Abstract
Objective
To describe the burden of comorbid conditions and comedications among people living with HIV (PLWH) vs. people living without HIV (PLWoH).
Methods
This was a case–control study conducted among insured patients using administrative claims data. Adult PLWH were identified by antiretroviral therapy (ART) claims or HIV/AIDS diagnosis codes from 1 January 2018 to 31 December 2018 (index date was set by the earliest claim). Continuous enrollment was required for ≥12 months pre-index (baseline) and ≥30 days post-index (follow-up). Patients were required to have ≥1 HIV diagnosis during baseline or follow-up. Those with only HIV prophylaxis were excluded. PLWoH were matched 2:1 to PLWH on demographic characteristics. Study outcomes were compared using z-tests with robust standard errors in an ordinary least squares regression or Rao–Scott tests.
Results
The study included 20,256 PLWH and 40,512 matched PLWoH, mean age 52 years. PLWH vs. PLWoH had higher mean (SD) Charlson comorbidity index scores (0.93 [1.59] vs. 0.61 [1.28]; p < .001) and a greater proportion had ≥1 comorbidity (69.1% vs. 54.5%, p < .001). The most prevalent comorbidities included hypertension (33.9% vs. 32.2%; p < .001), hyperlipidemia (29.4% vs. 24.6%; p < .001), chronic kidney disease (13.6% vs. 9.4%, p < .001), depression (13.1% vs. 7.3%, p < .001) and substance abuse (12.8% vs. 7.1%, p < .001). Mean (SD) non-ART prescription fills were higher among PLWH vs. PLWoH (11.9 [10.1] vs. 9.2 [9.4]; p < .001).
Conclusions
Multimorbidity and polypharmacy were more prevalent among PLWH vs. matched PLWoH. Findings support the need to consider comorbidities and comedications when choosing ART and to minimize drug–drug interactions and adverse events to improve patient outcomes.
Keywords:
Introduction
With significant improvements in treatments for HIV and improved outcomes for people living with HIV (PLWH), it is expected that increasing numbers of PLWH will survive into older age. In 2018, 51% of people with prevalent HIV and 17% of newly diagnosed patients were at least 50 years of ageCitation1. By 2030, 70% of PLWH are projected to be over 50 years of ageCitation2. In fact, the life expectancy of PLWH has recently approached that of the general populationCitation3–5.
However, older PLWH may experience age-related illnesses earlier in life, and/or in a more severe form than their non-HIV-infected counterpartsCitation6,Citation7. These comorbid conditions include cardiovascular disease, diabetes mellitus and renal failure, in addition to certain neuropsychiatric conditions and gastrointestinal problemsCitation7,Citation8. Furthermore, as patients are treated with antiretroviral therapy (ART) for many years, risks of potential adverse effects, drug–drug interactions and contraindications are increased. As life expectancy increases for PLWH, a corresponding rise in comorbidities will impose consequent increases in comedications.
Despite recent attention on older PLWH, there is still a need for more appropriate study utilizing a sample of a broad range of ages over a recent time period, especially compared with people living without HIV (PLWoH). Although more studies have included persons at least 50 years of age, few have included matched controls without HIVCitation9,Citation10. Understanding the burden of comorbidity and comedications is essential in managing healthcare for PLWH as they age. Documenting trends in proportions of PLWH experiencing comorbidity and comedication burden is especially relevant for recent years in which modern-era ARTs have been largely successful in suppressing viral replication.
The primary objective of this study was to describe the burden of comorbid conditions and comedications among a large sample of PLWH compared with a matched cohort of PLWoH. In addition, a 5-year trend in burden of comorbidity and comedication was analyzed for PLWH.
Methods
Data source and study design
This was a retrospective case–control study using administrative medical and pharmacy claims, enrollment, and linked socioeconomic data from the Optum Research Database (ORD) for the period of 1 January 2013 through 31 January 2019 (Supplementary Figure S1). The ORD, one of the largest databases in the US, comprises medical and pharmacy claims and linked enrollment data from 1993 to the present on more than 73 million lives. Patients included in the ORD are generally representative of the US insured population with respect to age, sex and geographic distribution. In 2018, the ORD represented 19% of the US commercially enrolled population, 21% of the Medicare Advantage population and 22% of the Medicare Part D populations.
