sponsors, congresses, and journals (). For instance, parallel publication can enhance both publication access rates and online activity surrounding the late-breaking presentationCitation1,Citation2. Authors and study sponsors are also able to leverage peer review comments on the manuscript to guide development of the congress presentation to be prepared for potential questions from the audienceCitation2. An additional key aspect is that the broader public has immediate access to the full study details, enhancing timely information dissemination and providing an accessible reference for data not contained within the congress presentation. In addition, the full publication can potentially facilitate doctor–patient discussions.
Figure 1. Examples of ways parallel publications can bring value to all stakeholders. Abbreviations. HCP, healthcare professional.
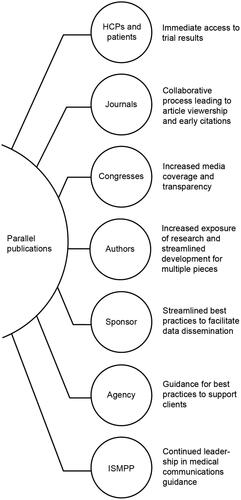
Many journals and congresses now have guidelines for parallel publications or make public calls for these submissions, including the American Association for Cancer Research, the American Heart Association, American College of Cardiology, Lancet family journals, Journal of Cardiac Failure, and American Society of Clinical Oncology congresses and journals including Journal of Clinical OncologyCitation2–6. With an increase in demand from authors, journals, and congresses alike to publish and present in parallel, the need for collaboration among stakeholders has evolved. The development process for parallel publication can present multiple challenges, including shortened timelines, resource constraints, requests from peer reviewers for additional analyses, journal rejection, and management of all processes and timelinesCitation7.
To address this need, this article highlights suggestions for streamlining the parallel publication development process using holistic insights representative of guidance from journal editors, congress officials, and study sponsors. With insights gathered from various publication professionals via surveys, online forums, and the personal experience of the authors, we highlight key pain points in the process and discuss strategies for success that may be broadly applicable to all therapeutic areas for industry-sponsored research.
Perspectives of key stakeholders
Journal and congress perspective
To better understand the needs of journal editors and congress officials in regards to parallel publication, a survey was developed with tailored questions on processes, experiences, and overall challenges, for which the full study results were previously reported at the International Society for Medical Publication Professionals (ISMPP) European meeting in 2021Citation8. Responses were received from 6 of 66 journals and 4 of 37 congresses (9.7% response rate). Of the 6 journals represented, the therapeutic areas of focus were oncology (n = 2), neurology (n = 1), autoimmune (n = 1), and general medicine or publishing (n = 2). Therapeutic areas represented by responding congresses were oncology (n = 2), endocrinology (n = 1), and autoimmune (n = 1). A range of challenges was reported, including lack of resourcing, difficulty in coordination, and challenges with timeline management ()Citation8. A key theme noted was the importance for authors and study sponsors to adhere to agreed-upon timelines, particularly from the journal’s perspective.
Figure 2. (A) Journal editor (white) and congress official (black) responses to survey of significant challenges surrounding parallel publication (A) and recommendations for streamlining the process (B). Abbreviations. LBA, late-breaking abstract.

One journal editor noted that the rushed timelines could negatively impact peer review. It is imperative that the goal of parallel publication should not be to obtain rapid data dissemination through reduced or compromised peer review due to rushed timelines. The integrity of the peer review process is paramount and should be maintained; generally, authors and study sponsors engage with high-tier journals for parallel publications. High-tier journals have rigorous review processes in place to ensure full and thoughtful reviews of the manuscript, even with compressed timelines. Further, authors and study sponsors prefer to present the associated published data at large, international meetings with specific late-breaking abstract guidance and strong scientific review committees. To prevent publications with insufficient evidence or quality from moving forward, journals must ensure that the full data are or will be available in advance of the congress date to allow for adequate time for peer review. The integrity of the review process can further be enhanced by the parallel review from congress officials of the associated abstract and late-breaking oral presentation. Multiple reviewers across these areas can help to ensure the integrity of the parallel elements. High-tier journals and congresses are well experienced in collaborating with authors and study sponsors to facilitate rapid peer review and adherence to accelerated timelines required for parallel publication. These journals and societies are well equipped to review and execute on key publications through a lens of best practices established by the industryCitation9,Citation10.
