Abstract
Objective
We conducted literature reviews to uncover differential effects of sex on sequelae from coronavirus disease 2019 (COVID-19) and on long COVID syndrome.
Methods
Two authors independently searched OvidSP in Embase, Medline, Biosis, and Derwent Drug File. Publications reporting original, sex-disaggregated data for sequelae of COVID-19 (published before August 2020) and long COVID syndrome (published before June 2021) were included in the reviews. The association between COVID-19 sequelae (i.e. lasting <4 weeks after symptom onset) and sex, and between long COVID syndrome (i.e. lasting >4 weeks after symptom onset) and sex, was determined by odds ratio (OR) and 95% confidence interval (CI) (statistical significance defined by 95% CI not including 1).
Results
Of 4346 publications identified, 23 and 12 met eligibility criteria for COVID-19 sequelae and long COVID syndrome, respectively. COVID-19 sequelae in the categories of psychiatric/mood (OR = 1.80; 95% CI: 1.35–2.41), ENT (OR = 1.42; 95% CI: 1.39–1.46), musculoskeletal (OR = 1.15; 95% CI: 1.14–1.16), and respiratory (OR = 1.09; 95% CI: 1.08–1.11) were significantly more likely among females (vs. males), whereas renal sequelae (OR = 0.83; 95% CI: 0.75–0.93) were significantly more likely among males. The likelihood of having long COVID syndrome was significantly greater among females (OR = 1.22; 95% CI: 1.13–1.32), with the odds of ENT (OR = 2.28; 95% CI: 1.94–2.67), GI (OR = 1.60; 95% CI: 1.04–2.44), psychiatric/mood (OR = 1.58; 95% CI: 1.37–1.82), neurological (OR = 1.30; 95% CI: 1.03–1.63), dermatological (OR = 1.29; 95% CI: 1.05–1.58), and other (OR = 1.36; 95% CI: 1.25–1.49) disorders significantly higher among females and the odds of endocrine (OR = 0.75; 95% CI: 0.69–0.81) and renal disorders (OR = 0.74; 95% CI: 0.64–0.86) significantly higher among males.
Conclusions
Sex-disaggregated differences for COVID-19 sequelae and long COVID syndrome were observed. Few COVID-19 studies report sex-disaggregated data, underscoring the need for further sex-based research/reporting of COVID-19 disease.
Keywords:
Introduction
It is well-known that biological sex can influence risks and vulnerability to diseases and their outcomesCitation1, and between-sex differences have long been recognized in medical research. In this regard, sex differences in outcomes and sequelae were reported in previous coronavirus epidemics (e.g. acute respiratory syndrome coronavirus [SARS-CoV], Middle East respiratory syndrome coronavirus [MERS-CoV])Citation2–5. Sex has also been recognized as a determinant of the progression and health outcomes of the coronavirus disease 2019 (COVID-19) infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)Citation6. Early in the COVID-19 pandemic, it was noted that severity of acute illness, rates of intensive care admission, and COVID-19-related mortality were greater among male patients than female patientsCitation7, whereas the opposite trend was observed with long COVID syndrome, where females are more often affectedCitation8.
After exposure to SARS-CoV-2, individuals can remain asymptomatic or become ill with symptoms ranging from mild and self-limited to severe, resulting in hospitalization, intensive care unit stay, and even death. As the pandemic evolved, it was noted that a portion of patients who survive the acute stage of COVID-19 illness experience new or persistent symptoms, involving most organ systems, that can linger for weeks to monthsCitation9. Even SARS-CoV-2-infected patients who experience only mild illness after exposure and do not require hospitalization for management of their acute illness can suffer from sequelae of COVID-19 infectionCitation10–12. Long COVID syndrome is characterized by a wide range of acquired sequela/complications such as extreme fatigue, dyspnea, low mood, pulmonary fibrosis, post-viral or inflammatory myocarditis, sleep disturbance, memory impairment, diabetes, chronic kidney disease, and a plethora of additional symptoms and conditionsCitation13–16 that begin or persist beyond 4 weeks from the onset of acute COVID-19 symptomsCitation12. The cause(s) of long COVID syndrome remains unclear; some researchers suggest the condition may develop as sustained damage from the initial infection, while others believe it may be the result of elevated autoantibodies in response to the initial infectionCitation17. The theory that long COVID syndrome could be related to an elevated immune response offers a potential explanation for why it appears to be more common in female patients, as research has shown females mount a faster and more robust innate and adaptive immune responsesCitation11–15. Sex differences in immune response have also been reported in other viral and bacterial infections with chronic sequelae, such as SARS CoV, MERS CoV, and Lyme disease, as well as in numerous rheumatological conditionsCitation18,Citation19.