To assess the primary objective of comparing comorbidities and comedications between PLWH and PLWoH, a 2018 calendar-year cohort was constructed by identifying eligible PLWH between 1 January 2018 and 31 December 2018 (identification period), the most recent full calendar year for which data were available at the time the study was conducted. A comparison cohort of PLWoH were matched 2:1 to PLWH in the 2018 calendar-year cohort on age group, sex, race, geographic region and health plan type.
To assess the secondary objective of 5-year trend of comorbidity and comedication burden in PLWH, annual cohorts were constructed for only PLWH during each calendar year (between 1 January and 31 December) from 2014 through 2018.
People living with HIV cohort
Inclusion criteria
For the primary objective, patients in the PLWH cohort were included on the basis of study inclusion and exclusion criteria for the 2018 calendar year. Eligible patients were identified via a pharmacy fill for an antiretroviral (ARV) agent or, if none were observed, by International Classification of Diseases, 9th and 10th Editions, Clinical Modification (ICD-9-CM and ICD-10-CM) diagnosis codes for HIV or AIDS. An index date was set as the date of the earliest claim that did not coincide with an HIV antibody test.
Continuous enrollment with medical and pharmacy benefits was required for at least 12 months pre-index (baseline period) and at least 30 days after and including the index date (follow-up period). Included PLWH were required to be at least 18 years of age as of the index date and have ≥1 claim with an HIV diagnosis code during the baseline or follow-up period.
Exclusion criteria
Individuals were excluded if they had evidence of pre-exposure prophylaxis (PrEP) for HIV during the baseline or follow-up periods (pharmacy claims for tenofovir disoproxil fumarate [TDF] plus emtricitabine [FTC] or tenofovir alafenamide [TAF] plus FTC and no pharmacy claims for another ARV agent). In addition, individuals with index dates based on ART claims were excluded if they had a diagnosis code for post-exposure prophylaxis (PEP; ICD-10-CM diagnosis codes Z11.4 and Z20.6) during the baseline or follow-up periods.
For the secondary objective, PLWH meeting inclusion and exclusion criteria for at least one of the calendar years from 2014 through 2018 were included in building annual cohorts. A patient may have been included in more than one annual cohort.
People living without HIV cohort
A PLWoH cohort was constructed only for the 2018 calendar year. Individuals without medical claims with HIV/AIDS diagnoses or pharmacy claims for ART (other than claims for only TDF/FTC or TAF/FTC, or with a diagnosis code for PEP therapy at any time) were matched 2:1 to PLWH based on age group, sex, race, geographic region and health plan type. The index dates for PLWoH were set as the index dates of their matched PLWH.
PLWoH were required to be enrolled in a health plan for at least 30 days in 2018 and have identical continuous enrollment criteria as PLWH. They were also required to have at least one claim during the baseline or follow-up period to ensure that selected patients had pharmacy or medical activity.
Study measures
For both the primary and secondary objectives, the following patient characteristics were obtained during baseline: age, sex, insurance type, US geographic regionCitation11 and race/ethnicity. A baseline Quan–Charlson comorbidity score based on 12 condition categories (excluding HIV/AIDS) was calculated and expressed as a continuous score and a categorical variableCitation12.
In addition, selected comorbid conditions were identified for both analyses using diagnosis codes on medical claims where there was at least one inpatient admission or at least two ambulatory claims on different dates based on ICD-9/10-CM codes in the 12-month baseline period. A list of 67 comorbid conditions most relevant to PLWH was compiled on the basis of literature review. Where feasible, the definition for each comorbid condition was based on condition categories from the Chronic Conditions Data Warehouse published by the Centers for Medicare and Medicaid Services, or alternatively on a clinician’s review of relevant diagnosis codes. Conditions described included broad categories of cardiovascular diseases, renal diseases, liver diseases, cancer, mental health and pain, lung diseases, autoimmune conditions, endocrine disorders and gastrointestinal disorders (Supplementary Table S1; Chronic Conditions Data Warehouse https://www2.ccwdata.org/web/guest/condition-categories). The number of comorbid conditions from the full list (Supplementary Table S1) observed was summated for a categorical score (0, 1, 2, 3+).
