Abstract
Objective
To report on the use of antihyperglycemic agents (AHAs) by age (i.e. <65, ≥65 years) in patients with type 2 diabetes (T2D) and cardiovascular disease (CVD) or cardiovascular risk (CV risk) factors in the United States.
Methods
Patients with T2D and CVD (CVD cohort) or T2D and an additional CV risk factor without pre-existing CVD (CV risk cohort) were identified from 2015 to 2019 in a claims database. Patients were followed from their first observed CVD diagnosis or CV risk factor for each year they were continuously enrolled or until occurrence of a CVD diagnosis (CV risk cohort only). Classes of AHAs received were reported by year, cohort, and age group.
Results
From 2015 to 2019, the percentage of patients <65 years on glucagon-like peptide-1 receptor agonists (GLP-1 RAs) increased (CVD: 9–17%, CV risk: 9–17%) and was approximately twice that of those ≥65 years (CVD: 4–8%, CV risk: 4–8%); the percentage of patients <65 years on sodium-glucose cotransporter-2 (SGLT2) inhibitors increased (CVD: 11–16%, CV risk: 11–17%) and was approximately triple that of those ≥65 years (CVD: 3–6%, CV risk: 4–7%).
Conclusions
The use of GLP-1 RAs and SGLT2 inhibitors increased during the study period; however, most patients did not receive these medications. Patients aged ≥65 years were particularly disadvantaged.
1. Introduction
It is estimated that more than 30 million Americans, or 9.4% of the population of the United States (US), have diabetes [Citation1]. Type 2 diabetes (T2D) is estimated to account for more than 95% of all diabetes cases in the US and most often develops in people over the age of 45 years [Citation2]. Cardiovascular disease (CVD) has been shown to be a major cause of death and disability among patients with diabetes [Citation3,Citation4], with adults with diabetes tending to have a higher prevalence rate of CVD compared with adults without diabetes [Citation5]. A recent worldwide study estimated that CVD affects approximately one-third of all patients with T2D and was the cause of death in almost 50% of patients with T2D [Citation6]. Furthermore, previous research has suggested that the economic impact of CVD is substantial and contributes to between 20 and 49% of the total direct costs associated with treating T2D [Citation7].
Historically, the focus of T2D treatment has been on maintaining good glycemic control. Starting in 2015, there was evidence published on the cardiovascular benefits of sodium-glucose cotransporter-2 (SGLT2) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1 RAs), with American Diabetes Association (ADA) recommending the use of empagliflozin or liraglutide for patients with long-standing, suboptimally controlled T2D and established atherosclerotic cardiovascular disease, in 2017 [Citation8]. Currently, two classes of antihyperglycemic agents (AHAs) approved for the treatment of T2D also have demonstrated cardiovascular (CV) benefits: SGLT2 inhibitors (i.e. empagliflozin, canagliflozin, and dapagliflozin) and GLP-1 RAs (i.e. liraglutide, semaglutide, and dulaglutide). Furthermore, the 2020 ADA guidelines recommend treatment with empagliflozin, canagliflozin, dapagliflozin, liraglutide, semaglutide, or dulaglutide for patients with T2D and either established atherosclerotic CVD or multiple atherosclerotic CVD risk factors to reduce the risk of major adverse CV events and heart failure hospitalization [Citation1].
Despite the available published evidence on the benefits of certain SGLT2 inhibitors and GLP-1 RAs since 2015 and 2016, respectively, and the recommendations for their use in patients with atherosclerotic CVD in the ADA guidelines published since 2017 [Citation8], previous studies have reported that only a low percentage of patients with T2D and CVD receive AHA therapy with CV benefits [Citation9–11]. However, Hamid et al. and Pantalone et al. conducted their studies in individual medical centers between 2013 and 2019, while Arnold and colleagues, whose data were included in an ongoing registry between 2016 and 2018, evaluated patients with atherosclerotic CVD and T2D. Results generated from these previous analyses may not be nationally representative of the general T2D population with CVD and CV risk. Additionally, there is a lack of real-world published data on the longitudinal changes in prescribing patterns for GLP-1 RAs and SGLT2 inhibitors as well as an understanding of patient characteristics for patients with T2D and CVD or patients with T2D and additional CV risk factors. Furthermore, to our knowledge, no published information exists regarding treatment patterns of AHAs among patients with T2D and CV risk factors.
This retrospective observational study was undertaken to provide more recent information and fill noted gaps in the literature regarding the utilization of classes of AHAs by year (over a 5-year time period) in patients with T2D and CVD and CV risk factors. Specifically, the key objective of this analysis of administrative health insurance claims data was to evaluate two cohorts of patients with T2D (i.e. patients with T2D and CVD and patients with T2D and at least one additional CV risk factor without pre-existing CVD) to assess trends in AHA prescribing patterns between 2015 and 2019, stratified by age group (i.e. <65 years, ≥65 years).