Knowledge about fundamental sex differences underpinning the clinical manifestations, disease progression, and health outcomes of COVID-19 is crucial for the identification and rational design of effective therapies and public health interventions that are inclusive of and sensitive to the potential differential treatment needs of both sexes. Such evidence will inform sex-based management of COVID-19 and long COVID syndrome, and as such will optimize the probability of successful outcomes for both males and females. In the future, researchers should anticipate studying sex as a variable in infectious outbreaks to ensure optimal treatment of both sexes. With this goal in mind, we conducted a literature review to uncover insights into the differential effect of sex on the sequelae of COVID-19 disease, long COVID syndrome, and the variety of clinical presentations to identify information gaps where additional research and data are needed.
Methods
The study protocol for the literature review was registered (July 2020) in PROSPERO: ID CRD42020190366. The protocol was amended based on the rapidly evolving science around COVID-19 infection, prompting us to redefine the research questions, search strategy, and endpoints. Reporting of the findings followed PRISMA guidelinesCitation20, as feasible, based on the study protocol and results. The research question, according to PICO criteriaCitation21, is presented in Supplemental Content, Table S1.
Identification and selection of studies
The published medical literature was searched to identify research studies of COVID-19 infection that included human patients 18 years of age and older who had tested positive for the presence of antigen or antibodies to SARS-CoV-2. Endpoints of interest included sequelae of COVID-19 (defined as COVID-related new condition or chronic symptom a patient developed after the acute phase of the infection had terminated, with the acute phase typically lasting no longer than 4 weeks from the onset of COVID-19 symptoms) and long COVID syndrome (defined by the persistence of symptoms, development of sequelae, and delayed or long-term complications beyond 4 weeks from the onset of COVID-19 symptomsCitation12).
Studies identified by the searches were reviewed, according to a pre-specified checklist (provided in Supplemental Content Table S2), for methodological validity prior to inclusion in the review.
Search strategy
This review considered published observational, case-control, cohort, randomized controlled (RCT), and retrospective and prospective real-world experience studies of COVID-19 infection that included at least 20 participants and reported complete data by sex (i.e. included total event counts by sex and total counts of male and of female participants to allow calculation of prevalence rates).
Studies that reported data without sex disaggregation and studies that reported data only in female patients or only in male patients were excluded because they were not applicable to the aim of the current investigation. Conference abstracts, conference papers, conference reviews, and case reports were also excluded, as were manuscripts that were published in non-peer-reviewed scientific journals.
Publications were identified by searching electronic databases and scanning reference lists of selected articles. No limits were applied based on language. The electronic database search was conducted on OvidSP in Embase (1974 to present), Medline (1946 to present, including ePub/in press and articles in press/in data review), Biosis (1993 to present), and Derwent Drug File (1964 to present). Review articles were excluded from the analysis, but the reference list of selected review articles was reviewed to identify relevant primary publications.
With respect to sequelae of COVID-19 infection, articles published or listed in pre-print services between 1 December 2019 and 31 July 2020 (inclusive) were eligible for inclusion in this narrative review. As the pandemic evolved and the risk of long COVID syndrome became clear, a second search was conducted, from which articles published or listed in pre-print servers between 31 December 2019 and 31 May 2021 (inclusive) were eligible for inclusion in this literature review.
The search strategy was developed in collaboration with librarians/information professionals from PharmIntell Consulting, LCC (Branchburg, NJ). The following search terms were used:
“coronavirus disease 2019”, “coronavirus-disease-2019”, “COVID-19”, “COVID19”, “novel coronavirus 2019”, “novel coronavirus 19”, “coronavirus disease 2019”, “coronavirus disease 19”, “coronavirus 2019”, “coronavirus 19”, COVID 2019”, “COVID 19”, “SARS-CoV-2”, “SARS-COV2”, “SARSCOV2”, “SARSCOV-2”, “nCOV 2019”, “nCOV-19”, “Severe Acute Respiratory Syndrome Coronavirus 2”, and “SARS coronavirus 2”. In addition to these search terms, the following terms were added to capture articles on long COVID syndrome: “post-COVID”, “long COVID”, “long-hauler”, “COVID long-haul”, “long haul COVID”, “long term COVID”, “post-acute sequelae”, “chronic COVID”, “long-term sequelae”, “long term symptoms”, “long term effect”, “ongoing symptomatic COVID”, “post-acute viral syndrome”, “post-acute COVID”, “postviral syndrome”, “post-viral syndrome”, “post-viral illness”, “postviral illness”, “post-viral symptom”, “postviral symptom”, and “postviral fatigue syndrome”.
