Abstract
Background
The extent of short-acting β2-agonist (SABA) overuse in Africa remains poorly documented. As part of the SABA use IN Asthma (SABINA) III study, we assessed SABA prescriptions/clinical outcomes in 3 African countries.
Methods
Data on disease characteristics/asthma treatments were collected from patients (≥12 years) using electronic case report forms. Patients were classified by investigator-defined asthma severity (guided by the 2017 Global Initiative for Asthma) and practice type (primary/specialist care). Multivariable regression models analyzed associations between SABA prescriptions and outcomes.
Results
Data from 1778 patients (mean age, 43.7 years) were analyzed. Most patients were female (62.4%) and had moderate-to-severe asthma (63.3%), with 57.1 and 42.9% of patients treated in specialist and primary care, respectively. Asthma was partly controlled/uncontrolled in 66.2% of patients, with 57.9% experiencing ≥1 severe exacerbation in the previous 12 months. Overall, 46.5% of patients were prescribed ≥3 SABA canisters in the preceding 12 months (over-prescription); 26.2% were prescribed ≥10 canisters. SABAs were purchased over-the-counter by 32.6% of patients, of whom 79.3% had received SABA prescriptions; 71.9% and 40.1% for ≥3 and ≥10 canisters, respectively. Higher SABA prescriptions (vs. 1–2 canisters) were associated with increased incidence rate of severe exacerbations and lower odds of having at least partly controlled asthma (except 3–5 canisters).
Conclusions
Findings from this African cohort of the SABINA III study indicate that SABA over-prescription and SABA over-the-counter purchase are common and associated with poor asthma-related outcomes. This highlights the need for healthcare providers/policymakers to align clinical practices with the latest treatment recommendations.
Introduction
Asthma is a serious global health problemCitation1 estimated to affect approximately 339 million people worldwideCitation2 and over 119 million people in the African continentCitation3. Many patients with asthma require long-term medication daily to control the underlying airway inflammation and prevent symptoms and exacerbationsCitation1,Citation4. Although inhaled corticosteroids (ICS) are used to treat the underlying airway inflammation, short-acting β2-agonists (SABAs) provide rapid symptom relief by reducing airway narrowingCitation1. However, SABAs have no inherent anti-inflammatory activityCitation5,Citation6, and their overuse (≥3 canisters/yearCitation7) is associated with an increased incidence of exacerbations, mortality, and healthcare costsCitation8–10. Consequently, owing to safety concerns, the Global Initiative for Asthma (GINA) no longer recommends as-needed SABAs without concomitant ICS for patients aged ≥12 yearsCitation1.
Despite the availability of effective treatment optionsCitation1, asthma remains poorly controlled in a substantial proportion of patients worldwide, with long-term management being insufficient to meet the goals put forward in the GINA recommendationsCitation11. Therefore, asthma remains a major health problem, particularly in low- and middle-income countries, and represents a greater problem in Africa than originally thoughtCitation2 owing to weak healthcare systems, including poor infrastructure; inadequate resources and healthcare provider (HCP) capacity; and low budget allocationCitation3,Citation12. In addition, regional factors including diagnostic challenges, low level of awareness of the disease burden, non-availability and unaffordability of ICS, nonadherence to prescribed medications (when available), lack of patient education, poor communication between HCPs and patients, lower educational levels, and inherent sociocultural misconceptions regarding asthma and its treatment negatively impact asthma management in African countriesCitation3,Citation12,Citation13.
To date, the prevalence of asthma has been reported in only a few parts of Africa, with data indicating a gradual increase in morbidity due to asthmaCitation14. Furthermore, there is a scarcity of data concerning prescription patterns for asthma medications, in particular the prevalence of SABA use and its consequences, in Africa. Understanding how access to and use of medications impact asthma care, particularly in Africa, where improving access to affordable asthma medication represents an unmet needCitation2,Citation15, remains of paramount importance. Moreover, an assessment of SABA prescription patterns can help guide policy decisions to align local treatment guidelines with the latest evidence-based treatment recommendationsCitation1 and therefore ensure that patients have sufficient access to essential asthma medications. However, a lack of comprehensive healthcare databases has limited access to patient-level data and evaluation of trends in medication use across the African continent.
The SABA use IN Asthma (SABINA) III study is part of a series of real-world observational studies conducted globally to describe SABA prescription patterns across countries in the Asia Pacific, Africa, the Middle East, Latin America and in Russia using electronic case report forms (eCRFs) to capture patient-level data from HCPsCitation16. Here, we report SABA prescription patterns and their association with clinical outcomes in the African cohort (Egypt, South Africa, and Kenya) of the SABINA III study.
Methods
Study design
SABINA III was a cross-sectional, multi-country, multi-centre observational study conducted in 24 countries across 5 continentsCitation16. The primary objective was to describe SABA prescription patterns in the African cohort of the SABINA III study at an aggregated multi-country level. The secondary objective was to determine the associations between SABA prescriptions and asthma-related health outcomes in this cohort. The methodology for SABINA III has been described previouslyCitation7,Citation16. The study was conducted in accordance with the study protocol, the Declaration of Helsinki, and local ethics committees. Signed informed consent was obtained from all patients or their legal guardians per local ethics review committee regulations.
Study population
Patients aged ≥12 years who met the following criteria were eligible for enrollment: a documented physician diagnosis of asthma in their medical records, ≥3 prior consultations with their HCP, and medical records containing data for ≥12 months before the study visit. Patients with a diagnosis of other chronic respiratory diseases, such as chronic obstructive pulmonary disease, or with an acute or chronic condition that, in the investigator’s opinion, would limit the patient’s ability to participate in the study were excluded. Primary and specialist care study sites were selected using purposive sampling with the aim of obtaining a sample representative of asthma management within each participating country by a national coordinator, who also facilitated the selection of investigators.
Study variables
During the cross-sectional study visit, retrospective data were obtained from existing medical records, and patient data, including an assessment of current asthma symptom control, were collected and entered into an eCRF by the investigator. SABA prescriptions recorded during the 12 months before the study were categorized as 0, 1–2, 3–5, 6–9, 10–12, and ≥13 canisters. Over-prescription was defined as a prescription of ≥3 SABA canisters in the 12 months prior to the study visitCitation7. For consistency, across the whole SABINA program, one SABA canister was assumed to contain 150 inhalationsCitation7. ICS canister prescriptions in the previous 12 months were recorded and expressed according to the prescribed average daily dose—low, medium, or high (Supplemental Table 1)Citation17.