2. Methods
2.1. Data source
This study used deidentified data from the IBM Watson Health Analytics’ MarketScan (MarketScan) Commercial Claims and Encounters (CCAE) and the Medicare Supplemental databases. The CCAE database contains medical and drug utilization data for nearly 60 million unique individuals, encompassing enrollees in both employer-sponsored and private health insurance plans. In total, more than 100 large employers and 12 unique health plans across the US are represented in the database. Similarly, the Medicare Supplemental database contains medical and drug utilization data for individuals with supplemental insurance paid by employers and includes both the Medicare- and employer-covered portions of payment.
For this study, data between 1 January 2015, and 30 September 2019 (which represented the 5 most recent years of data available at the time this study was conducted), for all patients with at least one diagnosis of T2D (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] codes 250.x0, 250.x2 or International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] code E11.xx), representing 4,859,172 unique individuals, were used.
Because the data used in this analysis were deidentified, retrospective, and preexisting, RTI International’s Institutional Review Board determined that this study did not classify as research with human subjects.
2.2. Patient selection criteria and study cohorts
Two cohorts of patients with T2D were evaluated in this study: all patients with CVD (CVD cohort) and all patients with at least one additional CV risk factor without pre-existing CVD (CV risk cohort). Patients were selected for inclusion in the CVD cohort if they had a medical claim with evidence of CVD (i.e. a diagnosis of acute coronary syndromes [excluding myocardial infarction], carotid arterial disease, coronary artery disease, heart failure, ischemic stroke, left ventricular hypertrophy, myocardial infarction, peripheral artery disease, transient ischemic attack, or a procedure code for a coronary artery bypass graft or percutaneous coronary interventions) between 1 January 2015, and 30 September 2019 (). The date of the first observed claim with evidence of CVD during the study period was termed the CVD index date and the calendar year during which the CVD index date occurred was termed the index year. Patients in the CVD cohort were required to have at least two claims with a diagnosis of T2D (ICD-9-CM codes 250.x0, 250.x2; or ICD-10-CM code E11.xx) at least 30 days apart either before or during the index year. Patients in the CVD cohort were also required to be ≥18 years of age on their CVD index date and to have continuous health plan enrollment for the index year. Patients in the CVD cohort were followed for each calendar year they remained continuously enrolled in the health plan or until the end of the database, whichever occurred first.
Figure 1. Study schemas. (A) CVD cohort. (B) CV risk cohort. Abbreviations. CV, cardiovascular; CVD, cardiovascular disease; T2DM, type 2 diabetes mellitus.
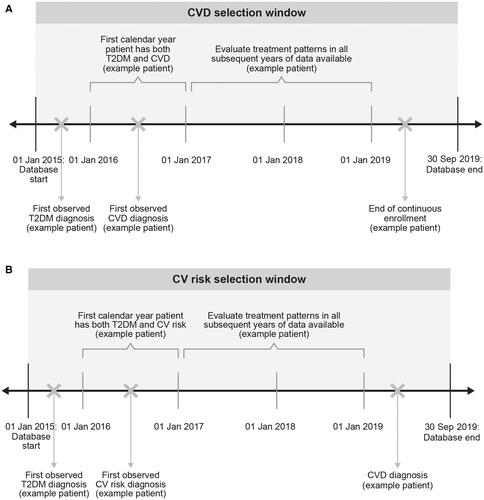
Patients were included in the CV risk cohort if they had evidence of a CV risk factor (i.e. a diagnosis on a medical claim for hyperlipidemia, hypertension, obesity, a history of smoking, or microalbuminuria or macroalbuminuria or receipt of a prescription for a likely CV-related medication [i.e. aspirin, beta blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, antithrombotics, or antihyperlipidemics]) without pre-existing CVD, in addition to the diagnosis of T2D, between 1 January 2015, and 30 September 2019, with the date of the first observed CV risk factor defining the CV risk index date (and the calendar year during which the CV risk index date occurred termed the index year) (). Patients in the CV risk cohort were required to have at least two claims for T2D on separate dates at least 30 days apart either before or during the index year, be aged ≥18 years on the CV risk index date, and have continuous health plan enrollment for the entire index year. Patients in the CV risk cohort were excluded from the study if they had a CVD event or received an antiplatelet medication (excluding aspirin monotherapy, as aspirin monotherapy may have been used for primary prevention) either before or during the index year. Patients in the CV risk cohort were followed for each calendar year they remained continuously enrolled in the health plan and did not have a CVD diagnosis or until the end of the database, whichever occurred first.
