Abstract
Objective
To understand physician preferences for various attributes of pediatric combination vaccines.
Methods
An online survey was completed by 400 US physicians (pediatricians and family physicians) who routinely administer vaccines to infants aged 1–12 months in outpatient settings. Respondents completed a discrete choice experiment (DCE) by selecting their preferred options from different hypothetical vaccine profiles with systematic variation in the levels of five attributes: vaccine presentation, number of injections administered at a single visit, completion rates, timeliness rates (within 30 days of recommended age), and years of availability for routine use, assuming similar cost, safety, and efficacy. Odds ratios and relative attribute importance scores were estimated using a random parameters logit model.
Results
Physicians (mean age 50.4 years, 52.5% women) preferred combination vaccines that reduced the number of injections administered at a single visit, facilitated higher completion and timeliness rates for the primary DTaP series, were available as a pre-filled syringe rather than a vial needing reconstitution and had been available for routine use for more than 1 year. All odds ratios were statistically significant. Physicians were twice as likely to prefer administering three injections in a single visit instead of four. The most important attribute was the number of injections administered at a single visit (relative importance 38%), followed by timeliness, completion rates, and vaccine presentation; years a vaccine has been available was the least important attribute.
Conclusion
US physicians prefer pediatric combination vaccines that enable fewer injections to be administered at a single visit, facilitate higher completion and timeliness rates, are offered as a pre-filled syringe, and have been available for routine use for more than 1 year. The most important attribute of pediatric combination vaccines was a reduction in the number of injections administered at a single visit.
Introduction
Childhood vaccination is widely considered to be one of the most effective public health interventionsCitation1. Within the US, it is estimated that, for children born during 1994–2013, childhood vaccinations prevented 322 million illnesses, 21 million hospitalizations, and 732,000 deathsCitation2. There are lifelong health and economic benefits associated with vaccination from improved physical well-being and developmentCitation2–4. The United States (US) Advisory Committee on Immunization Practices (ACIP) recommends up to 10 vaccines in the first 2 years of a child’s life to protect against 14 diseasesCitation5. A minimum of 14 injections and three oral doses are required to vaccinate an infant from birth to 18 months according to the recommended schedule with vaccines licensed in the US before 2018Citation5.
Combination vaccines merge antigens that protect against different diseases or protect against multiple strains of the same pathogen into a single productCitation6. DTP, which combines the whole cell pertussis vaccine with diphtheria and tetanus toxoids, was first licensed in 1948Citation7. The replacement of whole-cell pertussis (wP) with less reactogenic acellular pertussis (aP) antigens in the mid-1990s enabled DTaP to be combined with other routine vaccines, including inactivated poliovirus vaccine (IPV), Haemophilus influenzae type b (Hib), and Hepatitis B (HepB), in the pediatric immunization scheduleCitation7. In the early 2000s, two pentavalent vaccines were licensed for use in the US—Pediarix (DTaP-HepB-IPV, which protects against diphtheria, tetanus, pertussis, poliomyelitis, and HepB) in 2002 and Pentacel (DTaP-Hib/IPV, which protects against diphtheria, tetanus, pertussis, poliomyelitis, and invasive Hib), in 2008Citation8,Citation9. More recently, a hexavalent combination vaccine, Vaxelis (DTaP-HepB-IPV-Hib), which protects against six diseases (diphtheria, tetanus, pertussis, poliomyelitis, HepB, and invasive Hib), was licensed for use in the US in 2018 and recommended by the ACIP for routine use in infants 2, 4, and 6 months old in 2019Citation10,Citation11.
Combination vaccines are recommended by the ACIP over their equivalent individual component vaccines to facilitate adherence to the vaccination guidelinesCitation6. Combination vaccines reduce the number of injections in a single visit and are associated with improved vaccination coverage and timeliness ratesCitation12–15. The use of a hexavalent DTaP-HepB-IPV-Hib vaccine can reduce the total number of injections required to complete the primary DTaP, IPV, Hib, and HepB series by 2 or 3, depending on the pentavalent and complementary monovalent vaccines previously used by the practice. There are also other vaccine attributes that may drive physician preferences for one vaccine over another. Several studies have demonstrated that healthcare providers prefer ready-to-use (RTU) vaccines over vaccines that need to be reconstituted (need for reconstitution [NFR]) as they save preparation time, reduce needlestick injuries, dosage errors, and opportunities for needle contaminationCitation16–19.