Secondary variables included practice type (primary or specialist care), investigator-classified asthma severity (guided by GINA 2017; steps 1–2: mild asthma; steps 3–5: moderate-to-severe asthma)Citation17, asthma duration, and asthma treatment in the preceding 12 months (SABA monotherapy, SABA in addition to maintenance therapy, ICS, fixed-dose combination of ICS with long-acting β2-agonists [LABAs], long-term oral corticosteroid [OCS] treatment [any OCS treatment for >10 days], OCS burst treatment [defined as a short course of intravenous (IV) corticosteroids or OCS administered for 3–10 days or a single dose of an intramuscular (IM) corticosteroid to treat an exacerbation], and antibiotics prescribed for asthma). In addition, data for SABA purchase over-the-counter [OTC] without a prescription was based on patient recall and obtained directly from patients at the study visit, which was subsequently entered into the eCRF by the investigator.
Other variables included healthcare insurance (not reimbursed [out-of-pocket expenses], partially reimbursed [expenses partially covered by insurance], or fully reimbursed [expenses fully covered by insurance]), education level (primary and secondary school, high school, or university and post-graduate), body mass index (BMI), number of comorbidities, and tobacco smoking status.
Outcomes
Asthma symptom control was evaluated according to the GINA 2017 assessment of asthma control and categorized as well controlled, partly controlled, and uncontrolledCitation17. Severe exacerbations in the 12 months before the study visit were based on the American Thoracic Society/European Respiratory Society recommendationsCitation18 and defined as deterioration in asthma resulting in hospitalization or emergency room treatment, or the need for IV corticosteroids or OCS for ≥3 days, or a single IM corticosteroid dose.
Statistical analysis
Patient-level analyses are presented as country-aggregated descriptive statistics. The association of SABA prescriptions (3–5, 6–9, 10–12, and ≥13 vs 1–2 canisters) in the previous 12 months with the incidence rate of severe exacerbations and the odds of achieving at least partly controlled asthma (uncontrolled asthma as the reference) was analyzed using negative binomial and logistic regression models, respectively. Patients with 0 SABA prescriptions were excluded as it was not possible to determine the reliever medication used. All regression models used complete-case analyses and were adjusted for prespecified covariates (country, age [continuous variable], sex, and tobacco smoking status) and potential confounders (GINA treatment step, healthcare reimbursement, education level, comorbidities, asthma duration [continuous variable], and BMI [continuous variable]). All statistical tests were 2-sided, at a 5% level of significance, and were performed using the R statistical software (version 3.6.0).
Patient and public involvement
It was not appropriate or possible to involve patients or the public in the design, conduct, reporting, or dissemination plans of our research.
Results
Patient disposition
Of the 1794 patients enrolled, 16 were excluded because their asthma duration was less than 12 months; therefore, 1778 patients were included in the analysis (Supplemental Figure 1). Most patients were recruited from Egypt (n = 872; 49.0%), followed by South Africa (n = 501; 28.2%) and Kenya (n = 405; 22.8%; Supplemental Figure 2). A slightly higher proportion of patients were treated by specialists (57.1%) than by primary care physicians (42.9%; Supplemental Figure 1).
Patient and disease characteristics
Overall, the mean (standard deviation [SD]) age of the patients was 43.7 (16.0) years, with most patients evenly distributed across all age groups. In specialist care, most patients were aged 18–34 years (29.9%), while in primary care most were aged ≥55 years (30.2%). The majority of patients were female (62.4%), classified with moderate-to-severe asthma (GINA steps 3–5; 63.3%), had a BMI of ≥25 kg/m2 (68.4%), and had never smoked tobacco (80.9%; ). More than one-quarter of patients had received only high school education (28.1%), while 33.7% had obtained university and post-graduate education. Most patients with mild asthma (67.1%) were treated in primary care, while most patients with moderate-to-severe asthma (86.1%) were treated in specialist care. More than half of all patients (53.2%) had no healthcare reimbursement. Overall, nearly half of all patients had ≥1 comorbidity (47.7%). The level of asthma symptom control was assessed as well-controlled in 33.8% of patients, partially controlled in 39.8% of patients, and uncontrolled in 26.4% of patients. Asthma symptom control was generally comparable across asthma severities in patients treated in primary care; however, in specialist care, asthma was well-controlled in a greater percentage of patients with mild asthma than in those with moderate-to-severe asthma (66.2 vs 35.7%; ). Patients reported a mean (SD) of 1.4 (2.4) severe exacerbations, with 57.9 and 16.1% having experienced ≥1 and ≥3 severe asthma exacerbations, respectively, in the previous 12 months. In primary care, a comparable proportion of patients with mild and moderate-to-severe asthma experienced ≥1 severe exacerbation in the previous 12 months (55.6 and 53.2%, respectively). In contrast, in specialist care, a higher proportion of patients with moderate-to-severe asthma than those with mild asthma experienced ≥1 severe exacerbation (62.4 and 46.5%, respectively).
Table 1. Demographics and baseline clinical characteristics of the SABINA III African population by investigator-classified asthma severity and practice type.
Table 2. Asthma characteristics of the SABINA III African population according to investigator-classified asthma severity and practice type.
Asthma treatment in the 12 months before the study visit
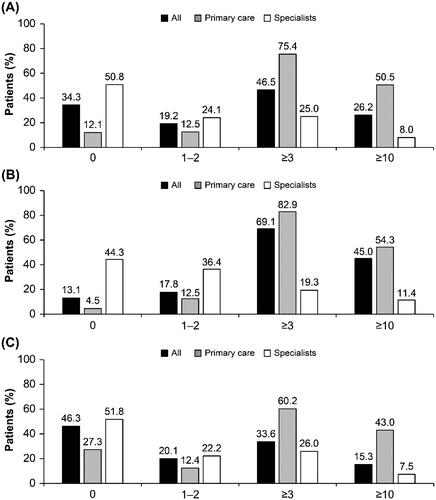
Overall, 46.5 and 26.2% were prescribed ≥3 and ≥10 SABA canisters, respectively, in the previous 12 months; over one-third of patients (34.3%) were prescribed 0 SABA canisters (). Compared with patients with moderate-to-severe asthma, a higher proportion of patients with mild asthma were prescribed ≥3 (69.1 vs. 33.6%) and ≥10 (45.0 vs. 15.3%) SABA canisters, respectively. A higher proportion of patients with mild asthma treated in primary care vs specialist care were prescribed ≥3 (82.9 vs. 19.3%) and ≥10 (54.3 vs. 11.4%) SABA canisters in the preceding 12 months, respectively. Similarly, a higher proportion of patients with moderate-to-severe asthma treated in primary care vs specialist care were prescribed ≥3 (60.2 vs. 26.0%) and ≥10 (43.0 vs. 7.5%) SABA canisters in the 12 months prior, respectively.
