Abstract
Objective
The R.Evolution project aimed to reach a consensus on the main challenges of conducting clinical research in Italy and possible strategies and approaches to address them and optimize clinical research management.
Methods
A scientific board of experts initially discussed potentially critical areas in clinical research conduct and further explored them through an online national survey. The survey results were further examined by a group of 35 panelists representing different clinical research stakeholders. A Nominal Group Technique and a Delphi approach (two rounds) were used to generate a consensus on critical factors, tools and strategies in clinical research.
Results
Four main critical areas were identified: study feasibility, authorization procedures, operational aspects and patient management. The main issues are scarce awareness of the value of clinical research, lack of trained workforce and excessive complexity of protocols and administrative procedures. The Delphi panel identified six intervention areas: culture and patient involvement; procedures; staff, contracts, training and incentives; organization and infrastructure; administrative procedures; and ethics committee.
Conclusion
According to the R.Evolution project, possible strategies to improve clinical research management in Italy include a deeper understanding of the value of clinical research, the creation of long-term plans for hiring, training, organizing and motivating clinical trial staff, the simplification and harmonization of administrative procedures, as well as protocol design, and the development of stronger networks of centers and stakeholders.
1. Introduction
In the last decades, clinical research has progressively become more challenging. The main causes are the growing number of regulatory requirements, the unprecedented number of sites (and patients) involved in large trials and the need to maintain relationships between multiple stakeholders, including institutions, vendors, and sponsors. According to a report published in 2017, protocol design elements associated with clinical trial feasibility, such as the number and frequency of study procedures, have rapidly grown from 2001–2005 to 2011–2015, further contributing to a substantially increased workload for the research centersCitation1.
In 2017, the European Clinical Research Infrastructure Network (ECRIN) project analyzed the major barriers faced by clinical research stakeholders in Europe and the possible ways to overcome such barriers. The main issues identified by the ECRIN Integrating Activity project were inadequate knowledge and understanding of clinical research and trial methodology, insufficient funding, the excessive need for monitoring, restrictive interpretation of privacy law, lack of transparency, overly complex or inadequate regulatory requirements and inadequate infrastructuresCitation2.
The European Medicines Agency (EMA) recognizes the complexity of managing clinical trials and provides guidance on how to overcome them. The ICH guideline E8 (R1) on general considerations for clinical studies provides guidance on the clinical development lifecycle, including designing quality into clinical studiesCitation3.
The relevance of challenges may be even higher in Italy due to the lack of dedicated funds (for infrastructure, workers and specific training) and penalizing bureaucratic procedures, which reduce Italy’s attractiveness for clinical research in Europe. Compared with other European countries, Italy’s complex regulatory framework causes inhomogeneity in the study documentation and duplication of requests that slow the process and increase costs. The main factors that weaken the ability of the Italian system to conduct clinical research seem to be the time required for the initiation of the clinical trial and the study approval; the lack of study coordinators hired with a national contract; and the lack of research nurses and sufficient Phase 1 units throughout the country. Another critical point is the delay in the adoption of the new European Regulation 536/2014, which aims, among other things, to simplify the bureaucracy process and might help to improve the Italian regulatory frameworkCitation4. A study conducted in 2015 to explore stakeholders' perception of Italy as a place to conduct clinical trials revealed that Italy’s governance of clinical trials is considered suboptimal and non-competitive compared to other major European countries. The main issues identified were the lack of accessibility and transparency of information required to run clinical trials and a slow, unpredictable, and fragmented approval process due to extremely laborious ethics and contracting processesCitation5. Different nation-based surveys have been conducted in Europe, highlighting common themes and different obstacles in conducting clinical trials among European states. A survey of the UK Clinical Research Collaboration registered Clinical Trials Units conducted in 2018 identified obtaining research and development approvals, finalizing the contract, obtaining other approvals, and meeting recruitment targets as the most relevant barriers to clinical research conductCitation6. In the same year, a survey conducted by Leem, the French association of pharmaceutical companies, determined that the attractiveness of France in clinical research was mainly hampered by long trial setup times, while hospital contract times and performance indicators, such as enrolment rates, seemed to be improvedCitation7.