Patients were excluded from the CVD and CV risk cohorts if they had evidence of type 1 diabetes (T1D). Because miscoding of T1D in a T2D population may be common, patients were allowed to remain in the study if they had a T1D diagnosis so long as the total number of T1D diagnoses observed on all claims during the index year was lower than the total number of T2D diagnoses observed on all claims during the index year. Patients also were excluded from the CVD and CV risk cohorts if they had a diagnosis of end-stage renal disease (ICD-9-CM code 585.6 or ICD-10-CM code N18.6) or stage 5 chronic kidney disease (ICD-9-CM code 585.5 or ICD-10-CM code N18.5) at any point in time.
2.3. Study measures
Study measures for both cohorts included patient demographics, clinical characteristics, and classes of AHAs received. Demographic characteristics were measured at the index date and included age, sex, insurance type (i.e. commercial, Medicare), health plan type, year of index date, and geographic location.
Clinical characteristics included comorbidities, diabetes severity, and CVD or CV risk characteristics. To summarize patients’ overall comorbidity burden, the Charlson Comorbidity Index (CCI) score was calculated during the index year [Citation12–14]. Diabetes severity was measured during the index year using the Diabetes Complications Severity Index (DCSI) [Citation15,Citation16]. The numbers and percentages of patients with diagnoses of other conditions of interest (i.e. obesity, hypertension, hyperlipidemia) during the index year were also reported.
Among patients in the CVD cohort, the types of CVD events observed during the index year were reported. Similarly, among patients in the CV risk cohort, the types of CV risk factors observed during the index year were reported. The percentages of patients receiving any antihyperglycemic medication along with the classes of AHAs that were received in each calendar year were reported for patients in both cohorts. Classes of AHAs included biguanides, GLP-1 RAs overall, GLP-1 RAs with CVD benefit (i.e. albiglutide, dulaglutide, liraglutide, semaglutide), SGLT2 inhibitors (i.e. empagliflozin, ertugliflozin, canagliflozin, and dapagliflozin; note: all SGLT2 inhibitors except ertugliflozin have CVD benefit), insulin, sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, and thiazolidinediones (note: the percentages of patients receiving meglitinides and alpha-glucosidase inhibitors were small and did not tend to vary over time; therefore, data for these medication classes were not shown). Patients were considered to have received medication in a class if they had at least one prescription claim for the medication at any point in the calendar year.
2.4. Data analysis
Analyses of patient demographics, clinical characteristics, CVD and CV risk characteristics, and treatment patterns were descriptive and entailed the tabular display of mean values, medians, ranges, and standard deviations for continuous variables and frequency distributions and percentages for categorical variables. All analyses were reported by cohort and stratified by patient age group (i.e. <65 years, ≥65 years).
The generalized estimating equations (GEEs) method was used to evaluate factors associated with the use of SGLT2 inhibitors and GLP-1 RAs over time. This method accounted for missing data as well as for the correlation of outcomes arising from repeated measures across individual patients in multiple years of follow-up. Odds ratios (ORs) were reported for all variables of interest. Dependent variables were flags for the presence of a pharmacy claim for an SGLT2 inhibitor or GLP-1 RA in each calendar year of follow-up observed for the patient. Covariates that were included in the GEE models included year of measurement, demographics (i.e. age, sex, geographic region), clinical characteristics (i.e. diagnoses of obesity, hyperlipidemia), and treatment history (i.e. previous use of an AHA, antihypertensive, and cholesterol-lowering medications).
All analyses were conducted using SAS version 9.4 (Cary, NC, USA).
3. Results
3.1. CVD cohort
Of the total 4,859,172 patients with at least 1 diagnosis of T2D, 1,208,938 patients (25%) were identified as having a CVD event. Of these patients, 693,910 patients met all of the selection criteria for the CVD cohort, with 55.6% of these patients aged <65 years. Demographic characteristics of these patients are presented in .
Table 1. Patient demographics, by cohort and age group.
The most common CVD conditions observed in the first year of follow-up were coronary artery disease, peripheral artery disease, and heart failure (). Both the CCI and DCSI scores were slightly higher for patients aged ≥65 years compared with patients aged <65 years (). Approximately one-third of patients had a diagnosis of obesity in the first year of follow-up (39.1% aged <65 years and 23.1% aged ≥65 years). Diagnoses of hyperlipidemia and hypertension were common in the first year of follow-up; these percentages did not tend to vary by patient age.
Table 2. Clinical characteristics, by cohort and age group.