Study selection and data extraction
Authors screened the articles identified by the searches and then performed a full text review (Full Text Screening Criteria provided in Supplemental Content Table S2) of those that appeared relevant to the research topic based on their title and abstract. The studies were then assessed independently by two reviewers for methodological validity prior to inclusion in the review using a study-specific checklist. Disagreements that arose between the reviewers were resolved through discussion, and in the case of continued disagreement, with assistance from a third reviewer.
Data on study characteristics (author, year, study design, setting, time frame), population characteristics (age, sex, comorbidities), clinical factors (definitions and measurement methods), interventions, and outcomes were extracted.
Data analysis
Sequelae of COVID-19 disease and long COVID syndrome were grouped into categories using DistillerSR (Evidence Partners, Ottawa, Canada). The nature of an association between sequelae of COVID-19 disease and sex was determined by odds ratio (OR) and 95% confidence interval (CI). Of note, 95% CIs that do not include 1 correspond to a two-sided P-value that is less than 0.05 (i.e. statistical significance). CIs were presented rather than P-values as they provide information on the direction and magnitude of the effect. The between-sex difference on the acquisition of long COVID syndrome and the varied clinical presentations were also analyzed by OR and 95% CI.
Results
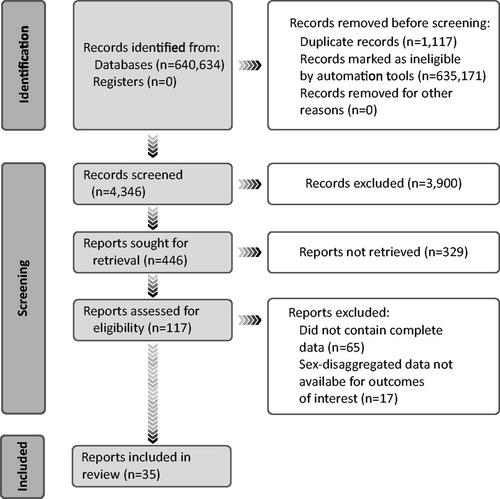
Of 4346 articles identified by literature searches and additional checks, 4311 were excluded based on not meeting all the key inclusion criteria or all required data were not reported. Of the remaining 35 articles, 23 reported data on sequelae of COVID-19 infectionCitation22–44; 5 reported data on counts of long COVID syndrome onlyCitation45–49; 3 reported data on only sequelae of long COVID syndromeCitation13,Citation50,Citation51; and, 4 reported data on both sequelae of COVID-19 disease and long COVID syndromeCitation52–55 (). The 35 articles reported on studies that were conducted before COVID-19 vaccination was widely available; none reported the vaccination status of study participants. All 35 articles met the eligibility criteria and reported sex-disaggregated data. Assuming that each participant in each of the articles is a unique individual, the total sample size is 1,393,355 (1.32 million patients in 1 studyCitation40) Sequelae of COVID-19 disease and long COVID syndrome are listed by category of disorder in .
Table 1. Sequelae of COVID-19 and Long COVID Syndrome by Category of Disorder.
Sex differences in sequelae of COVID-19 disease (<4 weeks after onset of symptoms)
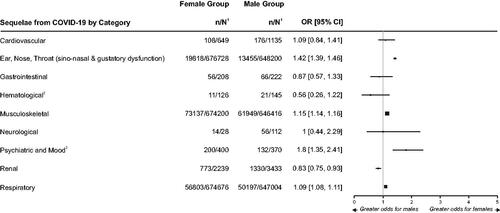
In our review, female (versus male) patients were significantly more likely to have sequelae of COVID-19 disease in the categories of psychiatric and mood (i.e. depression) (OR = 1.80; 95% CI: 1.35–2.41), ear, nose or throat (ENT; OR = 1.42; 95% CI: 1.39–1.46), musculoskeletal (i.e. myalgia) (OR = 1.15; 95% CI: 1.14–1.16), and respiratory (OR = 1.09; 95% CI: 1.08–1.11). Male (versus female) patients were significantly more likely to have sequelae in the category of renal disorders (i.e. acute kidney injury) (OR = 0.83; 95% CI: 0.75–0.93). Point estimates of OR for cardiovascular sequelae favored female patients and point estimates for gastrointestinal (GI) and hematological sequelae (i.e. thrombocytopenia) favored male patients, although the 95% CI indicated the between-sex difference was not significant for these categories of sequelae (). The point estimate for OR indicates similar likelihood of neurological sequelae between sexes.