The objective of this study was to examine US physicians’ preferences for combination vaccine attributes in the context of the routine infant immunization schedule.
Methods
Study design and population
This was a cross-sectional online survey of pediatricians and family physicians in the US conducted between April and May 2021. Eligibility criteria included being a pediatrician or family physician who practiced in the US (50 states or Washington, DC), routinely prescribed vaccines to infants (1–12 months) in an outpatient setting, and worked in a practice that used a DTaP-based pentavalent combination vaccine for the infant series.
Quota sampling was used to recruit physicians based on criteria for age, sex, practice region, and location using recently published survey data on the characteristics of US pediatricians and family physiciansCitation20–22. Quotas were re-evaluated throughout the recruitment period to ensure the feasibility of timely study completion. Recruitment was conducted through an online physician panel. Interested physicians were directed to an online screener to determine eligibility. Eligible physicians were presented with a study summary explaining the nature of the research, benefits/risks, and study procedures, and were required to provide consent before participating in the survey. To minimize bias, respondents were blinded to the study sponsor. Respondents were paid an honorarium that was determined to be within a fair market value range for their time. The anonymized dataset was used for analysis. The study was submitted to Advarra institutional review board (IRB) and determined to be exempt from IRB oversight because of minimal risk to participants.
Survey development
Discrete choice experiments (DCEs) quantify preferences through analysis of the choices respondents make when presented with pairs of hypothetical product profiles or scenarios. In the current DCE, physicians were presented with different vaccine profiles with systematic variation in the levels of five vaccine attributes product formulation (RTU syringe vs. NFR vial); the number of injections administered at a single visit (2, 3, or 4 injections); completion rates for the primary DTaP, IPV, Hib, and HepB series (85%, 90% and 95%); timeliness rates (within 30 days of recommended age) for the primary DTaP, IPV, Hib and HepB series (80%, 90% and 100%); and the number of years a vaccine has been available for routine use (<1, 1–3, and >3 years) (). The selection of vaccine attributes and levels was informed by a literature searchCitation16–19, interviews with 10 physicians recruited from the online physician panel, and expert opinion. presents details of the vaccine attributes included in the study.
Table 1. Attributes and levels of vaccine attributes selected to be part of the discrete choice experiment survey.
The combinations of attributes and levels that were presented to respondents in the choice tasks were generated from the D-efficient experimental design using the Ngene software (version 1.2.1, by ChoiceMetrics)Citation23. Respondents were asked to assume the similar cost, safety, and efficacy profiles when reviewing hypothetical vaccine profiles (Supplemental Figure 1). They were presented with a total of 14 choice tasks, 12 of which were included in the analysis. Of the other two, one was a warm-up exercise, and the other was a dominance check with one choice superior to the other on every attribute (fewer injections, higher completion rate, etc.) that served as a quality control measure. Failing the dominance task did not automatically invalidate respondents’ data since the random utility model assumes an error component to account for seemingly irrational behavior.
Data analysis
All analyses were conducted using SAS 9.4, Microsoft Excel, STATA 14.2, and R Studio 3.5.0. The sample size for the DCE was based on the work of Orme and colleagues, which takes into account the number of questions presented, the number of attributes, and the number of levels for attributesCitation24. We assumed 12 choice sets with two alternatives each, and the largest number of levels being 4, our sample size of 400 met this rule of thumb formula ([400 × 12 × 2]/4 = 2400, which is >500). Descriptive statistics were used to summarize sociodemographic and practice characteristics. Categorical variables were summarized by counts and frequencies and continuous variables were summarized by mean, median, and distribution characteristics. Observed choices were analyzed using a random parameters logit (RPL, sometimes called “mixed logit”) model. The RPL model assumes that the probability of selecting a vaccine profile is a function of the attribute levels that describe the alternative and a random error that adjusts for individual-specific variations in preferences. It also assumes that there is a distribution of preference weights across the sample reflecting preference heterogeneity, and it models the parameters of that distribution for each attribute level.