For PLWH, ART regimens were identified as of the index date (or within 14 days after the index date), including integrase strand transfer inhibitor (INSTI); non-nucleoside reverse transcriptase inhibitor (NNRTI); protease inhibitor (PI); and nucleoside/nucleotide reverse transcriptase inhibitor (NRTI), as well as pharmacokinetic enhancer and CCR5 antagonist. Comedications were selected on the basis of literature review and/or clinical rationale and identified in the 90 days prior to and including the index date. The categories of interest were antidiabetic treatments, cardiovascular medications, mood disorder medications, chronic antibiotic use (defined as ≥2 prescription fills in the 90 days before and including the index date, or ≥1 prescription fill in the 90 days before and including the index date plus ≥1 additional fill in the 91–181 days before the index date), oral or injectable steroids, respiratory medications, proton pump inhibitors and osteoporosis medications (see Supplementary Table S2 for detailed list). The comedication burden was summarized as the number of prescription fills (by unique National Drug Codes [NDCs], excluding ARV) during the 12 month baseline period, expressed as continuous number and category (0, 1, 2, 3, 4, ≥5).
Statistical analyses
All study variables were analyzed descriptively. Numbers and percentages were provided for dichotomous and polychotomous variables. Means and standard deviations were provided for continuous variables.
For the primary analysis, baseline demographic characteristics, clinical characteristics, comorbidity and comedication burden were compared between PLWH and matched PLWoH using a z-test with robust standard errors in an ordinary least squares (OLS) regression for continuous variables or a Rao–Scott test for categorical variables.
For the 5-year trend analysis in the PLWH cohort, comorbidities and comedications were summarized in each of the calendar years (2014–2018) using descriptive statistics. In each year, the denominator consisted of all PLWH who met study inclusion criteria for that calendar year. The numerator consisted of all PLWH with indications for each of the comorbidities and comedications being evaluated. A p-for-trend test was computed using a Wald chi-square test in an OLS regression with a linear cohort for continuous measures, and logistic regression with linear cohorts for categorical measures. Trend tests accounted for clustering by individual identification across annual cohorts. The a priori alpha value used to determine significance was p < .05. All analyses were conducted using SAS software version 9.0.
Human research regulations
All databases that were used for this study were statistically certified as de-identified using techniques compliant with Health Insurance Portability and Accountability Act of 1996, and no identifiable protected health information was extracted. Therefore, institutional review board approval and informed consent were not required for this study.
Results
Sample selection and attrition
For the primary objective, among 58,836 patients identified by either ARV treatment (91.5%) or HIV diagnosis code (8.5%), 20,256 (34.4%) PLWH were retained after applying all inclusion/exclusion criteria (Supplementary Figure S2a). For the secondary objective, the number of PLWH for each annual cohort was as follows: 2014 (n = 14,222), 2015 (n = 14,527), 2016 (n = 16,310), 2017 (n = 18,571) and 2018 (n = 20,256).
A total of 11,553,403 enrollees not included in the PLWH cohort met the continuous enrollment and age requirements (Supplemental Figure S2b). After remaining criteria were met, a 2:1 sample of PLWoH was matched to the PLWH cohort on age group, sex, race, geographic region and health plan type, yielding a PLWoH cohort of 40,512 people.
Sample description
Demographic characteristics
Patients in the PLWH cohort were matched to PLWoH by age group, sex, race, region and health plan type, so there were no differences between cohorts in these characteristics ().
Table 1. Demographic and clinical characteristics among PLWH and PLWoH.