Figure 2. Forest plot of odds ratio for sequelae of COVID-19 between female and male patientsCitation22–44.
1. n = number in group with the outcome; N = total number in the group.
2. These categories represent only one kind of symptom/condition: Hematological (thrombocytopenia), Musculoskeletal (myalgia), Psychiatric and Mood (depression), Renal (acute kidney injury).
Notes: Sequelae is defined as new condition or chronic symptom a patient developed as a direct result of being infected with COVID-19 after the acute phase of the infection had terminated, with the acute phase typically lasting no longer than 4 weeks from the onset of COVID-19 symptoms.
Outcomes within each category of sequela are listed in .
The size of the squares used for the point estimates is proportional to the weight.
Sex differences in long COVID syndrome (>4 weeks after onset of symptoms)
In our review, the prevalence of long COVID syndrome varied widely across studies, ranging from 9.0% to 81.6%. Overall, the likelihood of having long COVID syndrome was significantly greater among female versus male patients (OR = 1.22; 95% CI: 1.13–1.32).
shows clinical variation in relation to long COVID syndrome by sex. Among the clinical categories reported for long COVID syndrome, ENT (OR = 2.28; 95% CI: 1.94–2.67), GI (OR = 1.60; 95% CI: 1.04–2.44), psychiatric and mood (OR = 1.58; 95% CI: 1.37–1.82), neurological (OR = 1.30; 95% CI: 1.03–1.63), dermatological (OR = 1.29; 95% CI: 1.05–1.58), and other (OR = 1.36; 95% CI: 1.25–1.49) disorders were significantly more likely among female patients as compared to male patients, the latter driven in large part by differences in rheumatological complications (OR = 3.31; 95% CI: 1.27–8.65) and fatigue (OR = 2.34; 95% CI: 1.83–2.98) (). Point estimates for OR indicate higher odds of musculoskeletal (OR = 1.17) complications of long COVID syndrome among female patients, although the lower bound of the 95% CI was less than 1 (). In contrast, males were significantly more likely to have complications in the clinical categories of endocrine (i.e. diabetes) (OR = 0.75; 95% CI: 0.69–0.81) and renal disorders (OR = 0.74; 95% CI: 0.64–0.86). The point estimate for OR indicate similar likelihood of hematological (i.e. hemorrhagic complications), respiratory, and cardiovascular complications between sexes.
Figure 3. Forest Plot of Odds Ratio for Long COVID Syndrome Between Female and Male PatientsCitation13,Citation50–55.
1. n = number in group with the outcome; N = total number in the group.
2. Venturelli and associatesCitation54 provided disaggregated data for dermatological complications and rheumatologic complications (the latter included in the category of Other); the data were combined in their primary report.
3. These categories represent only one kind of symptom/condition: Endocrine (diabetes), Hematological (hemorrhagic complications).
Notes: Long COVID syndrome is defined by the persistence of symptoms or development of sequelae and delayed or long-term complications beyond 4 weeks from the onset of acute symptoms of COVID-19.
Outcomes within each category of sequela are listed in .
The size of the squares used for the point estimates is proportional to the weight.
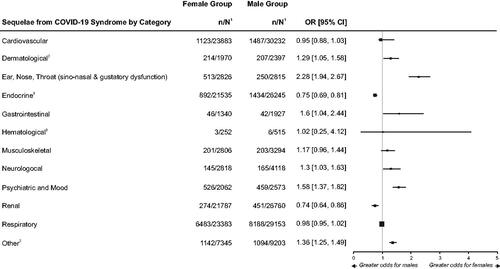
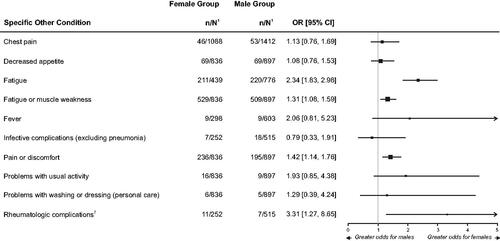
Figure 4. Forest Plot of Odds Ratio for Other Sequelae of Long COVID Syndrome Between Female and Male PatientsCitation50,Citation51,Citation53,Citation54.