In order to optimize clinical trial management in each country, it is therefore critical to acquire a deep awareness of the challenges and limitations faced by clinical trial stakeholders and promote a close collaboration among all the components interested in the promotion and management of clinical research.
The R.Evolution (Research and pipeline: when innovation translates into partnership) project aimed to identify the critical issues stakeholders face in clinical research in Italy, with a particular focus on sponsored phase III–IV trials. Besides discussing the main problems in clinical research conduct, the project used a Nominal Group Technique (NGT) and a Delphi approach to summarize and rank the opinions of multiple stakeholders in order to reach a consensus on possible strategies and approaches to address these challenges and optimize clinical research management.
2. Methods
A Scientific Board comprised of 11 members (including five physicians, two experts in health care professional-patient relationships, two representatives of Ethics Committees [ECs], one pharmacist and one clinical research coordinator) was established to design the project, review and discuss the survey results, and help to generate the final statements for adapted Delphi survey. A total of 35 panelists (listed in Supplemental Appendix A) representing different roles in clinical research, such as physicians, data managers, pharmacists, nurses, hospital management representatives and administrative personnel, as well as representatives of patients’ associations, met with the Scientific Board and actively contributed to the implementation of the project. The assistance of a non-clinical chair from an independent scientific consultancy agency (Polistudium srl, Milan, Italy) was also provided to facilitate meetings and take care of material preparation and scientific accuracy.
The study was promoted by Boehringer Ingelheim Italia S.p.A., a pharmaceutical company that focuses on three main therapeutic research areas, namely respiratory/rheumatic diseases, oncology, and cardio-metabolic diseases. The project included mainly physician experts in oncology, pulmonology and cardiology, specialists in quality assurance, regulatory affairs and clinical trial management, and different patient associations working in the cardiology, oncology, and pulmonology hematology, scleroderma or obesity field.
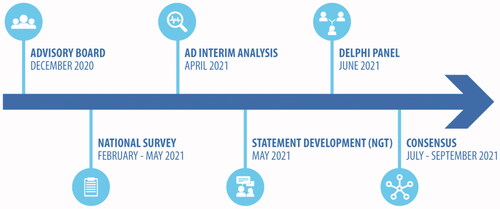
The project timeline and structure are summarized in . Briefly, based on the items raised during an advisory board with the Scientific Board, an online national survey was developed to assess the main challenges faced by stakeholders in clinical research; the results of the survey were further discussed to identify possible solutions and strategies through an NGT followed by a two-round Delphi survey to generate a consensus on critical factors, tools and strategies in clinical research. This combination of the NTG and Delphi approaches was already applied elsewhereCitation8.
2.1. Online survey
An online survey was conducted from February to June 2021 to identify, from the perspective of Italian clinical stakeholders, potentially critical areas in conducting clinical research and particularly sponsored phase III–IV trials. Questions were drafted based on items previously discussed with the 11 Scientific Board members and involved four main critical areas: study feasibility, authorization procedures, operational aspects, and patient management.
The survey was developed using the CHERRIES guidelines as a referenceCitation9. The survey was extensively disseminated on the national territory through newsletters, advertisements in online journals and social networks of the sponsoring company and the associations involved in the project (e.g. Gruppo Italiano Data Manager [GIDM], Amici Obesi Onlus, Associazione Italiana Scompensati Cardiaci [AISC], Gruppo Italiano per la Lotta alla Sclerodermia [GILS], Women Against Lung Cancer in Europe [WALCE]). Respondents included personnel directly involved in clinical research, such as physicians, data managers, pharmacists, nurses, hospital management, hospital administrative staff and institutions, as well as representatives and members of patients’ associations. In terms of specialty, the majority of respondents directly involved in clinical research were specialized in cardiology (38.5%), followed by oncology (32.5%), diabetology (23.5%), pulmonology (15%), rheumatology (7%) or other therapeutic areas (23%), whereas respondents from patients’ association were mainly involved in cardiology, lung cancer, sclerodermia or obesity.
2.2. NGT discussion and statement definition
The survey results were discussed during an Exchange Meeting held in May 2021. Participants included the Scientific Board and the 35 panelists.