Approximately three-quarters of patients received at least one AHA and this proportion remained approximately the same over time (overall: range, 72.7% in 2019 to 75.0% in 2015; age <65 years: range, 73.7% in 2019 to 77.9% in 2015; age ≥65 years: range, 70.5% in 2019 to 72.8% in 2016 [data not shown]). Regardless of the calendar year or age group, metformin was the most common AHA received (). A quarter of the patients received insulin and sulfonylureas in 2015, and the percentages of patients receiving these medications decreased slightly over time; these results were observed overall and in both patient age groups. Approximately 16.6% of patients received DPP-4 inhibitors in 2015; this percentage did not tend to change over time, and results were consistent for both patient age groups. In 2015, the percentage of patients receiving thiazolidinediones was low (less than 5%), and this percentage did not vary over time or by patient age group.
Figure 2. Antihyperglycemic treatment classes received in the CVD cohorta. (A) Overallb. (B) Patients aged <65 yearsc. (C) Patients aged ≥65 yearsd. Abbreviations. CVD, cardiovascular disease; DPP-4, dipeptidyl peptidase-4; GLP-1 RA, glucagon-like peptide-1 receptor agonist; SGLT2, sodium-glucose cotransporter-2. aGLP-1 RAs included albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide (oral and subcutaneous). GLP-1 RAs with labeled CVD benefit included dulaglutide, liraglutide, and semaglutide (subcutaneous only). SGLT2 inhibitors included canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. All these medications, except ertugliflozin, have proven CVD benefit. bThe percentage of patients receiving any AHA ranged from 72.7% in 2019 to 75.0% in 2015. cThe percentage of patients receiving any AHA ranged from 73.7% in 2019 to 77.9% in 2015. dThe percentage of patients receiving any AHA ranged from 70.5% in 2019 to 72.7% in 2015.
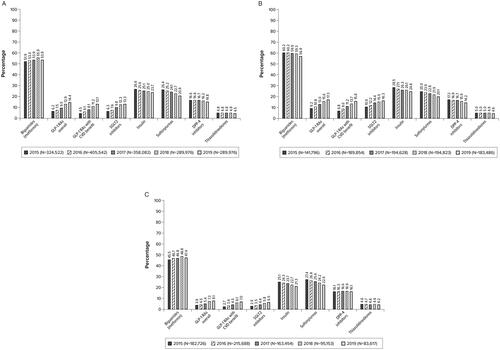
Overall, the percentage of patients receiving any GLP-1 RA (regardless of whether the medication had CV benefit or not) increased from 6.2% in 2015 to 14.4% in 2019. The percentage of patients receiving GLP-1 RAs with CV benefit increased from 4.5% in 2015 to 13.1% in 2019. Similarly, the percentage of patients receiving SGLT2 inhibitors increased from 6.3% in 2015 to 13.3% in 2019. The percentage of patients receiving any GLP-1 RA and the percentage of patients receiving GLP-1 RAs with CV benefit ranged from 9.2% in 2015 to 17.3% in 2019 and 6.8% in 2015 to 15.8% in 2019, respectively, among patients aged <65 years and ranged from 3.9% in 2015 to 8.1% in 2019 and 2.7% in 2015 to 7.0% in 2019, respectively, among patients aged ≥65 years. Similarly, the percentage of patients receiving SGLT2 inhibitors ranged from 10.6% in 2015 to 16.3% in 2019 among patients aged <65 years and ranged from 2.9% in 2015 to 6.5% in 2019 among patients aged ≥65 years.
The likelihood of receipt of GLP-1 RAs and SGLT2 inhibitors was found to increase with increasing calendar year, even after results were controlled for previous receipt of the medication ( and ). Females were slightly more likely to receive GLP-1 RAs and slightly less likely to receive SGLT2 inhibitors compared with males, while patients aged ≥65 years were less likely to receive either GLP-1 RAs or SGLT2 inhibitors compared with patients aged <65 years. A higher CCI score was also associated with an increased likelihood of receipt of either GLP-1 RAs or SGLT2 inhibitors, with the ORs tending to increase with increasing CCI score. Diagnoses of obesity and hyperlipidemia in the calendar year of the index date were also associated with an increased likelihood of receipt of either GLP-1 RAs or SGLT2 inhibitors, while receipt of a cholesterol-lowering medication or any antihypertensive agent was associated with a decreased likelihood of receipt of either a GLP-1 RA or an SGLT2 inhibitor.
Table 3. Factors associated with use of SGLT2 inhibitors, by cohorta.
Table 4. Factors associated with use of GLP-1 RAs, by cohort.