Odds ratios (OR) for preference weights were also calculated using the Broyden-Fletcher-Goldfarb-Shanno (BFGS) algorithmCitation25. Relative importance scores were calculated to compare the relative influence of each attribute on physicians’ choices. The relative attribute importance score is the proportion of total variance explained by the individual attribute, expressed as a percentage. The higher the score, the greater the influence of that attribute on decision-making. In addition, the rate at which physicians were willing to make trade-offs between the attributes of combination vaccines was examined through a marginal rate of substitution (MRS) analysisCitation26.
Sensitivity analyses were conducted by excluding respondents based on the following criteria: (1) failed the warm-up task and their overall survey completion time was extreme (<50 or >300% of median overall survey completion time), (2) failed the dominance task embedded within the survey and their overall survey completion time was extreme, or (3) always chose the alternative option presented to them either on the left or the right side, suggesting they may not have attended to each question adequately.
Results
A total of 400 physicians completed the survey. Respondents were similar to the US physician population of interest, indicating the success of the quota sampling. Mean age was 50.4 years, 52.5% were female, 41.5% practiced in an urban location, and 58.8% were part of a group practice. describes the respondents in more detail. Quotas were relaxed after the first two weeks of data collection once minimums had been met for geographic and practice setting location.
Table 2. Physician characteristics.
Odds ratios for preference weights
summarizes the findings from the RPL model. Preferences for every switch from the reference level were significant. Physicians significantly preferred combination vaccines that reduced the number of injections administered in a single visit, facilitated higher completion and timeliness rates for the primary DTaP, IPV, Hib, and HepB series, were available as a pre-filled RTU syringe rather than an NFR vial, and had been available for routine use for more than one year.
Table 3. Physician preferences for vaccine attributes.
Physicians were more than three times as likely to prefer administering two injections per visit instead of four injections (OR 3.12, 95% CI 2.62–3.70) and almost twice as likely to prefer administering three injections instead of 4 (OR 1.97, 95% CI 1.76–2.20). Similarly, physicians were 1.8 times (OR 1.81, 95% CI 1.59–2.05) as likely to prefer higher timeliness rates (100% vs. 80%), 1.7 times (OR 1.74, 95% CI 1.61–2.00) as likely to prefer higher completion rates (95% vs. 85%), and 1.5 times (OR 1.51, 95% CI 1.36–1.69) as likely to prefer a pre-filled RTU syringe to an NFR vial. There was a small but significant preference for vaccines that have been available for routine use for more than 1 year (1–3 years: OR 1.30, 95% CI 1.11–1.47 and >3 years: OR 1.20, 95% CI 1.05–1.38), with the confidence levels overlapping for the two non-reference levels (1–3 and >3 years). The preference weights are presented in Supplemental Table 1.
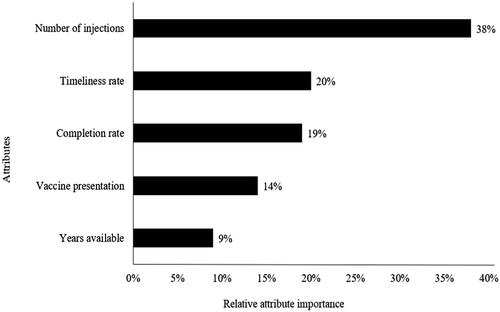
Relative importance of attributes
presents results on the relative importance of each attribute to physicians’ decision-making. The most important attribute was the number of injections administered at a single visit (38%), which was more than twice as important to physicians as timeliness (20%) and completion rates (19%). Years available on the market were the least important attribute, contributing only 9% to preferences.