Participants were divided into four groups to discuss the survey results; each group focused on a specific area: study feasibility, authorization procedures, operational aspects, and patient management. The NGT method was used to structure and facilitate the discussionCitation8 and generate the Delphi panel statementsCitation10.
Briefly, the results of the survey were presented, and participants were asked to share their opinion by answering the following questions:
"What tools, strategies, resources, and models could be implemented to address critical factors related to the area under discussion?"
"In your opinion, are there other critical factors related to the area under discussion? What solutions (tools, strategies, resources, and models) could be implemented?"
All the opinions (items) generated by the members of the Scientific Board and the Panelists were collected and shared with the participants; items were then re-elaborated with the help of a professional facilitator, merged with similar ones and ranked in terms of priority and relevance through an anonymous online survey.
The items with lower relevance scores were excluded. The scientific consultancy agency used the others to generate the list of statements for the Delphi questionnaire under the supervision and guidance of the Scientific Board.
2.3. Delphi panel
Between June and September 2021, the 11 members of the Scientific Board and the 35 panelists previously involved in the Exchange Meeting participated in the Delphi online survey and indicated their level of agreement with the statements generated through the NGT. Anonymity was kept during the entire Delphi process.
The Delphi Method is a standard method of consensus generally used when insufficient or contradictory evidence is available. It allows to assess the level of agreement (consensus quantification) interactively and anonymously and resolve differences of opinion (consensus development) on specific topics through online questionnaires. It takes place through several phases or rounds of expression and evaluation of opinions by a group of appropriately selected expertsCitation11. Each participant’s level of agreement is expressed using a Likert scale (1–5; 1 = total disagreement; 5 = total agreement), and the consensus is reached when at least 75% of voters express a vote equal to 4 or 5, according to the guidelines recommended by the Italian Ministry of HealthCitation12. Two rounds of the Delphi survey were held; after the first round, some of the items that did not reach the consensus were revised as deemed particularly relevant by participants.
2.4. Statistical analysis
All data were analyzed with descriptive statistics.
3. Results
3.1. Online survey and NGT discussion
The main items covered by the survey and discussed during the Exchange Meeting by the Scientific Board and the panelist are presented below. The survey questionnaire and the results are available as Supplementary Material.
One thousand and four respondents, including physicians (60.7%), data managers (12.3%), pharmacists (8.2%), nurses (7.3%), hospital management (4.5%), hospital administrative representatives (3.1%), institutions representatives (1.6%) and others (2.3%), and 76 representatives of patients’ associations, replied to the survey.
3.1.1. Feasibility
The main issues in terms of study feasibility were the lack of dedicated staff (e.g. study nurse, study coordinator), the lack of involvement of hospital health/general management in clinical research and the disproportionate number of studies compared with available resources. Other less impactful concerns were the lack of involvement of all necessary departments from the beginning of the study and the patient recruitment challenges. Most of the responses were provided by physicians, data managers and other staff directly involved in clinical research conduct, whereas patient’s association responses were “unknown” in 28% of the cases.
During the NGT discussion, 29 items were identified (eight very relevant, 14 relevant and seven less relevant). The most relevant items concerned the need to create a research culture, standardize bureaucratic procedures, and create a research network to improve the exchange of information, knowledge, and materials between centers. In addition, it was underlined the need for trained and specialized staff with stable contracts and for adequate motivation and dedicated spaces to support clinical research.
3.1.2. Authorization procedures
The “authorization procedures” area appeared as the most critical. The main issues raised by participants were the complex bureaucracy, the timing of the EC’s review and study contract finalization, the lack of involvement/support from the hospital general/healthcare management and the lack of dedicated spaces for clinical research. Clinical study staff and members of patients’ associations provided similar responses.
Overall, 35 items were generated (three very relevant, 13 relevant and 19 less relevant). One of the main strategies proposed by participants was a long-term plan for hiring and training dedicated staff, such as study coordinators and pharmacists and establishing a clinical trial center at each site. Another important aspect was the need for standardized, simplified and harmonized procedures and templates for submission of a clinical study to the EC. The group also discussed the importance of raising awareness of the importance of clinical research, improving communication among clinical research stakeholders, and possibly increasing the participation of patients’ associations in clinical trial design.