Figure 1. Proportion of patients (%) receiving SABA prescriptions in the 12 months before the study visit according to investigator-classified asthma severity and practice type in the SABINA III African cohort (N = 1778): (A) all patients, (B) mild asthma, and (C) moderate-to-severe asthma. *Patients without SABA prescriptions did not report which reliever they were using. Abbreviations. SABA, short-acting β2-agonist; SABINA, SABA use IN asthma.
SABA monotherapy
Overall, 6.5% of patients were prescribed SABA monotherapy, with a mean (SD) of 6.1 (4.4) canisters in the previous 12 months (). Among these patients, 66.1% were prescribed ≥3 canisters and 33.0% were prescribed ≥10 canisters in the preceding 12 months. A higher proportion of patients in primary care (12.6%), all of whom had mild asthma, were prescribed SABA monotherapy compared with those treated by specialists (1.8%). SABA monotherapy was prescribed to 18.8 and 12.1% of patients with mild asthma in primary and specialist care, respectively, and to 0.1% of patients with moderate-to-severe asthma in specialist care. Overall, 72.9 and 33.3% of patients were prescribed ≥3 SABA canisters in the previous 12 months in primary and specialist care, respectively.
Table 3. Asthma treatments prescribed and SABA canisters purchased OTC in the 12 months before the study visit by patients in the SABINA III African cohort.
SABA in addition to maintenance therapy
Most patients (59.3%) were prescribed SABA in addition to maintenance therapy in the previous 12 months, with a mean (SD) of 7.0 (4.8) canisters (). Overall, 71.3% of patients were prescribed ≥3 SABA canisters and 40.6% were prescribed ≥10 SABA canisters in the 12 months prior. A higher proportion of patients treated in primary care vs specialist care were prescribed SABA in addition to maintenance therapy (75.3 vs. 47.4%). Overall, 87.9 and 61.2% of patients in primary care were prescribed ≥3 and ≥10 canisters, respectively, compared with 51.6 and 16.1% of patients in specialist care.
SABA obtained OTC without prescriptions
Overall, 32.6% of patients purchased SABA OTC, of whom 51.8 and 6.0% purchased ≥3 and ≥10 SABA canisters, respectively (). Among patients who purchased SABA OTC, 20.7% had no SABA prescriptions and 79.3% had also received SABA prescriptions (Supplemental Figure 3). Of patients with both SABA OTC purchase and SABA prescriptions, 71.9% had received prescriptions for ≥3 SABA canisters and 40.1% had received prescriptions for ≥10 SABA canisters in the previous 12 months. A higher proportion of patients treated in primary care had SABA OTC purchases compared to those treated in specialist care (36.8 vs 29.6%).
Other prescriptions of asthma medication in the 12 months before the study visit
ICS as sole maintenance therapy was prescribed to 28.3% of patients, with a mean (SD) of 10.0 (4.3) ICS canisters in the preceding 12 months (). Most patients were prescribed medium-dose ICS (60.4%); 28.2 and 11.4% of patients were prescribed low-dose and high-dose ICS, respectively. Over half of patients (53.5%) in primary care were prescribed ICS. In contrast, only 9.5% of patients in specialist care were prescribed ICS. In both primary care and specialist care, ICS was generally prescribed to patients with mild asthma (75.5 and 42.9%, respectively). A higher proportion of patients in primary care vs specialist care were prescribed medium-dose ICS (66.7 vs 33.0%; ).
An ICS/LABA fixed-dose combination as maintenance therapy was prescribed to 66.6% of patients, of whom over half (52.2%) were prescribed medium-dose ICS; 34.9 and 12.9% of patients were prescribed low-dose and high-dose ICS, respectively (). Compared with 91.0% of patients in specialist care who were prescribed an ICS/LABA combination, only 34.2% of patients treated in primary care were prescribed ICS/LABA. Primary care physicians prescribed ICS/LABA for 2.9% of patients with mild asthma (60.0% as low-dose ICS combinations) and for 98.0% of patients with moderate-to-severe asthma (57.2% as medium-dose ICS combinations). Specialists prescribed ICS/LABA for 41.4% of patients with mild asthma (81.0% as low-dose ICS combinations) and for almost all patients (99.0%) with moderate-to-severe asthma (53.3% as medium-dose ICS).
Overall, during the previous 12 months, an OCS burst was prescribed to 34.9% of patients (30.3% in primary care and 38.3% in specialist care). In primary care, a similar proportion of patients with mild (30.4%) and moderate-to-severe asthma (30.1%) were prescribed an OCS burst. However, in specialist care, a higher proportion of patients with moderate-to-severe asthma vs mild asthma were prescribed an OCS burst (40.6 vs. 23.9%; ).
Approximately one-third of all patients (32.7%) were prescribed antibiotics (). Prescriptions of antibiotics differed between primary care and specialist care providers, with 46.8% of patients in specialist care prescribed antibiotics compared with only 14.5% of patients in primary care.
In addition, in both care modalities, a higher proportion of patients with moderate-to-severe asthma (primary care, 19.8%; specialist care, 50.1%) were prescribed antibiotics compared to those with mild asthma (primary care, 11.8%; specialist care, 27.3%).
Patients prescribed concomitant OCS maintenance treatment and antibiotics for asthma
Overall, only 0.6% of patients (n = 10) were prescribed concomitant OCS maintenance treatment and antibiotics for asthma. Of these patients, the majority (80%) were treated by specialists. The demographics, baseline clinical characteristics and asthma characteristics of patients prescribed OCS maintenance treatment and antibiotics is presented in Supplemental Tables 2 and 3. All patients prescribed concomitant OCS maintenance treatment and antibiotics were also prescribed SABA in addition to maintenance therapy, with 70% of these patients receiving prescriptions for ≥3 canisters in the previous 12 months (Supplemental Table 4). In addition, 90% of patients prescribed concomitant OCS maintenance treatment and antibiotics were prescribed ICS/LABA fixed-dose combination, with only 10% receiving an OCS burst prescription.