Trade-offs
The number of injections administered in a single visit also emerged as the most important attribute in the tradeoff analysis. Increasing the timeliness rate from 80% to 100% was less important (0.88) than reducing the number of injections from 3 to 2 in a single visit while increasing the completion rate from 85 to 95% was 0.82 times as important (). It would take increases in multiple attributes to equal the importance of shifting from 3 to 2 injections in a single visit: a shift in completion rate (from 85% to 90%) plus a shift in timeliness rate (from 80% to 90%) plus a shift in vaccine availability (from less than 1 year to more than 3 years) sums to 1.11 (0.54 + 0.30 + 0.27), which is almost equivalent to the importance placed on the reduction from 3 to 2 injections (1.0, reference value).
Table 4. Preference strength of attribute levels in relation to preference strength of moving from four or three injections to two injections per visit.
Sensitivity analysis
A total of eight (2.0% of the population sample) respondents were excluded from the sensitivity analysis, leaving 392 respondents. This analysis provided similar results in terms of direction, size, and statistical significance for all parameters compared to the mixed logit model with 400 respondents. Given the small number and the expectation that the error term in the RPL model could address non-rational choices, these eight respondents were retained in the analysis.
Discussion
To our knowledge, this is the first DCE survey to assess physician preferences for attributes of pediatric combination vaccines in the US. This online survey of 400 physicians demonstrated preferences for combination vaccines that reduced the number of injections administered in a single visit, facilitated higher completion and timeliness rates for the primary DTaP, IPV, Hib, and HepB series, and were available as a pre-filled RTU syringe rather than an NFR vial and had been available for routine use for more than 1 year. The number of injections administered at a single visit was more important than the other attributes. Similarly, the strength of preference for a shift from 3 to 2 injections per visit was greater than any other shift in levels within an attribute.
These findings are consistent with other studies outside the US on preferences for combination vaccine attributes. For example, a DCE was conducted to assess physician preferences for attributes of pediatric hexavalent vaccines in Germany and found that physicians were more than twice as likely to prefer vaccines that required 30 seconds of preparation compared to 2 minutes (OR 2.45, 95% CI 2.09, 2.87), vaccines that were available on the market for more than 3 years compared to less than 1 year (OR 2.26, 95% CI 1.94, 2.63), and vaccines available as a pre-filled RTU syringe rather than an NFR vial (OR 1.45, 95% CI = 1.31, 1.60)Citation19. Other studies in Italy, Spain and France have also demonstrated provider preferences for pre-filled RTU vs. NFR hexavalent vaccines because of easier and quicker preparation, reduced risk of errors, and lower risk of needle contamination and needlestick injuriesCitation17,Citation18,Citation27.
Combination vaccines may also generate operational efficiencies for medical practices. Time and motion studies conducted in Belgium and the US found that fewer injections in a single visit were associated with nurse time savings and a reduction in the child crying timeCitation17,Citation28, both of which may affect total visit time and room turnover. Median vaccination consultation time was longer for visits that included discussion of four injections compared to three. It is plausible that these time savings may be more important to busy practices.
The attributes preferred in this physician survey are consistent with characteristics of a hexavalent pediatric combination vaccine that recently became available in the US. In the US, concomitant administration with pneumococcal conjugate vaccine and rotavirus vaccine was testedCitation11. The use of a hexavalent DTaP-HepB-IPV-Hib vaccine reduces the total number of injections required to complete the primary DTaP, IPV, Hib, and HepB series by 2 or 3, depending on the pentavalent and complementary monovalent vaccines previously used by the practice. Studies have demonstrated improved coverage and timeliness rates following the introduction of pediatric pentavalent vaccines in the USCitation12–15; incremental benefits might be expected for a hexavalent vaccine but will need to be evaluated.