For the secondary analysis, the mean age of the PLWH cohort increased statistically significantly from 2014 (48.9 years) to 2018 (52.4 years) (p < .001) (Supplementary Table S3). The proportion comprising women increased from 17.2% in 2014 to 20.0% in 2018 (p < .001). The proportion of Medicare Advantage enrollees increased from 19.3% in 2014 to 34.6% in 2018 (p < .001).
Comorbidity burden
The PLWH cohort had a greater burden of comorbid conditions, by several measures, compared with the PLWoH cohort in 2018. The mean (SD) [interquartile range] Charlson comorbidity score (excluding HIV) was higher among PLWH compared to PLWoH: 0.93 (1.59) [0.0–1.0] vs. 0.61 (1.28) [0.0–1.0]; p < .001 (). The PLWH cohort had greater proportions of all categorical scores above zero than did the PLWoH cohort (PLWH: 38.7% vs. PLWoH: 27.9%, p < .001). As for comorbid conditions, greater proportions of the PLWH cohort had counts of 1 or more condition (PLWH: 69.1% vs. PLWoH: 54.5%, p < .001).
Table 2. Comorbidity burden measures, PLWH vs. PLWoH.
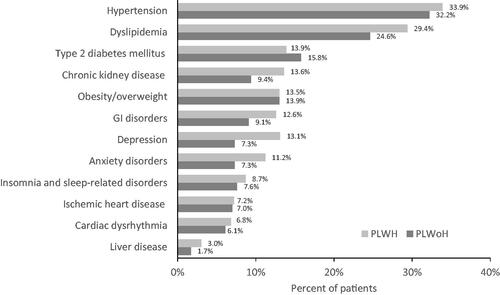
The most prevalent comorbidities were cardiovascular-related conditions, including hyperlipidemia (PLWH: 29.4% vs. PLWoH: 24.6%; p < .001) and hypertension (PLWH: 33.9% vs. PLWoH: 32.2%; p < .001) ( and Supplementary Table S4). Chronic kidney disease was identified among 13.6% of PLWH and 9.4% of PLWoH (p < .001). Neuropsychiatric conditions including depression, anxiety disorders and insomnia/sleep disorders were also more prevalent in the PLWH cohort compared with the PLWoH cohort (all p < .001). Gastrointestinal disorders, including diarrhea, nausea/vomiting and esophageal reflux, were more common in the PLWH cohort compared with the PLWoH cohort (all p < .001).
Figure 1. Proportion of cohorts with selected comorbid conditions, PLWH vs. PLWoH*Ϯ. Abbreviations. GI, Gastrointestinal; PLWH, People living with HIV; PLWoH, People living without HIV. *These listed were the most prevalent or relevant of the 67 conditions selected among those in the Chronic Conditions Data Warehouse. Dyslipidemia (includes hyperlipidemia or lipid disorders); GI disorders (includes diarrhea, nausea/vomiting, peptic ulcer disease and esophageal reflux); and liver disease (includes cirrhosis, fatty liver disease and other conditions, except for viral hepatitis) represent several specific conditions. ϮWith the exception of obesity/overweight (p = 0.206) and ischemic heart disease (p = 0.539), all p < 0.001.
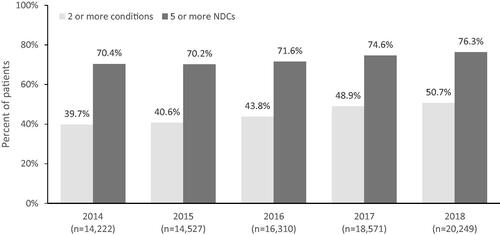
Comorbidity burden increased for the PLWH cohort over the 5-year period: mean (SD) Charlson comorbidity score increased from 0.72 (1.36) in 2014 to 0.93 (1.59) in 2018 (p < .001), and the proportion of PLWH having scores of 1 or higher increased from 32.3% in 2014 to 38.7% in 2018 (p < .001). The proportion of PLWH who had three or more comorbid conditions increased from 25.8% in 2014 to 37.2% in 2018 (p < .001).