To circumvent potential issues, early communication with the journal and congress is recommended. Authors may utilize presubmission inquiries to state their intentions, allowing the journal to recommend next steps to successfully achieve parallel publication while not sacrificing rigorous peer review. Additionally, journals may invite authors to submit manuscripts for consideration for parallel publication, at which point both parties can discuss submission and peer-review timelines and post-publication digital dissemination opportunities that may promote a successful outcome. Having a single point of contact for the study sponsor coordinating communications between all stakeholders may also help improve alignment and transparency. As each project, journal, and congress will have unique requirements, clear and consistent communication is critical. While these data are limited by the low response rate (∼10%), there was general alignment across respondents regarding various pain points and recommendations. Increased transparency and communication between journals, congresses, study sponsors, and authors could set the stage for successes in parallel publication across all functional areas.
Community insights
The online discussion forums and live chat during a seminar on parallel publications were used to capture broader insights and additional feedback from publication professionals with agency and pharma experience. A query was posted on the ISMPP community forum as well as in the chat of an ISMPP University presentation asking members about their parallel publication experience. Four members responded to the forum post, and 28 attendees responded during the presentation. Overall, these stakeholders noted rushed or challenging timelines and the review process as frequent challenges faced in the parallel publication process (). These results and the survey results of journals and congress officials demonstrate the importance of ensuring timelines are clear to all involved and key members, particularly lead authors. It is equally important to establish a clear role for the lead author who may be responsible for steering the authorship group and encouraging all authors to remain motivated and engaged to adhere to these timelines.
Figure 3. (A) Community insights from surveyed publication professionals (N = 32) on challenges presented with parallel publications. (B) Recommendations for addressing key challenges based on the role of the stakeholder and processes.
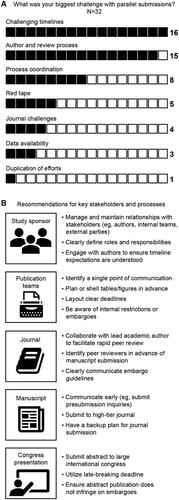
Managing these challenges and accelerated timelines is key for a successful parallel publication process. Strategies for optimizing the process were provided by some respondents, primarily those responding to the online forum. Publication professionals recommended various steps to be taken by study sponsors, journals, congress officials, and publication teams alike (). A key theme that emerged was the importance of relationship management for both internal and external contributors. Study sponsors and publication leads or teams have an important role in managing and maintaining collaborative relationships with authors, cross-functional team members, and external publication support teams. Ensuring roles and responsibilities for all stakeholders are clearly defined at the outset is a best practice for publication development overall and is critical for parallel publications. Additional recommendations were given to help manage the complex timelines involved, including early communication with the journals, submitting abstracts at the late-breaking deadline, and developing shell drafts of the publications, including tables and figures, for authors to review prior to data release. It was also suggested that a backup journal be selected to expedite turnaround if the initial manuscript submission was rejected to meet the goal of parallel publication. Together, these recommendations highlight strategies for overcoming key pain points in the development process.
Considerations for the study sponsor
The results from the surveys are corroborated by the first-hand experience of the authors and outline the importance of the key elements of parallel publication, including the target journal and data being reported. Parallel publication is well suited for pivotal clinical trials, as highlighted in the case studies below. Cutting-edge data can potentially have implications on clinical practice, and therefore rapid dissemination and broad awareness is crucial. Accordingly, high-tier journals often reach out to authors and/or study sponsors about publishing in their journal after acceptance of a late-breaking abstract. As such, it is important for study sponsors and publication teams to partner closely with journals to realize the final publication goal while adhering to company policies and compliance. Journals, when alerted in advance of a major study readout, can ensure alignment on peer reviewers prior to manuscript submission. Early and transparent communication allows journal editors to conduct required processes swiftly while adhering to best practices.