3.2. CV risk cohort
Of the 4,859,172 patients with at least 1 diagnosis of T2D, 3,853,320 patients (79%) were identified as having an additional CV risk factor. Of these patients, 1,244,610 patients met all of the inclusion criteria for the CV risk cohort, with 82.5% of these patients aged <65 years. Demographic characteristics of these patients are presented in . The most common additional CV risk factors were prescribed pharmaceuticals (i.e. a prescription claim for aspirin, beta blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, antithrombotics, or antihyperlipidemics), diagnoses of hyperlipidemia, and diagnoses of hypertension (). Almost one-third of patients had a diagnosis of obesity in the index year (30.5% among patients aged <65 years and 17.7% among patients aged ≥65 years) ().
Approximately three-quarters of patients received any AHA, and this percentage remained approximately the same over time (overall: range, 76.2% in 2019 to 77.5% in 2015; age <65 years: range, 76.4% in 2019 to 78.5% in 2015; age ≥65 years: range, 73.6% in 2019 to 74.3% in 2018 [data not shown]). Regardless of the calendar year or patient age, metformin was the most common AHA received (). In 2015, one-quarter of patients received sulfonylureas, and this proportion decreased slightly over time; these results were observed overall and in both patient age groups. Additionally, the percentages of patients receiving insulin or DPP-4 inhibitors were similar and did not vary over time or by patient age group. In 2015, the percentage of patients receiving thiazolidinediones was low, and the percentage of patients receiving this medication remained approximately the same over time overall and by patient age group.
Figure 3. Antihyperglycemic treatment classes received in the CV risk cohorta. (A) Overallb. (B) Patients aged <65 yearsc. (C) Patients aged ≥65 yearsd. Abbreviations. CV, cardiovascular; CVD, cardiovascular disease; DPP-4, dipeptidyl peptidase-4; GLP-1 RA, glucagon-like peptide-1 receptor agonist; SGLT2, sodium-glucose cotransporter-2. aGLP-1 RAs included albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide (oral and subcutaneous). GLP-1 RAs with labeled CVD benefit included dulaglutide, liraglutide, and semaglutide (subcutaneous only). SGLT2 inhibitors included canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. All these medications, except ertugliflozin, have proven CVD benefit. bThe percentage of patients receiving any AHA ranged from 76.2% in 2019 to 77.5% in 2015. cThe percentage of patients receiving any AHA ranged from 76.4% in 2019 to 78.5% in 2016. dThe percentage of patients receiving any AHA ranged from 73.6% in 2019 to 74.3% in 2018.
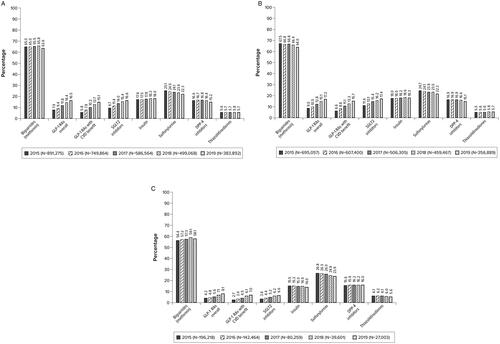
Overall, the percentage of patients receiving any GLP-1 RA (regardless of whether the medication had CV benefit or not) increased from 7.9% in 2015 to 16.5% in 2019. The percentage of patients receiving GLP-1 RAs with CV benefit increased from 5.8% in 2015 to 15.1% in 2019. Similarly, the percentage of patients receiving SGLT2 inhibitors increased from 9.7% in 2015 to 16.6% in 2019. The percentage of patients receiving any GLP-1 RA and the percentage of patients receiving GLP-1 RAs with CV benefit ranged from 9.0% in 2015 to 17.2% in 2019 and 6.6% in 2015 to 15.7% in 2019, respectively, among patients aged <65 years and ranged from 4.2% in 2015 to 8.1% in 2019 and 2.7% in 2015 to 7.0% in 2019, respectively, among patients aged ≥65 years. Similarly, the percentage of patients receiving SGLT2 inhibitors ranged from 11.4% in 2015 to 17.4% in 2019 among patients aged <65 years and ranged from 3.6% in 2015 to 6.6% in 2019 among patients aged ≥65 years.