Over the years 2014 to 2018 (Supplementary Table S5), the prevalence rose for cardiovascular-related comorbidities, including hypertension and hyperlipidemia (from 24.3% to 34.0% and 24.0% to 29.5%, respectively; p < .001 for both). The prevalence of obesity and overweight rose by 2.4-fold and 4.5-fold, respectively (p < .001 for both). Chronic kidney disease was observed among 6.5% of patients in 2014 and among 13.7% of patients in 2018 (significant trend at p < .001).
Comedication burden
The PLWH cohort had a greater mean (SD) number of unique non-ARV prescription fills by NDC at 11.9 (10.1), compared with the PLWoH cohort at 9.2 (9.4), p < .001. Furthermore, 76.3% of the PLWH cohort had five or more non-ARV comedications, compared with 60.8% of the PLWoH cohort.
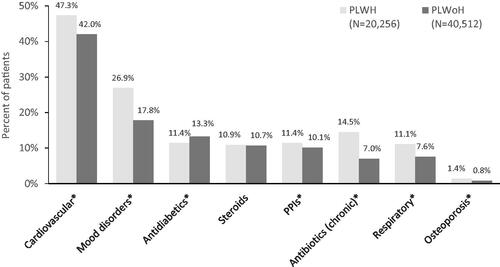
Statistically significantly greater (p < .001) proportions of the PLWH cohort, compared with the PLWoH cohort, were prescribed cardiovascular (47.3% vs. 42.0%, respectively), mood disorder (27.0% vs. 17.8%, respectively) and chronic antibiotic (14.5% vs. 7.0%, respectively) medications ().
Figure 2. Proportion of PLWH and PLWoH with selected comedications. *p < 0.001. Mood disorders include depression and anxiety. Abbreviations. PLWH, People living with HIV; PLWoH, People living without HIV; PPI, Proton pump inhibitor.
In a trend analysis (), increasing proportions of PLWH had two or more comorbid conditions and five or more non-ARV comedications.
Discussion
Improved treatments have led to longer lives for PLWH, yet they are experiencing a greater burden of comorbidity and comedication as they age. Increasing longevity and comorbidities expose PLWH to longer ART periods with multiple medications, for which drug–disease and drug–drug interactions are of particular concern. The current study compared comorbidities and comedications between a large, diverse sample of PLWH and a matched group of individuals without HIV, and analyzed how comorbidities and comedications changed from 2014 through 2018 among PLWH.
PLWH had a greater burden of comorbidity and comedication than matched PLWoH. Trends observed in this study over 5 years demonstrated increases in comorbid conditions and comedication burden over time. A study by Priest et al. reported data from administrative claims spanning 2007–2017, describing a sample of PLWH regarding ARV treatment, comorbid conditions and pill burdenCitation10. Their sample was of similar age, sex distribution, insurer distribution and comorbidity scores to the current study. The most common comorbidities and comedications were similar to those observed in the current study. In their sample, the comorbidities were more common in patients with Medicare insurance, which aligns with an older age group. The results from both studies together suggest continuation of trends observed back to 2007.
Comorbidity burden
The most prevalent category of comorbidities overall was cardiovascular-related conditions. Hypertension was observed among approximately one-third of persons in each cohort. Hyperlipidemia was observed among a significantly larger proportion of PLWH than PLWoH. Chronic kidney disease was also significantly more prevalent among PLWH than PLWoH, with approximately one-quarter of patients affected. These findings align with those of previous studiesCitation7,Citation13. However, Type 2 diabetes mellitus (T2DM) was more prevalent among the PLWoH cohort in 2018. Yet, within the PLWH cohort, the proportion of patients with T2DM increased considerably between 2014 and 2018. Comparisons between PLWH and PLWoH were only conducted for 2018 for this study; future researchers may need to examine long-term trends for T2DM among individuals with or without HIV, considering many risk factors for diabetes, including obesity and HIV medicationsCitation14.