As in the case of any industry-sponsored publication, transparent and ethical publication practices are vital to maintain the integrity of the scientific content in parallel publicationsCitation9,Citation10. Transparency in author conflict of interests/disclosures is essential and should be obtained early and documented for publication.
Case study insights
An additional challenge inevitably facing parallel publishing is that no two scenarios will be identical, with each situation requiring a unique approach per its specific needs. The three case studies shown below highlight some of the unique challenges and solutions that may be required in development of parallel publications.
Case 1: optimizing journal selection and publication development
In all circumstances, journal selection is a key factor for the potential success of parallel publications. In this case, authors needed to decide if the late-breaking abstract that had been accepted to a congress should be submitted to 1 of 2 high-tier journals. The manuscript draft was initially submitted to the authoring team’s first choice high-tier journal for fast-track review. In tandem, anticipating the potential for a rejection, the authors reformatted the manuscript for the second choice high-tier journal. After 3 weeks, the first journal informed authors they would not accept the manuscript for publication. The preparation of the reformatted manuscript in advance allowed the authors to quickly resubmit to the second journal, where the manuscript was eventually accepted for parallel publication. The initial foresight and preparation of the backup plan enabled authors to continue moving forward and achieve their goals.
Case 2: adjusting for data availability
In the second case, authors sought to complete a parallel publication with data that were not available at the time of the late-breaking abstract deadline for the desired congress. In this case, relationship management and communication between the study sponsor and the congress was critical. The authors contacted the society affiliated with the congress expressing their intentions and the data availability situation. Working with the authors, the congress accepted the concept for an oral presentation without an abstract. Following this notification, the authors then proceeded with submission to their journal of choice. This example highlights the importance of early communication with stakeholders including the congress scientific session representative, as well as the level of flexibility some congresses may have depending on the significance and perceived impact of data to be presented. This level of flexibility is evident through major congresses, with the American Society of Clinical Oncology noting directly in their guidelines for the 2022 meeting that key Phase III clinical data not available by the late-breaking deadline may be granted an exception for a later submission, provided the authors submit a shell abstract and communicate with the scientific committee to discuss alternate options.
Case 3: recognizing and preparing for the unpredictable
In the final case, we highlight the inevitable unpredictability of the development process, with the global COVID-19 pandemic illustrating an unprecedented example of events causing interruptions. Authors had submitted a high-impact abstract to a congress and were developing the manuscript for submission when the COVID-19 pandemic began, causing significant uncertainty in congress proceedings. In the rapidly changing environment with uncertainty of congress dates, authors chose to retract the abstract from the initial congress and resubmit to a second congress. At the same time, they submitted the manuscript to a high-tier journal. Due to increases in submissions during the pandemic, there was a notable delay in response from the journal. This required reviewer comments to be incorporated and resubmitted within 1 week of being received in order to continue on the timeline needed for parallel publication. Despite adherence to journal timelines, parallel publication and presentation was not initially achieved. However, planning and foresight by the study sponsor and authors provided for the submission of a secondary abstract to another key congress, which ultimately did result in parallel publication with the later presentation. Additionally, close collaboration with the publication support agency as well as publication contingency planning was needed to manage multiple moving pieces for the congress presentation and manuscript development, resubmission, and final publication.
Conclusion
In a digital era where the speed of information availability is increasing, authors and study sponsors are more frequently using parallel publication as an avenue to ensure healthcare professionals and patients have access to full study results at the time of congress presentation. With a shift to digital solutions driven by advances in technology and the COVID-19 pandemic, parallel publication has become and will likely continue to be an important information dissemination strategy for authors and study sponsors. The need for and utility of rapid information dissemination was highlighted during the COVID-19 pandemic, during which manuscript uploads to preprint servers prior to publication became a frequent strategy for sharing emerging novel data in certain fieldsCitation11. However, the general practice of posting preprints is not widely utilized for pharmaceutical-sponsored research from interventional clinical trialsCitation12. This may be attributed to multiple reasons, including internal company policies only allowing manuscripts to be published after peer review. Rigorous peer review helps ensure the integrity of the research reported, which is not a standard process for preprints. The gold standard for data dissemination remains a peer-reviewed publication, and parallel publication allows study sponsors an option to accelerate data dissemination while retaining integrity and rigor of the peer review process.