This study was subject to some limitations. First, the sample was designed to reflect the demographic characteristics of physicians in the US, with soft quotas used to drive sampling. However, even though the final sample reflected the population of pediatricians and family physicians in the US, physicians who choose to participate in online panels like this one may be inherently different than physicians who do not participate. This may have influenced both the selection of vaccine attributes as well as the results. Second, the DCE required that respondents evaluate hypothetical profiles and assume comparable safety, efficacy, and cost when comparing vaccines, all of which may not reflect real-world vaccine profiles. However, through literature searches, qualitative work, and pre-testing, we attempted to develop vaccine profiles that were clinically relevant and plausible. Third, we did not conduct a subgroup analysis to determine whether pediatricians and family physicians have different preferences for combination vaccine attributes because of sample size restrictions.
Conclusion
US physicians prefer pediatric combination vaccines that enable fewer injections to be administered at a single visit, facilitate higher completion and timeliness rates for the primary DTaP, IPV, Hib, and HepB series, are offered as a pre-filled syringe, and have been available for routine use for more than 1 year. The most important attribute of combination vaccines in this study was a reduction in the number of injections administered at a single visit.
Transparency
Declaration of funding
MSP Vaccine Company is funded by a partnership between Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and Sanofi Inc., Swiftwater, PA, USA.
Declaration of financial/other relationships
SS, TP, and AH are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and own stock in Merck & Co., Inc., Rahway, NJ, USA. MM and CBN are employees of Sanofi Swiftwater, PA, USA. At the time of this study, JA, EZ, and AP were employees of OPEN Health, an institution that received funding from MSP Vaccine Company. GSM has been an investigator on clinical trials funded by GlaxoSmithKline, Merck, Novartis, Pfizer, Sanofi and Seqirus, and has received honoraria from these companies for service on advisory boards and honoraria from Pfizer and Sanofi for non-branded presentations.
All authors have no other conflicts of interest to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
SS, TP, AH, EZ, JZ, MM, CBN, and GSM contributed to the study design and survey development. EZ, JA, and AP helped deploy the survey and generate results. All authors were involved in the interpretation of the results. SS, TP, JA, and AP drafted the manuscript. AH, EZ, MM, CBN, and GSM provided comments and interpretation.
Acknowledgements
The authors wish to thank Jenna Bhaloo for her contribution to survey design, Ning Ning for statistical analysis, and Jordana Schmier for her manuscript writing.
Supplemental Material
Download MS Word (15.5 KB)Data availability statement
The data that supports the findings of this study are available from the corresponding author, SS, upon reasonable request.
References
- World Health Organization. Immunization; 2019. Available from: https://www.who.int/news-room/facts-in-pictures/detail/immunization
- Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the vaccines for children program era – United States, 1994–2013. MMWR Morb Mortal Wkly Rep. 2014;63(16):352–355.
- Li X, Mukandavire C, Cucunubá ZM, et al. Estimating the health impact of vaccination against ten pathogens in 98 low-income and Middle-income countries from 2000 to 2030: a modelling study. Lancet. 2021;397(10272):398–408.
- Modlin JF, Schaffner W, Orenstein W, et al. Triumphs of immunization. J Infect Dis. 2021;224(12 Suppl 2):S307–S308.
- Centers for Disease Control and Prevention. Recommended child and adolescent immunization schedule for ages 18 years or younger United States 2019; 2019 [cited 2021 Nov 19]. Available from: https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
- Elaine Miller APW. General best practice guidance for immunization; 2021. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/genrec.html
- Klein NP. Licensed pertussis vaccines in the United States. History and current state. Hum Vaccin Immunother. 2014;10(9):2684–2690.