Previous studies of common comorbidities have included large samples of PLWH older than 65, with similar findings for 2006–2009Citation15 relative to the current study. A trend analysis was also published comparing comorbidities among PLWH between 2003 and 2013 in the USCitation9. These studies also observed higher prevalence of common comorbidities for PLWH, compared with individuals without HIV, over time, with the highest prevalence observed among Medicare patients. In conjunction with the current study results, rates of more prevalent comorbid conditions among PLWH have been reported to increase from as far back as 2003.
PLWH have been reported to have a greater burden of neuropsychiatric conditions than PLWoH in previous studiesCitation10,Citation16, although exact prevalence rates vary widely by study methodology. In addition, neurocognitive disorders have been noted to be associated with older age in PLWH, and to worsen with treatment with efavirenz, which can cause acute psychosis, nightmares, irritability, difficulty concentrating and increased risk of suicideCitation17. Some antiretroviral (ARV) agents have been associated with increased risks of chronic kidney disease, cardiovascular disease, fractures/osteoporosis and weight gainCitation18–22. Furthermore, HIV infection itself causes chronic inflammation that may contribute to the development of comorbid conditions at earlier ages than among uninfected individualsCitation23,Citation24.
Polypharmacy burden
Polypharmacy (excluding ARV medications) was observed to a greater extent among the PLWH cohort than the PLWoH cohort, measured both as the mean number of unique NDCs and the proportion of patients having five or more comedications. The most prevalent medications among all patients were cardiovascular-related conditions, which aligns with the prevalence of hypertension and hyperlipidemia among both cohorts. Among PLWH, increasing numbers of patients were prescribed cardiovascular medication, antidiabetic medication, steroids and respiratory medications from 2014 through 2018; and the prevalence of polypharmacy (five or more non-ARV comedications) increased from 70.4% to 76.3%. Many studies report a high polypharmacy burden among PLWH, similar to the current studyCitation25–27.
Treating comorbid conditions among elderly PLWH involves several risks, including potentially inappropriate treatment such as less prescribed statin therapy despite a higher risk of high cholesterol observed among PLWHCitation28. Polypharmacy or inappropriate prescribing increases risk of adverse events/side effects; the risk is higher among older PLWHCitation13,Citation27. Furthermore, studies have demonstrated consequences unique to drug–drug interactions with ARV medications, which may lessen the impact of ART or efficacy of treatment for comorbid conditionsCitation29–31. More guidance is needed to balance consideration of individual risk factors and drug–drug interactions among PLWH, especially elderly patients. Further analysis of comedication burden by age group, and any trends observed, may be informative to the continuing discussion about optimizing care for PLWH.
Limitations
The interpretation of these study data is subject to limitations common to retrospective analyses conducted with administrative claims. For example, the presence of a claim for a prescription fill does not guarantee the medications were taken as prescribed. Medications filled over the counter or provided as samples by the physician or in a clinical trial are not observed in the claims data. Criteria used to detect certain medications may have imposed a limitation as well. PEP therapy is typically defined as treatment with ARV regimens for a period of less than 30 days. Therefore, reliance on medical claims for PEP codes may have resulted in some misclassification bias where patients on PEP therapy were not excluded from the sample. If present, this would likely bias associations to the null.
In addition, the presence of a diagnosis code on a medical claim is not positive proof of disease. Misspecification of codes may result in misclassification of comorbid status. To avoid misclassification, we included only patients with at least one inpatient or two ambulatory claims with diagnosis codes. Identification of chronic conditions from medical claims may not capture all of the conditions that a patient has, and this may be especially true in individuals with multimorbidity. We used a 12-month window to identify chronic conditions, which should help mitigate this source of misclassification bias.
Furthermore, clinical variables that influence the choice of treatment, such as disease severity, medication side effects, clinical rationale and personal preference, are not captured in the claims database; nor are characteristics such as socioeconomic status, length of infection or ARV regimens occurring prior to the study period, which also may have affected the results. Future studies utilizing registry data and/or electronic medical records could help elucidate the impact of these factors on the comorbidity and comedication burden among PLWH. Individuals without HIV who had no medical claims or prescriptions during the 12-month baseline or follow-up periods were excluded from the matching process. It is possible these individuals had other health care plans as the primary payer. This could have introduced a misclassification bias in which healthy people were excluded from the PLWoH cohort.