As the practice of parallel publication becomes commonplace, cross-entity collaborations will inevitably become more streamlined. Though it is not a trivial process, there are multiple steps study sponsors, journals, congresses, and agencies can take to streamline the process for optimal success and lessen the burden for all parties involved. We believe the recommendations described here provide publication professionals a toolbox to plan, execute, and realize the goal of parallel publishing, as well as aid journals and congresses in the organization of these communications.
Transparency
Declaration of funding
Development of the survey and community forum post was sponsored by Pfizer, Inc.; further refinement and execution of the survey as well as development of the ISMPP U presentation was conducted with support from MedThink SciCom.
Declaration of funding/other relationships
KWS is an employee of and receives stock as part of compensation from Pfizer, Inc., Collegeville, PA, USA. MIK is an employee of AstraZeneca, Gaithersburg, MD, USA. CIR and NM are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. RCS and TP are employees of MedThink SciCom, Cary, NC, USA. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
KWS developed the journal and congress survey and community forum post. RCS and TP refined the survey and forum post and executed the surveys. KWS, RCS, and TP analyzed the results of the survey and forum post. KWS, MIK, CIR, NM, and TP developed the ISMPP U presentation and individual case studies. All authors were involved in the drafting and critical review of the manuscript. All authors approved the final draft of the manuscript and agree to be accountable for all aspects of the work.
Acknowledgements
None.
References
- Shepherd A, Draghi N, Gary B. An exploratory analysis of online activity surrounding simultaneous publication and congress presentation. Presented at: 2018 European Meeting of ISMPP; London, UK; January 23–24, 2018.
- Kumbhani DJ, Hill JA. Publications simultaneous with meeting presentation. Circulation. 2019;139(3):307–309.
- Journal of cardiac failure supports simultaneous publications. Heart failure society of america. Accessed December 14, 2021. https://hfsa.org/journal-cardiac-failure-supports-simultaneous-publications.
- American association for cancer research. 2022 meeting abstract guidelines; [cited 2022 Jan 20]. Available from: https://www.aacr.org/meeting/aacr-annual-meeting-2022/abstracts/.
- Journal of clinical oncology. Manuscript preparation guidelines; [cited 2022 Jan 20]. Available from: https://ascopubs.org/jco/authors/submit-manuscript.
- JAAC journals. Simultaneous publications – AAC.20; [cited 2022 Mar 1]. Available from: https://www.jacc.org/events/conferences/acc-2020.
- Narayanan R, Ganpathy P, Pitre S, et al. Challenges and insights on parallel publication of manuscript with conference presentation: a case study. Presented at: The Annual Meeting of ISMPP; Virtua; June 16–18, 2020.
- Schuler K, Sellnow R, Parker T. Challenges of simultaneous publications: journal and congress perspectives. Presented at: 2021 European Meeting of ISMPP; Virtual; January 26–27, 2021.
- Recommendations for the conduct, reporting, editing and publication of scholarly work in medical journals. International committee of medical journal editors; [cited 2022 Apr 27]. Available from: https://www.icmje.org/recommendations/.
- Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464.
- Fraser N, Brierley L, Dey G, et al. The evolving role of preprints in the dissemination of COVID-19 research and their impact on the science communication landscape. PLoS Biol. 2021;19(4):e3000959.
- Wieting S, Bestall S, Wager K, et al. Current trends in pharmaceutical industry-affiliated medRxiv and bioRxiv preprints. Presented at: European Meeting of ISMPP; Virtual; January 25–26, 2022.