Association of SABA prescriptions with asthma-related health outcomes
In prespecified regression analyses (Supplemental Figure 4), higher SABA prescriptions (3–5, 6–9, 10–12, and ≥13 vs. 1–2 canisters) in the previous 12 months were associated with an increase in the incidence rate of severe exacerbations, although not statistically significant for all SABA prescription categories (). Compared with patients prescribed 1–2 SABA canisters, no increase in the incidence rate of severe exacerbations was observed in patients prescribed 3–5 (adjusted incidence rate ratio [IRR], 1.02; 95% confidence interval [CI], 0.80–1.31; p-value, .870) and 6–9 (IRR, 1.01; 95% CI, 0.79–1.30; p-value, .914) SABA canisters. However, the prescription of 10–12 SABA canisters (vs 1–2 canisters) was associated with a significant 28.0% increase in severe exacerbations (IRR, 1.28; 95% CI, 1.04–1.59; p-value, .029). Although the prescription of ≥13 canisters (vs. 1–2 canisters) was associated with a 78.0% increase in the incidence rate of severe exacerbations, this did not reach statistical significance (IRR, 1.78; 95% CI, 0.98–3.37; p-value, .059).
Figure 2. Association of SABA prescriptions with (A) severe exacerbations in the 12 months before the study visit and (B) level of asthma control assessed during the study visit in the SABINA III African cohort. Abbreviations. BMI, body mass index; CI, confidence interval; GINA, global initiative for asthma; IRR, incidence rate ratio; OR, odds ratio; SABA, short-acting β2-agonist; SABINA, SABA use IN asthma. Based on the covariable significance in the models, IRRs are corrected by country, age, sex, BMI, tobacco smoking history, GINA step, healthcare insurance, prescriber type, comorbidity, asthma duration, and education level. ORs are corrected by country, age, sex, BMI, asthma duration, tobacco smoking history, comorbidity, GINA step, healthcare insurance, prescriber type, and education level.
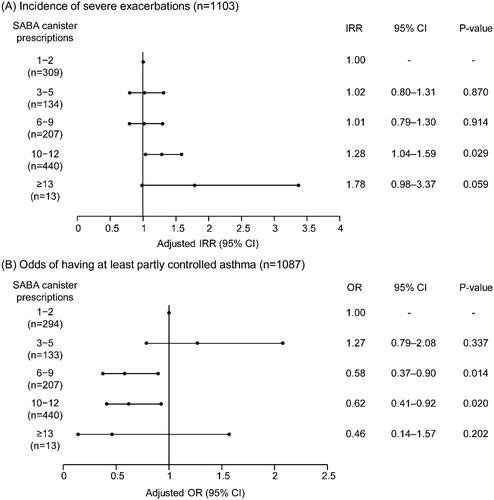
Additionally, the odds of having at least partly controlled asthma decreased with higher SABA prescriptions, with the exception of patients prescribed 3–5 SABA canisters (odds ratio [OR], 1.27; 95% CI, 0.79–2.08; p-value, .337; ). The prescription of 6–9 and 10–12 SABA canisters (vs 1–2 canisters) was associated with 42.0% (OR, 0.58; 95% CI, 0.37–0.90; p-value, .014) and 38.0% (OR, 0.62; 95% CI, 0.41–0.92; p-value, .020) significantly lower odds of having at least partly controlled asthma, respectively. Despite 54.0% lower odds of having at least partly controlled asthma, this association was not significant for prescription of ≥13 canisters (vs. 1–2 canisters; OR, 0.46; 95% CI, 0.14–1.57; p-value, .202).
Comparison of results between SABINA Africa and SABINA III
A comparison of data on sociodemographic and disease characteristics, asthma treatments, and asthma-related clinical outcomes in the previous 12 months between the SABINA Africa and the overall SABINA III population is summarized in Supplemental Table 5. The key differences are highlighted in the Discussion section.
Discussion
Asthma is an increasing health problem in Africa that has received relatively little attentionCitation3. Overall, there is a paucity of large-scale clinical trials from Africa, with most studies conducted in South African and Nigerian populationsCitation3. Furthermore, significant gaps remain in the current knowledge of asthma management in Africa. Therefore, the results from the African cohort of the SABINA III study in 1778 patients with asthma provide valuable real-world evidence on asthma management practices in this region. Notably, while the majority of patients were prescribed maintenance therapy in the form of either ICS or ICS/LABA fixed-dose combinations, SABA over-prescription was common, with 46.5% of patients overall being prescribed ≥3 SABA canisters in the preceding12 months, which was associated with poor asthma-related health outcomes in terms of an increased incidence of severe exacerbations (statistically significant for 10–12 canisters) and poor asthma control (statistically significant for 6–9 and 10–12 canisters).
Overall, the baseline patient and disease characteristics in this study were generally consistent with those observed in the SABINA III populationCitation16. However, compared with the overall population (mean age, 49.4 years)Citation16, patients in this African cohort were younger (mean age, 43.7 years). This finding may be explained in part by the reported increase in asthma prevalence among children in AfricaCitation3 and the fact that Africa has one of the world’s youngest populationsCitation19,Citation20. Although the mean BMI of this African cohort (28.2 kg/m2) was comparable to previous reports from this continentCitation13,Citation21, most patients (68.4%) had a BMI of ≥25 kg/m2. This finding is likely attributable to the fact that 62.4% of patients in this African cohort were female and is in line with the SABINA III population, where 68.1% of patients were female and 65.6% had a BMI of ≥25 kg/m2; based on previous research, older females with high BMI represent a distinct cluster of asthma patientsCitation22,Citation23. Importantly, and in contrast to the SABINA III populationCitation16, the African cohort had a relatively balanced distribution of patients treated in primary and specialist care (42.9 and 57.1%, respectively vs. 17.2 and 82.3%, respectively), thereby providing an understanding of how asthma is currently being managed and treated in Africa. Thus, compared with the overall SABINA III population, a lower percentage of patients from this African cohort had moderate-to-severe asthma (63.3 vs 76.%, respectively)Citation16. Overall, the majority of patients in this study had received secondary school education or higher (79.9%). Although this was substantially higher than that previously documented in Africa, where less than 50% of patients had reported secondary school education or higherCitation21,Citation24, these findings were comparable with the overall SABINA III population (76%)Citation16.