Finally, results from this study may not be generalizable to other US populations, such as uninsured persons or those insured by Medicaid, Medicare Fee-for-Service, or the AIDS Drug Assistance Program.
Conclusions
Multimorbidity and polypharmacy continue to be more prevalent among PLWH compared with PLWoH. The study findings support the need for greater consideration of the most common comorbid conditions, and the time frame in which they arise, throughout many years of HIV management. The burden of comorbid conditions and non-ART medications among PLWH is higher than that among PLWoH, in part because ARTs themselves are associated with side effects which increase risks of the most common comorbid conditions. Additionally, adverse events may also exacerbate existing conditions, necessitating a need for considering these risks when selecting ART and for informed decision making between patients and providers. The desired outcome of this work is to support optimal treatments based on individual risks of comorbid conditions, as well as minimizing drug–drug interactions, adverse events and pill burden, and thereby improve patient health in the long term.
Transparency
Declaration of funding
This work was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA.
Declaration of financial/other relationships
G.P. has disclosed that he is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA (Merck). J.M. has disclosed that he was an employee of Merck during the conduct of the study. S.G. has disclosed that she was working under an internship with Merck in partnership with the University of Mississippi, University, MS, USA. M.P., E.K.B. and K.M. have disclosed that during the study they were employed with Optum Inc., which was paid to conduct the study under contract with Merck. M.P. has disclosed that she is now employed by the Henry M. Jackson Foundation, Bethesda, MD, USA. P.K. has disclosed that she has received grant/research support from GSK, Merck and Gilead; has stock ownership with Merck, Pfizer, Johnson & Johnson, GSK and Gilead; and serves or has served as consultant/advisory board member with Amgen, GSK, Merck and Gilead.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval of the version to be published. M.P., G.P., S.G., J.M. and P.K. were involved in the conception and design of the study and data interpretation. E.K.B. and K.M. were involved in the acquisition of data. M.P., G.P., E.K.B. and P.K. were involved in the data analysis.
Ethics statement
This study used fully de-identified data extracts in a manner compliant with the Health Insurance Portability and Accountability Act of 1996. Institutional review board approval was neither required nor sought.
Supplementary_files_20May2022.docx
Download MS Word (134.2 KB)Acknowledgements
Writing, editorial support and formatting assistance were provided by Caroline Jennermann MS and Yvette Edmonds PhD, both employees of Optum, which was contracted and compensated by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA.
Data availability statement
Data used to generate these results cannot be disclosed publicly. Proprietary data obtained from the Optum Research Database may be accessed only with strictest data security and privacy protocols, and oversight with a restrictive license agreement.
Results in part were presented at the 2021 Conference on Retroviruses and Opportunistic Infections Virtual Meeting, 6–10 February 2021; and ID Week 2021 Virtual Meeting, 29 September–3 October 2021.
References
- Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2018. HIV Surveillance Report. p. 31 [Internet]. 2020 [cited 2021 Oct 14]. Available from: https://www.cdc.gov/hiv/libary/reports/hiv-surveillance/vol-31/index.html
- O’Keefe KJ, Scheer S, Chen M-J, et al. People fifty years or older now account for the majority of AIDS cases in San Francisco, California, 2010. AIDS Care. 2013;25(9):1145–1148.
- Centers for Disease Control and Prevention. HIV among people aged 50 and older. 2018 [cited 2021 Oct 14]. Available from: https://www.cdc.gov/hiv/group/age/olderamericans/index.html
- Cahill S, Valadéz R. Growing older with HIV/AIDS: new public health challenges. Am J Public Health. 2013;103(3):e7–e15.
- Lewden C, Bouteloup V, De Wit S, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥ 500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41(2):433–445.
- Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172.
- Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–1126.