3.1.3. Clinical operation/drug management
According to physicians, clinical trial staff, hospital management and institutions, the major challenge of clinical operation was the complex bureaucracy, followed by less impactful aspects, such as difficulty in recruiting patients, increased complexity of studies managed by Contract Research Organization (CRO) and lack of involvement of patients’ associations. This last concern was reported as the most critical by patients’ associations. In terms of drug management, the main critical aspects were the lack of dedicated pharmacists, the limited involvement of hospital pharmacy, the lack of adequate tools, as well as structural deficiencies and the lack of appropriate tracking of the experimental drug.
Overall, 28 items were generated (15 very relevant, 11 relevant and two less relevant). Similar to what was previously discussed, participants suggested simplifying and standardizing bureaucratic procedures, improving communication and access to information for patients, hiring, training and motivating specialized personnel, and creating dedicated areas to conduct clinical research in the hospital.
3.1.4. Patient management
Study staff and hospital members identified the most critical needs were the lack of an efficient network to facilitate patients' access to the most appropriate clinical trials, the complexity of study procedures, examinations and follow-up, and the poor collaboration between hospital units/departments. These items were also recognized as important by patients’ associations, who highlighted as major issues their limited involvement and the lack of information given to patients and the general population about the value of clinical research.
The NGT discussion led to the identification of 26 statements (three very relevant, 11 relevant, 12 less relevant). The importance of having a dedicated workforce for clinical trials also emerged in this area; some of the proposed approaches included recognizing the role of study coordinators and clinical research nurses, creating dedicated study teams, improving staff contracts, and including performance indicators as incentives for the staff. Moreover, participants mentioned the need for simplified bureaucratic procedures and stressed the importance of educating patients about clinical trials, for example, by creating networks and shared platforms.
3.2. Delphi panel
Some of the NGT items were related to critical factors, tools and strategies that emerged in the different working groups and merged overlapping items. From the initial 118 items, 55 statements grouped into six interventional areas were generated: culture and patients’ involvement (11 items), procedures (nine items), staff, contracts, education and incentives (nine items), organization and infrastructures (eight items), administrative procedures (10 items) and the EC (eight items).
The Delphi panel was composed of 11 members of the Scientific Board and the experts participating in the Exchange Meeting. The items that reached consensus are reported in , while lists all the items that did not reach a consensus. For all the items that reached a consensus, the percentages of the agreement are presented in .
Table 1. Statements that reached consensus: culture and patient involvement.
Table 2. Statements that reached consensus: procedures.
Table 3. Statements that reached consensus: staff, contracts, training, incentives.
Table 4. Statements that reached consensus: organization and infrastructure.
Table 5. Statements that reached consensus: administrative procedures.
Table 6. Statements that reached consensus: ethics committee.
Table 7. Statements without overall consensus.
Interventional area 1: culture and patient involvement
The main strategies on which the panel reached a consensus () included promoting the value of clinical research both at the institutional and patient levels, creating hospital networks to refer patients to the most relevant centers/clinical trials, training healthcare professionals on how to inform patients about clinical trial requirements and benefits/risks and developing platforms with all the relevant information on ongoing clinical trials.
Some proposals, mainly advocated by patients’ associations, did not reach a consensus; these included promoting courses and information materials for patients, sharing more information between sponsors, reference centers, and patients’ associations, and collecting information on patients’ perception of clinical research. Increasing patients’ mobility between centers through inter and intra-regional networks and strengthening general practitioner involvement in clinical research did not reach a consensus.
Interventional area 2: procedures
Only two of the proposed strategies obtained a sufficient consensus (); these included optimizing patient pathways by avoiding any instrumental/laboratory exams that did not respond to study objectives, performing different exams on the same day, as well as encouraging the use of telemedicine and home visits to simplify study procedures.
All the other approaches discussed did not reach a consensus; these included reducing the number of examinations, modifying inclusion and exclusion criteria, promoting innovative study designs to reduce study costs and the number of patients enrolled, supporting the role of community healthcare professionals, general practitioners and hospital pharmacists in clinical research.