The likelihood of receipt of GLP-1 RAs and SGLT2 inhibitors was found to increase with increasing calendar year, even after results were controlled for previous receipt of the medication ( and ). Females were slightly more likely to receive GLP-1 RAs and slightly less likely to receive SGLT2 inhibitors compared with males, while patients aged ≥65 years were less likely to receive either GLP-1 RAs or SGLT2 inhibitors compared with patients aged <65 years. A higher CCI score was also associated with an increased likelihood of receipt of either GLP-1 RAs or SGLT2 inhibitors. Diagnoses of obesity and hyperlipidemia in the calendar year of the index date were also associated with an increased likelihood of receipt of either GLP-1 RAs or SGLT2 inhibitors. Prior receipt of a cholesterol-lowering medication or any antihypertensive agent was associated with a decreased likelihood of receipt of a GLP-1 RA or an SGLT2 inhibitor.
4. Discussion
To our knowledge, this study is the first nationwide real-world analysis evaluating trends in AHA prescribing patterns between 2015 and 2019 in a large population of insured patients with T2D from across all four US census regions. Overall, this analysis found that the percentage of patients receiving GLP-1 RAs and SGLT2 inhibitors increased over time for patients in the CVD cohort (GLP-1 RA: range, 6.2–14.4%; SGLT2 inhibitor: range, 6.3–13.3%) and for patients in the CV risk cohort (GLP-1 RA: range, 7.9–16.5%; SGLT2 inhibitor: range, 9.7–16.6%). Additionally, this study also found that patients with a diagnosis of obesity or hyperlipidemia had an increased likelihood of receipt of either GLP-1 RAs or SGLT2 inhibitors.
Several previous studies have evaluated the use of SGLT2 inhibitors and GLP-1 RAs within single institutions or registries. For example, Pantalone and colleagues [Citation11] evaluated patients with T2D and CVD who were seen at the Cleveland Clinic and found that in 2016, utilization of GLP-1 RAs and SGLT2 inhibitors was low, with only 4.1 and 2.5% of patients with T2D and CVD receiving these medications, respectively. Arnold and colleagues [Citation9] evaluated patients with atherosclerotic CVD and T2D in a US-based registry between 2016 and 2018 and observed that at the point of entry into the registry, 9.0% of patients were receiving an SGLT2 inhibitor, while 7.9% of patients were receiving a GLP-1 RA. Hamid et al. [Citation10] evaluated patients with T2D and CVD between 2013 and 2019 at an academic medical center in Mississippi and observed that only 1.4% of patients received an SGLT2 inhibitor and only 1.6% of patients received a GLP-1 RA.
The small increase in the percentages of patients receiving SGLT2 inhibitors and GLP-1 RAs between 2015 and 2019 observed in the present analysis may be due to several factors, including increased awareness, additional physician experience with these medication classes, and the publication of studies showing the CV benefits of these medication classes during this time frame [Citation17–25].
The present study found that there is a difference in the use of GLP-1 RAs and SGLT2 inhibitors between patients aged <65 years and those aged ≥65 years. Specifically, for patients in both the CVD and CV risk cohorts, the percentages of those aged <65 years on GLP-1 RAs was approximately twice those observed for patients aged ≥65 years, while the percentages of patients aged <65 years on SGLT2 inhibitors was approximately triple those observed for patients aged ≥65 years. Although this study was not able to look at reasons for differences in prescribing patterns, the lower percentages of patients receiving GLP-1 RAs and SGLT2 inhibitors observed among patients aged ≥65 versus <65 years may be due to several factors. As the aim of antihyperglycemic treatment has historically been achievement of HbA1c goals, physicians may be less willing for older (and potentially frail) patients who have achieved good glycemic control on established medication classes to be switched to newer agents. Furthermore, Medicare reimbursement of these medications may lag behind commercial approval, thereby resulting in high out-of-pocket costs associated with these medications for Medicare beneficiaries. Luo and colleagues [Citation26] evaluated coverage, formulary restrictions, and out-of-pocket costs for GLP-1 RAs and SGLT2 inhibitors among patients in Medicare Part D. They found that coverage for these medications without prior authorization and without step therapy requirements ranged from 53.2 to 95.4%, indicating that patients had reasonable access to these medications; however, the mean annual out-of-pocket costs for these medications ranged from $1,211 to $2,447, which may be unaffordable for many older adults.
While this and previous studies have observed that relatively few patients with T2D and CVD receive AHA with CV benefit, this study found that a substantial proportion of patients (range, 26% in 2015 to 21% in 2019) were receiving sulfonylureas. Though the proportion of patients in the present analysis receiving sulfonylureas decreased over time, it is important to note that the percentage of patients receiving this class of AHA remained higher than the percentages of patients receiving GLP-1 RAs and SGLT2 inhibitors. This finding was observed despite sulfonylureas having been shown to be associated with hypoglycemia and weight gain in patients with T2D [Citation27].