Of concern, a high proportion of patients in this African cohort were prescribed SABA treatments, both SABA monotherapy and SABA with maintenance therapy. Indeed, compared with the SABINA III populationCitation16, a higher proportion of patients in this study were prescribed ≥3 SABA canisters as monotherapy (66.1 vs. 53.6%) or in addition to maintenance therapy (71.3 vs. 61.7%), in the previous 12 months, which is regarded as over-prescription. Worryingly, 33.0 and 40.6% of patients receiving SABA as monotherapy or with maintenance treatment, respectively, were prescribed ≥10 canisters in the preceding 12 months. Although more apparent in primary care, these trends were observed in both primary and specialist care. Such findings suggest an urgent need for educational initiatives targeted at both primary care physicians and specialists to align clinical practices in Africa with current treatment recommendations. This is of critical importance since many African countries have no standard protocols for the diagnosis and management of asthma in place, and where available, such guidelines are rarely widely disseminated and implementedCitation3. Indeed, it has been highlighted that educating both HCPs and patients is essential to address the current challenges posed by asthma in AfricaCitation25. A lack of full healthcare reimbursement may also have contributed to SABA over-prescription in this African cohort. In contrast to both the SABINA III studyCitation16, where 47.2% of patients had full healthcare reimbursement, and the ESMAA (Assessment of Asthma Control in Adult Asthma Population in the Middle East and North Africa) study, which included 3 African countries (Algeria, Egypt, and Tunisia), and reported that the majority of patients had medical insurance coverage, ranging from 52.5% (in Egypt) to 87.2% (in Algeria)Citation13, only 31.4% of patients in this African cohort were fully reimbursed. Notably, the proportion of patients with no healthcare reimbursement in this African cohort was ∼2-fold higher than that observed in the SABINA III population (53.2 vs. 27.3%), despite patients reporting comparable levels of education. Although higher education is associated with increased rates of healthcare insurance coverageCitation26, factors such as high premiums, limited understanding of entitlements, or insufficient healthcare benefitsCitation27 may have prevented patients in this African cohort from applying or receiving healthcare reimbursement. Consequently, there is a need to transform the way in which healthcare delivery is funded across Africa by moving away from out-of-pocket expensesCitation27, strengthening/pooling resources, providing support through existing legislation, enacting new laws or policies, and ensuring harmonization across different government departmentsCitation28. This is of particular importance since a lack of healthcare insurance has been linked to consistently poorer quality of asthma care, including a lower likelihood of receiving ICSCitation29.
Crucially, not all SABAs were obtained with prescriptions., Indeed, despite the fact that over three-quarters of patients (79.3%) had already been prescribed SABA, approximately one-third of patients (32.6%) in this African cohort purchased SABA OTC, which was considerably higher than the 18% observed in the overall SABINA III populationCitation16. This highlights patients’ over-reliance on SABA therapy and willingness to self-manage their worsening of asthma symptomsCitation30–32. However, this is a matter of grave concern since SABA purchase has been associated with low rates of consultation with family practitioners and specialists; low use of prescription-only medication, particularly ICS; and undertreatment of asthmaCitation33–35. Overall, these findings provide valuable insights into how patients in this African cohort self-manage their asthma; it would appear to be common practice for them to purchase SABA from a private pharmacy, which may further contribute to poor asthma control. Moreover, the high cost of ICS-containing combination inhalers compared with single SABA inhalers in African countriesCitation36–38 may have further contributed to out-of-pocket spending for OTC SABA purchase. Indeed, studies have consistently demonstrated that affordability of internationally recommended treatments for asthma in low-and middle-income countries, including Africa, remains a major challengeCitation36–38. Consequently, there is an urgent need to implement policies that regulate the purchase of SABA without prescription while ensuring that patients have access to affordable care and asthma medications, including adequate provision for maintenance therapy.
To our knowledge, this is the first study to assess the association between SABA prescription patterns and asthma-related health outcomes in an African population. Overall, our findings revealed that higher SABA prescriptions showed a significant association with an increase in the incidence rate of severe exacerbations (10–12 canisters) and lower odds of achieving at least partly controlled asthma (6–9 and 10–12 canisters). Although these associations were not statistically significant for all the SABA categories analyzed, most likely due to the small patient numbers in some of the subgroups, the results were generally consistent with those reported in the SABINA I and II studies (conducted in the United KingdomCitation8 and SwedenCitation9) the SABINA III studyCitation16, and other studies that have established a link between high SABA use and an increased risk of exacerbations and poor asthma controlCitation39–41.
Most patients in this African cohort were prescribed maintenance medication in the form of either ICS (28.3% of patients) or fixed-dose combination ICS/LABA (66.6% of patients). These findings were expected given the higher proportion of patients with moderate-to-severe asthma (63.3%). Likewise, the proportion of patients prescribed ICS (28.3%) was comparable to the percentage of patients at GINA step 2 (28.5%). Overall, a higher proportion of patients in primary care compared with specialist care were prescribed ICS (53.5 vs. 9.5%), which was in line with the proportion of GINA step 2-treated patients in both care modalities (53.2 vs. 10%). Nevertheless, the majority of patients in this African cohort who were prescribed ICS (GINA step 2) received prescriptions for medium-dose ICS (60.4%) instead of the recommended low-dose ICSCitation1. This trend was particularly apparent among patients treated by primary care physicians, where 66.7% were prescribed medium-dose ICS. This finding, which could be explained by the fact that primary care physicians are often not familiar with GINACitation42, indicates that prescribing practices in this cohort of patients did not always conform to internationally recommended guidelinesCitation1 and are in line with previous reports from Africa that have documented poor awareness of international guidelines and a low level of participation at update trainings on asthma managementCitation43–45. This nonadherence to international asthma management guidelines underscores the urgent need for extensive asthma campaigns to popularize the use of guidelines among physicians and the importance of continuing medical education in Africa. Fortunately, the National Asthma Education Programme (NAEP), whose mission is to provide asthma education to healthcare professionals, patients, and the lay public and whose benchmark asthma education course is the only accredited asthma course in South Africa, is expanding its footprint across South Africa and the African continentCitation46.