Interventional area 3: staff, contracts, training, incentives
As reported in , most of the items in this area reached a consensus, with high percentages (75.6–95.6%). Participants agreed that it is essential to hire and offer stable contracts to the staff working in clinical research. Staff should be dedicated, specialized, appropriately trained, and have diversified skills; this could be achieved by introducing appropriate university curricula and providing continuous training to all personnel involved in clinical research. It is also important to motivate staff working in the research field by providing economic incentives, possibly obtained from the sponsoring pharmaceutical company, through appropriate clinical trial agreements for covering all hospital costs, including appropriate research staffing.
Interventional area 4: organization and infrastructure
To improve clinical research organization, a stable and transversal research infrastructure (a network of dedicated clinical research units and offices) should be created to support the principal investigator and the EC. This could be achieved by creating a multifunctional clinical research center with dedicated space, facilities, and staff. Moreover, structured paths should be created to encourage and streamline the communication between various departments, ensuring that all responsible personnel, including the study coordinator, hospital pharmacist and research nurse, are timely involved in the trial organization and conduction. Finally, the panel agreed on the need to create dedicated platforms to share patients’ clinical data between centers to support real-world studies.
The importance of creating an infrastructure for drug storage and management, especially advocated by pharmacists, and the involvement of local patients’ associations in drafting clinical trial protocols, mainly requested by patients’ associations, did not reach a consensus.
The statements that reached the consensus are reported in .
Interventional area 5: administrative procedures
The strategies proposed by the panel concerned simplification, standardization, harmonization and improved communication between all the parties involved (clinical center, EC, sponsor and CRO). The panel proposed the development of shared platforms for ethics submission and sharing documentation and templates between centers. The panel also agreed on the need to speed up both EC approval and contract arrangements with the sponsor. Some participants also mentioned how the new clinical trial regulation might represent an important tool to improve and standardize the administrative process. However, the actual impact of the regulation could be better discussed only after its implementation.
Conversely, the proposal to introduce a quality certification to confirm the suitability of each center for participating in clinical trials did not reach sufficient consent, as well as the idea of providing more information or tools to clinicians to prepare the study documentation directly.
The statements that reached consensus are reported in .
Interventional area 6: ethics committees
Efforts should be made to standardize EC’s requests among different centers and organize dedicated meetings with investigators to rapidly address unclear aspects of a clinical study. The efforts of staff working in the EC should be recognized, especially for committees with a relevant workload; however, it is also essential that the timelines for EC evaluation are respected and that any delay is adequately explained to investigators. Finally, the involvement of patients’ associations and external consultants in the ethics evaluation of clinical trials was encouraged, while the presence of a clinical research coordinator was not deemed necessary by all the participants.
The statements related to Ethics Committee that reached consensus are reported in .
Discussion
According to our analysis, the main challenges faced by clinical research centers are related to study feasibility, authorization procedures, operational aspects and patient management. Similar strategies have been proposed to address issues identified in different areas, and ultimately six areas of interventions were identified. The main interventions proposed by the panel refer to the need for cultural and patient involvement; the importance of staff, contracts, training, but also organization and infrastructures, and the need for simplified procedures, both in relation to patients’ assessments and in terms of bureaucratic procedures and relationships with the EC.
One of the main challenges identified by panelists was the lack of awareness of the value of clinical research among patients and the general population and inside the institutions and among hospital managers. A high consensus level (93.3%) was reached on the need to promote clinical research culture at all levels by promoting a better understanding of its value in terms of benefits for the community and its requirements.
In particular, the panel agreed on the fact that patients should be more involved in clinical research, should be able to retrieve all the information on clinical trials through dedicated platforms, and should have facilitated access to the most relevant centers/trials through a collaborative network of clinical centers and receive complete explanations on the requirements, benefits and risks of clinical trials from trained healthcare professionals. Patients' associations proposed additional strategies to implement patients’ involvement in clinical trials but did not reach a consensus among all the experts.