1. n = number in group with the outcome; N = total number in the group.
2. Rheumatologic complications includes data provided by Venturelli and associatesCitation54; the data were combined with dermatological complications in their primary report.
Notes: Long COVID syndrome is defined by the persistence of symptoms or development of sequelae and delayed or long-term complications beyond 4 weeks from the onset of acute symptoms of COVID-19.
The size of the squares used for the point estimates is proportional to the weight.
Discussion
Sex differences in COVID-19 outcomes such as hospitalization, ICU admission, mechanical ventilation, and mortality have been reported in numerous studiesCitation56,Citation57, as have physiological differences between sexes in immune responseCitation58. Despite these findings, the majority of COVID-19 studies in the literature do not report disaggregated data to allow meaningful characterization of the sex differences. To our knowledge, this is one of few reviews conducted to characterize sex differences in early sequelae of COVID-19 disease as well as long COVID syndrome.
Our review uncovered between-sex differences for some of the early sequelae of COVID-19 infection and for long COVID syndrome. In the early phase of illness, psychiatric/mood, ENT, musculoskeletal, and respiratory sequelae were significantly more likely in female patients, and renal sequelae were significantly more likely in male patients. Studies published after the date cutoff for our narrative review have reported similar findings. For example, in a study of patients with mild-to-moderate COVID-19 illness, Lechien et al.Citation59 observed that females were significantly more affected by olfactory and gustatory dysfunctions than were males. Similarly, ElibolCitation60 reported that otolaryngological symptoms (e.g. anosmia and dysgeusia) were more common in female patients as compared to male patients. In a study of critically ill patients with COVID-19, Toth-Manikowski et al.Citation61 found that males had a higher risk of severe acute kidney injury (OR 1.92) than did the females.
Across the few studies that have reported sex-disaggregated data, we found that, overall, female patients were more likely to experience long COVID-19 syndrome than their male counterparts, a trend that was seen in all 9 studies included in our reviewCitation45–49,Citation52–55. In patients who experienced long COVID syndrome, ENT, GI, psychiatric/mood, dermatological, neurological, and other complications (primarily rheumatological complications and fatigue) were significantly more likely in female patients while endocrine and renal complications were significantly more likely in male patients. In line with our findings, other research groups reported that risk of persistent symptoms is higher among female than male patients previously hospitalized for COVID-19, with female sex a predictor of chronic fatigue and symptoms of mood/behavior disorders as well as symptoms of various other disordersCitation62,Citation63.
Differences in immune system function between females and males could be an important driver of sex differences in long COVID-19 syndromeCitation64,Citation65. Females mount more rapid and robust innate and adaptive immune responses, which can protect them from initial infection and severity. However, this same difference can render females more vulnerable to prolonged autoimmune-related diseasesCitation64,Citation65. Moreover, Stewart et al. hypothesized that sex hormone differences may also contribute to the asymmetry in risk and outcomes between sexes, based on overlapping symptoms of long COVID syndrome with those of perimenopause and menopauseCitation66. Expanding beyond the biological basis, sex differences in outcomes have been reported during previous coronavirus outbreaksCitation2–5. Therefore, differences in outcomes between females and males infected with SARS-CoV-2, as reported herein, could have been anticipated. Unfortunately, most studies did not evaluate or report granular data by sex, which limited sex-specific clinical insights that may be impacting treatment.
Limitations
Our findings are based on analyses of relatively small data sets from the few published studies that reported sex-disaggregated outcome data during the first 18 months of the COVID-19 pandemic. Our search for published papers that reported sex-disaggregated data identified only 23 studies of COVID-19 sequelae and 12 studies of long COVID syndrome. Global tracking of COVID-19 by sexCitation67 and ongoing studies of long COVID syndrome [Supplemental Content Table S3] should add to the evidence base. Other limitations of our work include no reporting of data by gender. It is well known that gender can also impact clinical outcomes, as we have seen during the COVID-19 pandemicCitation68. For example, women are more likely to have higher exposures to the virus in certain occupations such as nursing and education, which can predispose them to higher incidence of the disease. In addition, there may be disparities in access to care based on gender that could affect the natural history of the disease, leading to more complications and sequela. Gender is also important in diagnosis of diseases, particularly in chronic conditions where women are often diagnosed later than menCitation69. This is important for long COVID syndrome, as the symptoms in women may be dismissed as being psychologically similar to the myalgic encephalomyelitis or chronic fatigue syndrome observed in Lyme diseaseCitation70.