Overall, an OCS burst was prescribed to 34.9% of patients, potentially indicating OCS burst–treated exacerbations in these patients. A greater percentage of patients in specialist care (38.3%) compared with primary care (30.3%) were prescribed an OCS burst, likely reflective of the greater number of patients with moderate-to-severe asthma under specialist care. Interestingly, 32.7% of patients were prescribed antibiotics, with this occurring in a higher proportion of patients in specialist care compared to those in primary care (46.8 vs. 14.5%). The high rates of antibiotic prescriptions issued by specialists in this African cohort are consistent with the results of a study from Uganda which reported that more than half of all patients with asthma treated in chest and emergency units of a tertiary healthcare facility received antibioticsCitation44. This suggests a lack of understanding of the mechanisms underlying antimicrobial resistanceCitation47 and unfamiliarity with asthma management guidelines that do not support the routine use of antibiotics in the treatment of acute asthma exacerbations unless there is strong evidence of lung infectionCitation1. Moreover, antibiotics can increase the cost of prescription, may cause adverse effectsCitation48 and could delay the use of appropriate therapy. Thus, the extent of antibiotic use in this study represents a serious concern underscoring the need for targeted interventions, such as antimicrobial stewardship programs (ASPs), to tackle antibiotic resistance. Although there is a paucity of data on the implementation of ASPs in African countriesCitation49, the World Health Organization provides a practical toolkit for implementing antimicrobial stewardship in healthcare facilities to help low- and middle-income countries optimize antibiotic useCitation50. This toolkit focuses on improving awareness and understanding of antimicrobial resistance, strengthening knowledge and evidence base through surveillance, promoting sanitation and hygiene to prevent infections, and providing guidance to HCPs to change their antibiotic prescribing behaviorCitation50. In South Africa, implementation of a pharmacist-led stewardship program across a diverse group of 47 urban and rural private hospitals demonstrated that it was possible to substantially reduce antibiotic use despite limited resources and no prior stewardship experienceCitation51.
Of key importance, only one-third of patients (33.8%) in the current study had well-controlled asthma. While the level of asthma control was less than what is typically observed, with results from the Asthma Insight and Management (AIM) study reporting that globally, a median of 67.0% (range, 27.0 − 88.0%) of patients perceived their asthma as completely controlled and/or well-controlledCitation52, this finding is aligned with previous reports from AfricaCitation21,Citation24. For example, the ESMAA, reported that asthma was only controlled in 29.4% of 7179 evaluable patientsCitation13. Furthermore, the level of asthma control in this African cohort was poor when compared with that of the SABINA III population (the proportion of patients with well-controlled asthma was 43.3%)Citation16. Consequently, the burden of asthma in these three African countries was high, with 57.9% of patients experiencing at least 1 severe asthma exacerbation in the previous year. Therefore, the levels of asthma control in Africa remain below recommended standards and have contributed to the disease burdenCitation3. Of note, a high percentage of patients with mild asthma, across both primary and specialist care, experienced ≥1 severe exacerbation in the previous 12 months (55.6 and 46.5%, respectively). This could be due in part to a substantial proportion of patients with partly controlled/uncontrolled asthma (77 and 33.9%, respectively). However, consistent with previously published reportsCitation53–55, these results demonstrate that many patients with mild asthma experience suboptimal symptom controlCitation54 and are at risk of exacerbations. Such findings indicate potential under-estimation of asthma severity and over-estimation of disease control in patients with milder disease or the under-treatment of patients with “mild” asthma resulting in poor symptom controlCitation1,Citation56. However, to overcome this, a number of unique challenges faced by African countries will need to be overcome, including those arising from limited healthcare facilities and health planning; a lack of trained staff, diagnostic apparatus, and organized health promotion programs; the high cost and unavailability of essential asthma medications and devices; and a lack of patient self-monitoring equipment and educational materials, all of which have previously hindered efforts at improving asthma managementCitation3,Citation12,Citation13,Citation57. In addition, due consideration will need to be given to underlying risk factors, such as the rapid rate of urbanization, which has been linked to the increase in the burden of asthma across AfricaCitation3,Citation58. Crucially, inherent socio-cultural misconceptions will need to be addressed in order to enhance understanding of asthma and improve acceptance and use of asthma medications among patientsCitation3,Citation59.
Our study is not without limitations. Prescription data may not always reflect actual medication use or adherence and therefore SABA use may have been over-estimated or under-estimated. Owing to its observational nature, the study may also be prone to bias, e.g. therapies may be differently prescribed depending on disease severityCitation60. Indeed, 2.9 and 41.4% of patients classified as having mild asthma in primary and specialist care, respectively, had ICS/LABA prescriptions, suggesting differences in local treatment practices compared with GINA recommendations. Patient-reported data on SABA OTC purchase may have been subject to recall and non-response biasCitation60,Citation61. In addition, due to the small sample size for some SABA prescription categories, our results should be generalized with caution across these three African countries. Furthermore, disease severity was based on the GINA 2017 recommendations (in place at the time this study was conceived and implemented), where as-needed SABA was the preferred treatment option for patients at GINA step 1Citation17, which may have accounted for some of the high levels of SABA prescription observed in this study. Consequently, GINA step 1-treated patients who were prescribed ≥3 SABA/year would have been classified by investigators as having mild asthma if their symptoms were adequately controlled. Notably, this study was not designed to examine the impact of patient demographics or baseline clinical characteristics on SABA prescription patterns, although, such an analysis may be the subject of future research. Additionally, only the number of comorbidities (categorized as 0, 1–2, 3–4 and ≥5) were recorded in the eCRF, while data on the type and rate of comorbidities, were not captured. Finally, although data from a large patient population were analyzed, only 3 countries from Africa were included in the study. Therefore, results should be interpreted in the context of country-specific clinical practices and regulations and not generalized to the African continent as a whole. However, aggregated data from these 3 African countries enabled a detailed analysis of asthma treatment in a large patient population and an assessment of trends in SABA prescriptions and their impact on patient health in Africa. Taken together, these findings highlight that a concerted effort is required by national governments, HCPs, and patients to reduce the current burden of asthma and by healthcare policymakers to regulate SABA purchase without prescription in Africa so that clinical practices are aligned with current treatment recommendations. The future publication of individual country data from Egypt, South Africa and Kenya will provide further valuable real-world insights into asthma treatment practices at a country level.