Another critical aspect that clearly emerged from the R.Evolution project was the lack of adequately trained, contractually stable and dedicated personnel. Given the increasing complexity of clinical research, and the major changes foreseen in clinical trials when the Clinical Trials Regulation (No 536/2014) comes into forceCitation13, experienced and qualified personnel for supporting clinical research activities must be available in each center. This requires a political and organizational commitment and a long-term investment plan to create dedicated facilities and hire the required staff. Study coordinators, clinical research nurses, data managers and pharmacists need to be recognized as essential figures in clinical research.
In order to improve the qualification of staff involved in clinical trials, specific and standardized university curricula should be created. These professionals need continuous training and adequate financial and career recognitions to maintain and increase their motivation. Moreover, it is essential to ensure that all the necessary staff is involved in each trial and that in each center, structured paths of collaboration are created and followed by different units and departments. To this end, each hospital should be equipped with a clinical trial center, a stable and transversal research infrastructure with dedicated spaces, facilities and personnel to support the principal investigators and the ECs.
Indeed, the lack of staff and adequate incentives is also recognized at the EC level, especially for committees with high workloads. Especially in these cases, EC members should be adequately paid for their efforts; moreover, the help of external consultants and patients’ associations should be encouraged in the evaluation of clinical trial protocols by EC.
Last, the panelists proposed a strategy based on simplifying patients’ assessments and administrative procedures. Interestingly, most (7/9) of the items related to simplification of patients’ procedures did not reach a consensus; panelists agreed only on the need to focus clinical trials assessments on primary and secondary endpoints, thus avoiding unnecessary examinations and combining more exams on the same day, whenever possible.
Conversely, most (7/10) of the strategies proposed to simplify, harmonize and standardize the complex bureaucratic procedures received full consensus from panelists. Potential strategies include the creation of shared portals between all the stakeholders (center, sponsor, CRO and EC), drafting and sharing common templates (especially for the informed consent form) and harmonizing procedures for study submission through digitalization. It also appeared essential that timelines be respected regarding EC approval and finalization of clinical study agreements between centers and sponsors.
Overall, the strategies and tools identified by the R.Evolution project, which summarize the point of view of multiple subjects involved in clinical research, may help improve clinical research conduct, strengthen the relationships among stakeholders, and promote clinical research at the local, national, and international level. The results of this study could also represent a useful starting point to inform healthcare politicians on the challenges and possible solutions to clinical research's main issues, given the implementation of relevant Italian decrees in this field.
Obviously, the results of this study apply to the Italian context and are focused on the perspectives of centers conducting sponsored phase III–IV clinical trials; different results may be yielded in other European countries or with reference to phase I-II clinical trials, which present major complexities. Also, many differences among clinical trials addressed to different specialties exist, for example, in relation to main endpoints, phases of development and study construction. However, the drug's development phases (I, II, III, IV) are similar. Despite all this, the methodology used in this paper could be applied in different settings. In fact, the project was conducted using a qualitative approach, including the NGT technique and the Delphi model to produce rigorous data; moreover, multiple stakeholders were involved, including clinical staff, physicians, ethics committee and patients’ associations, so to convey the point of view of multiple subjects.
Conclusion
The results of the R.Evolution project suggest that the main critical areas in the conduct of clinical research in Italy are the lack of awareness of the value of clinical research, the lack of trained workforce at the center and the excessive complexity of protocols and administrative procedures. Possible strategies to overcome these obstacles include increasing the understanding of the value of clinical research among patients, institutions and the general population; implementing long-term plans for hiring, training, organizing and motivating clinical trial staff; reducing the complexity of protocol design by avoiding unnecessary tests; simplifying and harmonizing administrative procedures and increasing communication among centers and between different stakeholders.
Transparency
Declaration of funding
This study was funded by an unrestricted grant from Boehringer Ingelheim Italia S.p.A.
Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy and intellectual property considerations.
Declaration of financial/other relationships
ML: Payment or honoraria for lectures, presentations, advisory board, manuscript writing or educational events from BMS, Pfizer, Sanofi, Boehringer, Edwards Lifescience. D.C.: Speaker bureau and Scientific Advisor from Roche, AstraZeneca, BMS, MSD, Boehringer Ingelheim, Amgen, Novartis, Lilly. A.Co: Payment or honoraria for lectures, presentations, advisory board, manuscript writing or educational events from BMS, Pfizer, Sanofi, Boehringer Ingelheim, Amgen, Abbvie, Novartis, Lilly, UCB, Celgene, Janssen. FA, AB, AC, L.DM., RL, FL, MT.P., DT have no conflict of interest to declare.