Evidence from some, but not all, studies suggests that individuals who receive COVID-19 vaccination and subsequently become infected with SARS-CoV-2 are less likely to experience symptoms of long COVID syndrome than unvaccinated individualsCitation71,Citation72. The studies included in this review were conducted before COVID-19 vaccination was widely available, preventing us from assessing the impact of vaccination status on prevalence of long COVID syndrome by sex.
Implications for the future
The size of female cohorts and sex-disaggregated data analysis and reporting are insufficient in medical researchCitation73–76. The lack of studies reporting sex-disaggregated outcomes for COVID-19Citation77 speaks to the need for further, large-scale research that includes sex as an analytical variable and that reports data by sex. In this regard, the United Nations has issued an urgent global health mandate to characterize the differential impact of sex on COVID-19 detection and clinical managementCitation78. Devising basic research and clinical trial protocols using sex-specific methodologies, with a primary objective of prospectively evaluating aspects of COVID-19 by sex, will fill critical information gaps. A thorough understanding of how biological sex is influencing COVID-19 will have important implications for clinical management and mitigation strategies for this disease.
Conclusions
Our literature reviews of early, published research uncovered differences between sexes on early sequelae of COVID-19 and on long COVID syndrome, suggesting the opportunity to develop and implement prevention and treatment interventions tailored to each sex. In doing so, there is the potential to reshape the natural history of COVID-19.
Transparency
Declaration of funding
This work was supported by funding from Johnson & Johnson, New Brunswick, NJ, USA.
Declaration of financial/other relationships
Shirley V. Sylvester, Carly O’Keefe, Rada Rusu, and Susan Nicholson are employees of Johnson and Johnson and some hold company equity. Some of the contributions to this work by Biankha Chan and Martha Bellows were done while they were employed by Johnson & Johnson. All authors meet ICMJE criteria and all those who fulfilled those criteria are listed as authors. All authors had access to the study data and made the final decision about where to present these data. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
SVS, SN, MB, CO, BC, and RR were involved in the conception, design, analysis and interpretation of the data. All authors were involved in drafting of the paper or revising it critically for intellectual content; the final approval of the version to be published; and, agree to be accountable for all aspects of the work. All authors meet ICMJE criteria and all those who fulfilled those criteria are listed as authors.
Supplemental Material
Download MS Word (35 KB)Acknowledgements
The authors acknowledge Sandra Norris, PharmD, of the Norris Communications Group, LLC for medical writing assistance and Ellen Baum, PhD (Janssen Global Services, LLC), for additional editorial support. The authors thank Dr. Serena Venturelli and associates from Bergamo and Milan, Italy, for providing sex-disaggregated data on dermatological complications and on rheumatologic complications of long COVID syndrome, which were combined in their primary reportCitation54. The authors also thank Alan Fisher, DrPH, an independent consultant, for his assistance in screening articles and Marsha Wilcox, EdD, ScD for her input into the initial design of the review.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
References
- World Health Organization. Gender and genetics; [cited 2022 Feb 1]. Available from:https://www.who.int/genomics/gender/en/#:∼:text=Sex%20and%20gender%20are%20both,outcomes%20for%20women%20and%20men.
- Alghamdi IG, Hussain II, Almalki SS, et al. The pattern of Middle east respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi ministry of health. Int J Gen Med. 2014;7:417–423.
- Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159(3):229–231.
- Leung GM, Hedley AJ, Ho LM, et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141(9):662–673.
- Leong HN, Earnest A, Lim HH, et al. SARS in Singapore–predictors of disease severity. Ann Acad Med Singap. 2006;35(5):326–331.
- Heidari S, Ahumada C, Kurbanova Z. GENDRO gender, evidence and health network. Towards the real-time inclusion of sex- and age-disaggregated data in pandemic responses. BMJ Glob Health. 2020;5(10):e003848.
- Lancet . The gender dimension to COVID-19. The Lancet. 2020;395(10231):1168.
- Phillips S, Williams MA. Confronting our next national health disaster – long-haul Covid. N Engl J Med. 2021;385(7):577–579.