Conclusions
The results from the African cohort of the SABINA III study in over 1700 patients with asthma demonstrated that approximately 1 out of every 2 patients was prescribed SABA in excess of treatment recommendations (≥3 canisters in the previous 12 months). In addition, of the 32.6% of patients who purchased SABA OTC, just over 50.0% purchased ≥3 canisters in the preceding 12 months, with almost 80.0% already receiving prescriptions for SABA canisters. With some exceptions, higher SABA prescriptions (vs. 1–2 canisters) were associated with poor asthma-related outcomes. These findings from the African cohort of the SABINA III study highlight that SABA over-prescription remains a major public health concern, necessitating that HCPs and policymakers urgently work together to improve asthma care and education and ensure that clinical practices are aligned with the latest evidence-based treatment recommendations.
Transparency
Declaration of funding
AstraZeneca funded all the SABINA studies and was involved in designing the study, developing the study protocol, conducting the study and performing the analyses. AstraZeneca was given the opportunity to review the manuscript before submission and funded medical writing support.
Declaration of financial/other relationships
A.K. has nothing to disclose. A.M. has nothing to disclose. A.A. has received honoraria for lectures and advisory board meetings from Novartis. C.S. has nothing to disclose. C.J.M. has nothing to disclose. J.O.M. has received research funds from AstraZeneca. M.A. is an employee of AstraZeneca. M.J.H.I.B. was an employee of AstraZeneca at the time this study was conducted. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to data collection, data analysis, data interpretation, and writing. The study was designed by M.J.H.I.B.
Supplemental_Material_CLEAN.docx
Download MS Word (562.4 KB)Acknowledgements
AstraZeneca funded all the SABINA studies and was involved in designing the study, developing the study protocol, conducting the study, and performing the analyses. AstraZeneca was given the opportunity to review the manuscript before submission and funded medical writing support.
Writing and editorial support was provided by Praveen Kaul and Frances Gambling of Cactus Life Sciences (part of Cactus Communications, Mumbai, India) in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) and fully funded by AstraZeneca.
Data availability statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Global Initiative for Asthma [Internet]. Global strategy for asthma management and prevention; 2022 [cited 2022 Jun 17]. Available from: http://ginasthma.org/.
- Global Asthma Network (GAN) [Internet]. The global asthma report; 2018 [cited 2021 Jun 22]. Available from: http://www.globalasthmareport.org.
- Adeloye D, Chan KY, Rudan I, et al. An estimate of asthma prevalence in Africa: a systematic analysis. Croat Med J. 2013;54(6):519–531.
- World Health Organization [Internet]. Asthma, key facts; 2021 [cited 2021 Jun 22]. Available from: https://www.who.int/news-room/fact-sheets/detail/asthma.
- Aldridge RE, Hancox RJ, Robin Taylor D, et al. Effects of terbutaline and budesonide on sputum cells and bronchial hyperresponsiveness in asthma. Am J Respir Crit Care Med. 2000;161(5):1459–1464.
- O'Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017;50(3):1701103.
- Cabrera CS, Nan C, Lindarck N, et al. SABINA: global programme to evaluate prescriptions and clinical outcomes related to short-acting β2-agonist use in asthma. Eur Respir J. 2020;55(2):1901858.
- Bloom CI, Cabrera C, Arnetorp S, et al. Asthma-related health outcomes associated with short-acting β2-agonist use: an observational UK study as part of the SABINA global program. Adv Ther. 2020;37(10):4190–4208.
- Nwaru BI, Ekström M, Hasvold P, et al. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872.
- Silver HS, Blanchette CM, Kamble S, et al. Relationship between short-acting β2-adrenergic agonist use and healthcare costs. Am J Manag Care. 2011;17(1):19–27.
- Rabe KF, Adachi M, Lai CK, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114(1):40–47.
- Onyedum C, Ukwaja K, Desalu O, et al. Challenges in the management of bronchial asthma among adults in Nigeria: a systematic review. Ann Med Health Sci Res. 2013;3(3):324–329.
- Tarraf H, Al-Jahdali H, Al Qaseer AH, et al. Asthma control in adults in the Middle East and North Africa: results from the ESMAA study. Respir Med. 2018;138:64–73.
- Anandan C, Nurmatov U, van Schayck OC, et al. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010;65(2):152–167.
- Asher I, Bissell K, Chiang CY, et al. Calling time on asthma deaths in tropical regions-how much longer must people wait for essential medicines? Lancet Respir Med. 2019;7(1):13–15.
- Bateman ED, Price D, Wang HC, et al. Short-acting β2-agonist prescriptions are associated with poor clinical outcomes of asthma: the multi-country, cross-sectional SABINA III study. Eur Respir J. 2022;59(5):2101402.
- Global Initiative for Asthma [Internet]. Global strategy for asthma management and prevention; 2017 [cited 2021 Jun 22]. Available from: http://ginasthma.org/.
- Reddel HK, Taylor DR, Bateman ED, American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99.
- World Economic Forum [Internet]. 19 Of the world’s 20 youngest countries are in Africa; 2019 [cited 2021 Jun 22]. Available from: https://www.weforum.org/agenda/2019/08/youngest-populations-africa/.
- Office of the Special Adviser on Africa [Internet]. Youth empowerment; 2021 [cited 2021 Jun 22]. Available from: https://www.un.org/osaa/.
- Haouichat H, Benali R, Benyounes A, et al. Asthma control in adult Algerian patients. Comparison with other North African and Middle-East countries. Rev Mal Respir. 2020;37(1):15–25.
- Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224.
- Moore WC, Meyers DA, Wenzel SE, National Heart, Lung, and Blood Institute's Severe Asthma Research Program, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181(4):315–323.
- Zemedkun K, Woldemichael K, Tefera G. Assessing control of asthma in Jush, Jimma, South West Ethiopia. Ethiop J Health Sci. 2014;24(1):49–58.
- Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4S–12S.
- US Department of Health and Human Services [Internet]. Office of disease prevention and health promotion. Acces to health services; 2022 [cited 2022 Jun 17]. https://www.healthypeople.gov/2020/leading-health-indicators/2020-lhi-topics/Access-to-Health-Services/data.
- Macha J, Harris B, Garshong B, et al. Factors influencing the burden of health care financing and the distribution of health care benefits in Ghana, Tanzania and South Africa. Health Policy Plan. 2012;27(Suppl 1):i46–i54.