A reviewer on this manuscript has disclosed that they are a consultant for AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Eisai, Enteris BioPharma, HLS Therapeutics, Impel, IntraCellular Therapies, Janssen, Karuna, Lundbeck, Lyndra, Medavante-ProPhase, Merck, Neurocrine, Novartis,
Noven, Otsuka, Ovid, Relmada, Reviva, Sage, Sunovion, Supernus, Teva, University of Arizona, and has also done one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Authors are listed in alphabetic order because they all equally contributed to the paper. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Study conception and design: all the authors; collection and interpretation of data: all the authors manuscript editing: all the authors; approval to submit: all the authors; Drafting of the Statement: all the authors; Consensus: Scientific Board and Panelist
SUPPLEMENTARY_MATERIAL_2_-_SURVEY_results.pptx
Download MS Power Point (466.1 KB)SUPPLEMENTARY_MATERIAL_1_-_SURVEY_Translated.docx
Download MS Word (30.8 KB)APPENDIX_A_Revolution_revACFC_10.04.2022.docx
Download MS Word (19.7 KB)Acknowledgements
Francesca Cappellini, PhD, Ambra Corti, PhD, Sara di Nunzio, PhS Luca Giacomelli, PhD and Aashni Shah of Polistudium srl, Milan, Italy provided writing, scientific, editorial and methodological assistance under the authors’ conceptual direction and based on feedback from the authors; Polistudium srl was contracted and compensated by Boehringer Ingelheim Italia SpA.
The authors thank the panelist listed in Appendix 1 for the participation in the meetings and their intellectual contribution.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
References
- Getz KA, Campo RA. Trial watch: trends in clinical trial design complexity. Nat Rev Drug Discov. 2017;16(5):307.
- Djurisic S, Rath A, Gaber S, et al. Barriers to the conduct of randomised clinical trials within all disease areas. Trials. 2017;18(1):360.
- ICH guideline E8 (R1) on general considerations for clinical studies. EMA; 2021. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-8-general-considerations-clinical-trials-step-5_en.pdf.
- Laboratorio sul Management delle Sperimentazioni Cliniche. Il Valore delle sperimentazioni cliniche in Italia. Report; 2020. Available from: https://www.farmindustria.it/app/uploads/2021/04/Il-valore-della-sperimentazioni-COMPLETO.pdf.
- Gehring M, Jommi C, Tarricone R, et al. The italian SAT-EU study group. Towards a more competitive Italy in clinical research: the survey of attitudes towards trial sites in Europe (the SAT-EU StudyTM). Epidemiol Biostat Public Health. 2015;12(1):1–9.
- Duley L, Gillman A, Duggan M, et al. What are the main inefficiencies in trial conduct: a survey of UKCRC registered clinical trials units in the UK. Trials. 2018;19(1):15.
- Galaup A, Barthélémy P, Pouletty-Lefebvre B, et al. Attractivité de la France pour la recherche clinique internationale: résultats de la 8e enquête du leem [Attractiveness of France for international clinical research: 8th survey conducted by leem (french association for pharmaceutical companies)]. Therapie. 2018;73(5):367–376. French.
- Bosello SL, Beretta L, Del Papa N, et al. Interstitial lung disease associated with autoimmune rheumatic diseases: checklists for clinical practice. Front Med. 2021;8:732761.
- Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E-Surveys (CHERRIES). J Med Internet Res. 2004;6(3):e34.
- Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–380.
- Milholland AV, Wheeler SG, Heieck JJ. Medical assessment by a delphi group opinion technic. N Engl J Med. 1973;288(24):1272–1275.
- Candiani G, Colomb C, Daghini R, et al. Come organizzare una conferenza di consenso. Manuale metodologico Sistema nazionale per le Linee Guida. 2019. Available from: https://www.psy.it/wp-content/uploads/2018/02/Manuale-Metodologico-Consensus.pdf.
- EMA. Clinical Trials Regulation. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials/clinical-trials-regulation.