- Higgins V, Sohaei D, Diamandis EP, et al. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2021;58(5):297–310.
- Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264.
- Maamar M, Artime A, Pariente E, et al. Post-COVID-19 syndrome, inflammatory markers and sex differences. MedRxiv. 2021;2021:1–9.
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615.
- Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-COVID syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ. 2021;372:n693.
- Nabavi N. Long COVID: How to define it and how to manage it. BMJ. 2020;370:m3489.
- Oronsky B, Larson C, Hammond TC, et al. A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol. 2021;2021:1–9.
- Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–1273.
- Khamsi R. Rogue antibodies could be driving severe COVID-19. Nature. 2021;590(7844):29–31.
- Jarefors S, Bennet L, You E, et al. Lyme borreliosis reinfection: might it be explained by a gender difference in immune response? Immunology. 2006;118(2):224–232.
- Torcia MG, Nencioni L, Clemente AM, et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS One. 2012;7(6):e39853.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7(1):16.
- Meini S, Suardi LR, Busoni M, et al. Olfactory and gustatory dysfunctions in 100 patients hospitalized for COVID-19: sex differences and recovery time in real-life. Eur Arch Otorhinolaryngol. 2020;277(12):3519–3523.
- Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002.
- Santoliquido A, Porfidia A, Nesci A, et al. Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J Thromb Haemost. 2020;18(9):2358–2363.
- Pelayo J, Lo KB, Bhargav R, et al. Clinical characteristics and outcomes of community- and hospital-acquired acute kidney injury with COVID-19 in a US inner city hospital system. Cardiorenal Med. 2020;10(4):223–231.
- Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14.
- Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26.
- COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. Erratum in: Lancet. 2020.
- Whyte MB, Kelly PA, Gonzalez E, et al. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res. 2020;195:95–99.
- Zhang LI, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China. Prevalence, risk factors, and outcome. Circulation. 2020;142(2):114–128.
- Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121.
- Ma YF, Li W, Deng HB, et al. Prevalence of depression and its association with quality of life in clinically stable patients with COVID-19. J Affect Disord. 2020;275:145–148.
- Chen W, Li Z, Yang B, et al. Delayed-phase thrombocytopenia in patients with Coronavirus disease 2019 (COVID-19). Br J Haematol. 2020;190(2):179–184.
- Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218.
- Yao N, Wang SN, Lian JQ, et al. [Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region]. Zhonghua Gan Zang Bing Za Zhi. 2020;28(3):234–239. Chinese.
- Jiang S, Wang R, Li L, et al. Liver injury in critically ill and non-critically ill COVID-19 patients: a multicenter, retrospective, observational study. Front Med. 2020;7:347.
- Sierpiński R, Pinkas J, Jankowski M, et al. Sex differences in the frequency of gastrointestinal symptoms and olfactory or taste disorders in 1942 nonhospitalized patients with coronavirus disease 2019 (COVID-19). Pol Arch Intern Med. 2020;130(6):501–505.
- Biadsee A, Biadsee A, Kassem F, et al. Olfactory and oral manifestations of COVID-19: sex-related symptoms-a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722–728.
- Yu M, Liu Y, Xu D, et al. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020;21(6):746–755.
- Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance – United States, 22 January–30 May, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(24):759–765.
- Wei JF, Huang FY, Xiong TY, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106(15):1154–1159.
- Dogra S, Jain R, Cao M, et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29(8):104984.
- Kremer S, Lersy F, de Sèze J, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297(2):E242–E251.
- Aggarwal A, Shrivastava A, Kumar A, et al. Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care Centre in New Delhi. J Assoc Physicians India. 2020;68(7):19–26.
- Gaber TAK, Ashish A, Unsworth A. Persistent post-covid symptoms in healthcare workers. Occup Med. 2021;71(3):144–146.
- Prieto MA, Prieto O, Castro HM. Long COVID: cross sectional study. Rev Fac Cien Med Univ Nac Cordoba. 2021;78(1):33–36.
- Jacobson KB, Rao M, Bonilla H, et al. Patients with uncomplicated Coronavirus disease 2019 (Covid-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 2021;73(3):e826–e829.
- Perlis RH, Green J, Santillana M, et al. Persistence of symptoms up to 10 months following acute COVID-19 illness. medRxiv [Preprint]. 2021; Mar 8:2021.03.07.21253072.
- Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631.
- Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199(2):113–119.