- Department of Health, Republic of South Africa [Internet]. National health insurance for South Africa – towards universal health coverage; 2021 [cited 2021 Jun 22]. Available from: https://www.gov.za/sites/default/files/gcis_document/201707/40955gon627.pdf.
- Ferris TG, Blumenthal D, Woodruff PG, MARC Investigators, et al. Insurance and quality of care for adults with acute asthma. J Gen Intern Med. 2002;17(12):905–913.
- Partridge MR, van der Molen T, Myrseth SE, et al. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13.
- Douglass JA, Goeman DP, McCarthy EA, et al. Over-the-counter β2-agonist purchase versus script: a cross-sectional study. Respir Med. 2012;106(2):223–229.
- Campbell D, Ruffin R, Wilson D, et al. Over-the-counter purchase of inhaled β2-agonists: a qualitative study of asthma patients. Health Promot J Austr. 2000;10:38–42.
- Gibson P, Henry D, Francis L, et al. Association between availability of non-prescription beta 2 agonist inhalers and undertreatment of asthma. BMJ. 1993;306(6891):1514–1518.
- Reddel HK, Ampon RD, Sawyer SM, et al. Risks associated with managing asthma without a preventer: urgent healthcare, poor asthma control and over-the-counter reliever use in a cross-sectional population survey. BMJ Open. 2017;7(9):e016688.
- Henry DA, Sutherland D, Francis L. The use of non-prescription salbutamol inhalers by asthmatic patients in the hunter valley, New South Wales. Newcastle Retail Pharmacy Research Group. Med J Aust. 1989;150(8):445–449.
- Babar ZU, Lessing C, Mace C, et al. The availability, pricing and affordability of three essential asthma medicines in 52 low- and middle-income countries. Pharmacoeconomics. 2013;31(11):1063–1082.
- Kibirige D, Sanya RE, Nantanda R, et al. Availability and affordability of medicines and diagnostic tests recommended for management of asthma and chronic obstructive pulmonary disease in Sub-Saharan Africa: a systematic review. Allergy Asthma Clin Immunol. 2019;15:14.
- Sanyang B, Jagne E, Sefa N, et al. Availability, cost, and affordability of asthma and chronic obstructive pulmonary disease medications in the Gambia. JPATS. 2021;2(1):33–41.
- Gerald JK, Carr TF, Wei CY, et al. Albuterol overuse: a marker of psychological distress? J Allergy Clin Immunol Pract. 2015;3(6):957–962.
- Patel M, Pilcher J, Reddel HK, SMART Study Group, et al. Metrics of salbutamol use as predictors of future adverse outcomes in asthma. Clin Exp Allergy. 2013;43(10):1144–1151.
- Stanford RH, Shah MB, D'Souza AO, et al. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012;109(6):403–407.
- Nguyen VN, Nguyen QN, Le An P, et al. Implementation of GINA guidelines in asthma management by primary care physicians in vietnam. Int J Gen Med. 2017;10:347–355.
- Fawibe AE, Onyedum CC, Sogaolu OM, et al. Drug prescription pattern for asthma among Nigerian doctors in general practice: a cross-sectional survey. Ann Thorac Med. 2012;7(2):78–83.
- Kirenga JB, Okot-Nwang M. The proportion of asthma and patterns of asthma medications prescriptions among adult patients in the chest, accident and emergency units of a tertiary health care facility in Uganda. Afr Health Sci. 2012;12(1):48–53.
- Chikaodinaka Ayuk A, Ubesie A, Laura Odimegwu C, et al. Use of global initiative for asthma (GINA) guidelines in asthma management among paediatric residents in a Sub Saharan African country: a cross-sectional descriptive study. Pan Afr Med J. 2017;27:120.
- Verwey C. The national asthma education programme and asthma in Africa. S Afr Med J. 2019;109(7):453–454.
- Manderson L. Prescribing, care and resistance: antibiotic use in urban South Africa. Humanit Soc Sci Commun. 2020;7(1):77.
- Normansell R, Sayer B, Waterson S, et al. Antibiotics for exacerbations of asthma. Cochrane Database Syst Rev. 2018;6:CD002741.
- Akpan MR, Isemin NU, Udoh AE, et al. Implementation of antimicrobial stewardship programmes in African countries: a systematic literature review. J Glob Antimicrob Resist. 2020;22:317–324.
- World Health Organization [Internet]. Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries: a WHO practical toolkit; 2021 [cited 2021 Jun 22]. https://apps.who.int/iris/handle/10665/329404.
- Brink AJ, Messina AP, Feldman C, et al. Antimicrobial stewardship across 47 South African hospitals: an implementation study. Lancet Infect Dis. 2016;16(9):1017–1025.
- Nathan RA, Thompson PJ, Price D, et al. Taking aim at asthma around the world: global results of the asthma insight and management survey in the Asia-Pacific region, Latin America, Europe, Canada, and the United States. J Allergy Clin Immunol Pract. 2015;3(5):734–742 e5.
- Bateman ED, Reddel HK, O'Byrne PM, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378(20):1877–1887.
- Ding B, Small M. Disease burden of mild asthma: findings from a cross-sectional real-world survey. Adv Ther. 2017;34(5):1109–1127.
- FitzGerald JM, Barnes PJ, Chipps BE, et al. The burden of exacerbations in mild asthma: a systematic review. ERJ Open Res. 2020;6(3):00359-2019.
- Baddar S, Jayakrishnan B, Al-Rawas O, et al. Is clinical judgment of asthma control adequate?: a prospective survey in a tertiary hospital pulmonary clinic. SQUMJ. 2013;13(1):63–68.
- Martins P, Rosado-Pinto J, do Céu Teixeira M, et al. Under-report and underdiagnosis of chronic respiratory diseases in an African country. Allergy. 2009;64(7):1061–1067.
- van Gemert F, van der Molen T, Jones R, et al. The impact of asthma and COPD in sub-Saharan Africa. Prim Care Respir J. 2011;20(3):240–248.
- Jumbe Marsden E, Wa Somwe S, Chabala C, et al. Knowledge and perceptions of asthma in Zambia: a cross-sectional survey. BMC Pulm Med. 2016;16:33.
- Roche N, Reddel H, Martin R, Respiratory Effectiveness Group, et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc. 2014;11(Suppl 2):S99–S104.
- Wang X, Cheng Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest. 2020;158(1S):S65–S71.