The present study also found that a substantial proportion of patients received DPP-4 inhibitors, and the percentage of patients receiving DPP-4 inhibitors did not change over time (range, 16.6% in 2015 to 15.2% in 2019). A recent study by Newman and colleagues [Citation28] evaluated an administrative claims database in which patients with T2D switched from DPP-4 inhibitors to either a GLP-1 RA or an SGLT2 inhibitor. The analysis by Newman and colleagues [Citation28] observed that patients switching to a GLP-1 RA or an SGLT2 inhibitor had a lower incidence rate of inpatient admissions and lower total unadjusted medical costs compared with patients remaining on DPP-4 inhibitors. This suggests that patients may benefit (in the form of reduced inpatient admissions) from switching from DPP-4 inhibitors to either GLP-1 RAs or SGLT2 inhibitors, while health plans may benefit from switching in terms of reduced health care costs.
Despite the potential benefits of treatment switching and augmentation, clinical inertia, defined as a lack of treatment changes or intensification despite not reaching glycemic goals, is common in patients with T2D, and previous studies have estimated that this phenomenon is experienced by up to 50% of patients with T2D [Citation29]. While clinical inertia may partially explain the slow uptake in GLP-1 RAs and SGLT2 inhibitors, the prescribing of GLP-1 RAs and SGLT2 inhibitors may be further inhibited by the current health care system in the US and by a lack of comprehensive care plans for patients spanning multiple physician specialties [Citation30]. Furthermore, it has been hypothesized that a rebranding of SGLT2 inhibitors and GLP-1 RAs as cardiometabolic medications may further assist in the uptake of these medications through an increase in prescribing of these medications by cardiologists [Citation30].
This study has several limitations common to retrospective analyses of administrative claims data. Diagnoses in the databases were coded using ICD-9-CM and ICD-10-CM codes, which are subject to inaccuracies. Limited information was available on diagnoses that occurred before the start of the database; therefore, it was not possible to ensure patients in the CV risk cohort had not had a CVD event before the start of the study period. We required that all patients had at least 1 calendar year of continuous health plan enrollment. Therefore, this study may be biased toward a healthier population of patients with T2D and CVD or T2D and additional CV risk factors, as those patients who died within the calendar year (e.g. from acute myocardial infarction) would have been excluded from the study. No information was available in the database on laboratory test results, longitudinal weight, or smoking history. Evaluation of obesity and smoking history as CV risk factors was based on diagnosis codes, and these diagnoses likely were undercoded in the administrative claims data. As most patients in the overall T2D population were identified as having at least 1 CV risk factor (i.e. nearly 80% of the overall T2D population had at least 1 additional CV risk factor), no control population of patients without CVD or CV risk was evaluated. Finally, no information was available in the database on reason for prescription, so it was not possible to determine why a physician prescribed a medication or to evaluate their rationale for selecting one medication class versus another.
5. Conclusions
To our knowledge, this is the first nationwide, retrospective, real-world study evaluating AHA treatment patterns in the years leading up to and after publication of the 2017 ADA guidelines (when GLP-1 RAs and SGLT2 inhibitors were first recommended for patients with CVD). While the use of GLP-1 RAs and SGLT2 inhibitors increased during the study period, even in 2019, most patients in the CVD and CV risk cohorts did not receive these cardioprotective AHAs, and patients aged ≥65 years were particularly disadvantaged.
Preventing cardiovascular events in patients with T2D has the potential to reduce both additional disease burden and health care costs in patients. We recognize that a paradigm shift in the treatment of patients with T2D and CVD or CV risk factors would not be expected to occur immediately after release of new recommendations, and it will take time for physicians to alter their prescribing habits in the face of new evidence. Future studies should be conducted to describe the treatment utilization trends as more recent data become available.
Transparency
Declaration of funding
Financial support for the conduct of the research and preparation of the article were provided by Eli Lilly and Company. Employees of Eli Lilly and Company participated in the study design, interpretation of study data, in the writing of the report, and in the decision to submit the article for publication.
Declaration of financial/other relationships
R. Mody, M. Yu, and J. Levine are employees of Eli Lilly and Company. J. Meyers and K. Davis are employees of RTI Health Solutions. RTI Health Solutions received funding from Eli Lilly and Company to conduct this study. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
R. Mody was involved in the conceptualization, methodology, funding acquisition, supervision, and review and editing of the written manuscript; J. Meyers was involved in the methodology, formal analysis, and writing the original draft of the manuscript; M. Yu was involved in the conceptualization, methodology, and review and editing of the written manuscript; K. Davis was involved in the validation, methodology, supervision, and review and editing of the written manuscript; and J. Levine was involved in the conceptualization, methodology, and review and editing of the written manuscript.