- Cortés-Telles A, López-Romero S, Figueroa-Hurtado E, et al. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir Physiol Neurobiol. 2021;288:103644.
- Makaronidis J, Firman C, Magee CG, et al. Distorted chemosensory perception and female sex associate with persistent smell and/or taste loss in people with SARS-CoV-2 antibodies: a community based cohort study investigating clinical course and resolution of acute smell and/or taste loss in people with and without SARS-CoV-2 antibodies in London, UK. BMC Infect Dis. 2021;21(1):221.
- Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784.
- Venturelli S, Benatti SV, Casati M, et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149:e32.
- Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232.
- Gomez JMD, Du-Fay-de-Lavallaz JM, Setri Fugar S, et al. Sex differences in COVID-19 hospitalization and mortality. J Womens Health. 2021;30(5):646–653.
- Vahidy FS, Pan AP, Ahnstedt H, et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross-sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16(1):e0245556.
- Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320.
- Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261.
- Elibol E. Otolaryngological symptoms in COVID-19. Eur Arch Otorhinolaryngol. 2021;278(4):1233–1236.
- Toth-Manikowski SM, Caldwell J, Joo M, et al. Sex-related differences in mortality, acute kidney injury, and respiratory failure among critically ill patients with COVID-19. Medicine. 2021;100(50):e28302.
- Munblit D, Bobkova P, Spiridonova E, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy. 2021;51(9):1107–1120.
- Sigfrid L, Drake TM, Pauley E, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. Lancet Reg Health Eur. 2021;8:100186.
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638.
- Sharma G, Volgman AS, Michos ED. Sex differences in mortality from COVID-19 pandemic: Are men vulnerable and women protected? JACC Case Rep. 2020;2(9):1407–1410.
- Stewart S, Newson L, Briggs TA, et al. Long COVID risk – a signal to address sex hormones and women’s health. Lancet Regional Health. 2021;11:100242.
- Global Health 5050: The Sex, Gender and COVID-19 Project. Tracking differences in COVID-19 infection, illness and death among women and men and producing the world’s largest analysis of sex and gender in national COVID-19 health policies [internet]. Washington DC: ICRW; 2021. Oct 27. Available from: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/
- Nordhues HC, Bhagra A, Stroud NN, et al. COVID-19 gender disparities and mitigation recommendations: a narrative review. Mayo Clin Proc. 2021;96(7):1907–1920.
- Westergaard D, Moseley P, Sørup FKH, et al. Population-wide analysis of differences in disease progression patterns in men and women. Nat Commun. 2019;10(1):666.
- Wormser GP, Shapiro ED. Implications of gender in chronic Lyme disease. J Womens Health. 2009;18(6):831–834.
- UK Health Security Agency. The effectiveness of vaccination against long COVID: A rapid evidence briefing. February 2022; [cited 2022 May XX]. Available from:https://ukhsa.koha-ptfs.co.uk/cgi-bin/koha/opac-retrieve-file.pl?id=fe4f10cd3cd509fe045ad4f72ae0dfff.
- Ledford H. Do vaccines protect against long COVID? What the data say. Nature. 2021;599(7886):546–548.
- Bischof E, Wolfe J, Klein SL. Clinical trials for COVID-19 should include sex as a variable. J Clin Invest. 2020;130(7):3350–3352.
- Kocher K, Delot-Vilain A, Spencer D, et al. Paucity and disparity of publicly available sex-disaggregated data for the COVID-19 epidemic hamper evidence-based decision-making. Arch Sex Behav. 2021;50(2):407–426.
- Sugimoto CR, Ahn YY, Smith E, et al. Factors affecting sex-related reporting in medical research: a cross-disciplinary bibliometric analysis. Lancet. 2019;393(10171):550–559.
- World Health Organization. Breaking barriers towards more gender-responsive and equitable health systems, 18 October 2019; [cited 2022 Feb 1]. Available from: https://www.who.int/publications/m/item/breaking-barriers-towards-more-gender-responsive-and-equitable-health-systems.
- Brady E, Nielsen MW, Andersen JP, et al. Lack of consideration of sex and gender in COVID-19 clinical studies. Nat Commun. 2021;12(1):4015.
- United Nations Population Fund (UNFPA). COVID-19: A gender lens. Protecting sexual and reproductive health and rights, and promoting gender equality (March 2020); [cited 2022 Feb 1]. Available from: https://www.unfpa.org/resources/covid-19-gender-lens.