- Food and Drug Administration. PEDIARIX [Diphtheria and tetanus toxoids and acellular pertussis adsorbed, hepatitis B (recombinant) and inactivated poliovirus vaccine], suspension for intramuscular injection. GlaxoSmithKline Biologicals; 2019 [updated 2019 Oct; cited 2022 Apr 29]. Available from: https://www.fda.gov/media/79830/download
- Food and Drug Administration. Pentacel (diphtheria and tetanus toxoids and acellular pertussis adsorbed, inactivated poliovirus and Haemophilus b conjugate (tetanus toxoid conjugate). Vaccine: Sanofi Pasteur Limited; 2019 [updated 2019 Dec; cited 2022 Apr 29]. Available from: https://www.fda.gov/media/146048/download
- Food and Drug Administration. VAXELIS (diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, Haemophilus b conjugate and hepatitis B vaccine). MSP Vaccine Company; 2020. [updated 2020 Oct; cited 2022 Jan 24]. Available from: https://www.fda.gov/vaccines-blood-biologics/vaxelis
- Oliver SE, Moore KL. Licensure of a diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b conjugate, and hepatitis B vaccine, and guidance for use in infants. MMWR Morb Mortal Wkly Rep. 2020;69(5):136–139.
- Kurosky SK, Davis KL, Krishnarajah G. Effect of combination vaccines on completion and compliance of childhood vaccinations in the United States. Hum Vaccin Immunother. 2017;13(11):2494–2502.
- Marshall GS, Happe LE, Lunacsek OE, et al. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J. 2007;26(6):496–500.
- Centers for Disease Control and Prevention. Timing and spacing of immunobiologics; 2021 [cited 2021 Dec 20]. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/timing.html#simultaneous
- Happe LE, Lunacsek OE, Kruzikas DT, et al. Impact of a pentavalent combination vaccine on immunization timeliness in a state medicaid population. Pediatr Infect Dis J. 2009;28(2):98–101.
- Icardi G, Orsi A, Vitali Rosati G, et al. Preferences of healthcare professionals regarding hexavalent pediatric vaccines in Italy: a survey of attitudes and expectations. J Prev Med Hyg. 2020;61(3):E424–E444.
- De Coster I, Fournie X, Faure C, et al. Assessment of preparation time with fully-liquid versus non-fully liquid paediatric hexavalent vaccines. A time and motion study. Vaccine. 2015;33(32):3976–3982.
- Cuesta Esteve I, Fernandez Fernandez P, Lopez Palacios S, et al. Health care professionals' preference for a fully liquid, ready-to-use hexavalent vaccine in Spain. Prev Med Rep. 2021;22:101376.
- Lloyd AJ, Nafees B, Ziani E, et al. What are the preferences of health care professionals in Germany regarding fully liquid, ready-to-use hexavalent pediatric vaccine versus hexavalent pediatric vaccine that needs reconstitution? Patient Prefer Adherence. 2015;9:1517–1524.
- Kaiser Family Foundation. Professionally active primary care physicians by field; 2019. Available from: https://www.kff.org/other/state-indicator/primary-care-physicians-by-field/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
- American Board of Pediatrics. Pediatric physicians workforce data book 2017–2018. Available from: https://www.abp.org/sites/abp/files/pdf/pediatricphysiciansworkforcedatabook2017-2018.pdf
- Agency for Healthcare Research and Quality. The distribution of the U.S. primary care workforce. Primary care workforce facts and stats [updated 2018 Jul; cited 2021 Nov 19]. Available from: https://www.ahrq.gov/research/findings/factsheets/primary/pcwork3/index.html
- ChoiceMetrics. Ngene 1.2 user manual & reference guide; 2018. Available from: http://www.choice-metrics.com/NgeneManual120.pdf
- Orme B. Sample size issues for conjoint analysis. 4th ed. Madison (WI): Research Publishers LLC; 2010. (Getting started with conjoint analysis: strategies for product design and pricing research).
- Gould W, Pitblado J, Sribney W. Maximum likelihood estimation with Stata. 3rd ed. College Station (TX): Stata Press; 2006.
- Black J, Hashimzade N, Myles G. A dictionary of economics. 3rd ed. Oxford (UK): Oxford University Press; 2009.
- Bakhache P, Virey B, Bienenfeld C. Knowledge and practices regarding infant vaccination: results of a survey of French physicians. Eur J Pediatr. 2019;178(4):533–540.
- Pellissier JM, Coplan PM, Jackson LA, et al. The effect of additional shots on the vaccine administration process: results of a time-motion study in 2 settings. Am J Manag Care. 2000;6(9):1038–1044.