- Williams ND, Huser V, Rhame R, et al. The changing patterns of comorbidities associated with human immunodeficiency virus infection, a longitudinal retrospective cohort study of Medicare patients. Medicine. 2021;100(16):e25428.
- Gallant J, Hsue PY, Shreay S, et al. Comorbidities among US patients with prevalent HIV infection—a trend analysis. J Infect Dis. 2017;216:1525–1533.
- Priest JL, Burton T, Blauer-Peterson C, et al. Clinical characteristics and treatment patters among US patients with HIV. Am J Manag Care. 2019;25(12):580–586.
- US Department Commerce Economics and Statistics Administration. Census Regions and Divisions of the United States [Internet] [cited 2021 Oct 14]. Available from: https://www2.census.gov/programs-surveys/sahie/reference-maps/2017/us-regdiv.pdf
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Kong AM, Pozen A, Anastos K, et al. Non-HIV comorbid conditions and polypharmacy among people living with HIV age 65 or older compared with HIV-negative individuals age 65 or older in the United States: a retrospective claims-based analysis. AIDS Patient Care STDS. 2019;33(3):93–103.
- Hernandez-Romieu AC, Garg S, Rosenberg ES, et al. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2017;5(1):e000304.
- Friedman EE, Duffus WA. Chronic health conditions in Medicare beneficiaries 65 years old, and older with HIV infection. AIDS. 2016;30(16):2529–2536.
- Do AN, Rosenberg ES, Sullivan PS, et al. Excess burden of depression among HIV-infected persons receiving medical care in the United States: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One. 2014;9(3):e92842.
- Kolakowska A, Maresca AF, Collins IJ, et al. Update on adverse effects of HIV integrase inhibitors. Curr Treat Options Infect Dis. 2019;11(4):372–387.
- Guaraldi G, Prakash M, Moecklinghoff C, et al. Morbidity in older HIV-infected patients: impact of long-term antiretroviral use. AIDS Rev. 2014;16(2):75–89.
- Hemkens LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart J. 2014;35(21):1373–1381.
- Islam FM, Wu J, Jansson J, et al. Relative risk of renal disease among people living with HIV: a systematic review and meta-analysis. BMC Public Health. 2012;12:234.
- Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–2174.
- Triant VA, Brown TT, Lee H, et al. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93(9):3499–3504.
- Babu H, Ambikan AT, Gabriel EE, et al. Systemic inflammation and the increased risk of inflamm-aging and age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. Front Immunol. 2019;10:1965.
- Lv T, Cao W, Li T. HIV-related immune activation and inflammation: current understanding and strategies. J Immunol Res. 2021;2021:7316456. eCollection 2021.
- Greene M, Steinman MA, McNicholl IR, et al. Polypharmacy, drug–drug interactions and potentially inappropriate medications in older HIV-infected adults. J Am Geriatr Soc. 2014;62(3):447–453.
- McNicholl IR, Gandhi M, Hare CB, et al. A pharmacist-led program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older HIV-positive patients. Pharmacotherapy. 2017;37(12):1498–1506.
- Guaraldi G, Malagoli A, Calcagno A, et al. The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65–74 years and more than 75 years. BMC Geriatr. 2018;18(1):99.
- Emmons RP, Hastain NV, Miano TA, et al. Patients with HIV are less likely to receive appropriate statin therapy for cardiovascular disease risk reduction. J Pharm Pract. 2022;35(4):568–572.
- Murray MM, Lin J, Stein AB, et al. Relationship of polypharmacy to HIV RNA suppression in people aged ≥50 years living with HIV. HIV Med. 2021;22(8):742–749.
- Jernigan MG, Kipp GM, Rather A, et al. Clinical implications and management of drug–drug interactions between antiretroviral agents and psychotropic medications. Ment Health Clin. 2013;2(9):274–285.
- Sabourin AA, Patel T, Saad S, et al. Management of anticoagulation in patients with human immunodeficiency virus/acquired immunodeficiency virus. Thromb Res. 2021;200:102–108.