Acknowledgements
The study coauthors would like to thank Brian Calingaert for his assistance with the analysis of the study data.
Data availability statement
This study used deidentified data from the IBM Watson Health Analytics’ MarketScan (MarketScan) Commercial Claims and Encounters (CCAE) and the Medicare Supplemental databases. These data are available for purchase from IBM Watson.
References
- American Diabetes Association (ADA). Standards of medical care in diabetes—2020; 2020 [cited 2022 May 16]. Available from: https://diabetesjournals.org/clinical/article/38/1/10/32237/Standards-of-Medical-Care-in-Diabetes-2020.
- Centers for Disease Control and Prevention (CDC). National diabetes statistics report; 2020 [cited 2021 March 29]. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
- International Diabetes Federation (IDF). Diabetes and cardiovascular disease. Brussels: IDF; 2016. p. 1–144.
- International Diabetes Federation (IDF). IDF diabetes atlas. 7th ed. Brussels: IDF; 2015.
- Sarwar N, Gao P, Seshasai SR, Emerging Risk Factors Collaboration, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Emerging risk factors collaboration. Lancet. 2010;375(9733):2215–2222.
- Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018a;17(1):83.
- Einarson TR, Acs A, Ludwig C, et al. Economic burden of cardiovascular disease in type 2 diabetes: a systematic review. Value Health. 2018b;21(7):881–890.
- American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S135.
- Arnold SV, de Lemos JA, Rosenson RS, GOULD Investigators, et al. Use of guideline-recommended risk reduction strategies among patients with diabetes and atherosclerotic cardiovascular disease. Insights from getting to an improved understanding of low-density lipoprotein cholesterol and dyslipidemia management (GOULD). Circulation. 2019;140(7):618–620.
- Hamid A, Vaduganathan M, Oshunbade AA, et al. Antihyperglycemic therapies with expansions of US food and drug administration indications to reduce cardiovascular events: prescribing patterns within an academic medical center. J Cardiovasc Pharmacol. 2020;76(3):313–320.
- Pantalone KM, Misra-Hebert A, Hobbs TM, et al. Antidiabetic treatment patterns and specialty care among patients with type 2 diabetes and cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):54.
- Charlson ME, Charlson RE, Peterson JC, et al. The charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61(12):1234–1240.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- Quan H, Li B, Couris CM, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23.
- Glasheen WP, Renda A, Dong Y. Diabetes complications severity index (DCSI) – update and ICD-10 translation. J Diabetes Complic. 2017;31(6):1007–1013.
- Zinman B, Wanner C, Lachin JM, EMPA-REG OUTCOME Investigators, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128.
- Neal B, Perkovic V, Mahaffey KW, CANVAS Program Collaborative Group, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357.
- Marso SP, Daniels GH, Brown-Frandsen K, LEADER Steering Committee; LEADER Trial Investigators, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016a;375(4):311–322.
- Marso SP, Bain SC, Consoli A, SUSTAIN-6 Investigators, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016b;375(19):1834–1844.
- Hernandez AF, Green JB, Janmohamed S, Harmony Outcomes committees and investigators, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomized placebo-controlled trial. Lancet. 2018;392(10157):1519–1529.
- Gerstein HC, Colhoun HM, Dagenais GR, REWIND Investigators, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130.
- Pfeffer MA, Claggett B, Diaz R, ELIXA Investigators, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257.
- Holman RR, Bethel MA, Mentz RJ, EXSCEL Study Group, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239.
- Luo J, Feldman R, Rothenberger SD, et al. Coverage, formulary restrictions, and out-of-pocket costs for sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in the Medicare part D program. JAMA Netw Open. 2020;3(10):e2020969.
- Sola D, Rossi L, Schianca GPC, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11(4):840–848.
- Newman TV, Munshi KD, Neilson LM, et al. Health care utilization and costs associated with switching from DPP-4i to GLP-1 RA or SGLT2i: an observational cohort study. J Manag Care Spec Pharm. 2021;27(4):435–443.
- Blonde L, Aschner P, Bailey C, Global Partnership for Effective Diabetes Management, et al. Gaps and barriers in the control of blood glucose in people with type 2 diabetes. Diab Vasc Dis Res. 2017;14(3):172–183.
- Adhikari R, Blaha M. New insights into prescribing of SGLT2 inhibitors and GLP-1 receptor agonists by cardiologists in 2020: major barriers limiting role. 2021 [cited 2022 June 10]. Available from: https://www.acc.org/latest-in-cardiology/articles/2021/01/19/14/27/new-insights-into-prescribing-of-sglt2-inhibitors-and-glp-1-receptor-agonists-in-2020#.YAr3dDQXc0Y.twitter.
