Abstract
The term, “prediabetes”, describes a state of hyperglycaemia that is intermediate between true normoglycaemia and the diagnostic cut-offs for indices of glycaemia that are used to diagnose type 2 diabetes. The presence of prediabetes markedly increases the risk of developing type 2 diabetes. Numerous randomized, controlled evaluations of various agents have demonstrated significant prevention or delay of the onset of type 2 diabetes in subjects with prediabetes. Intensive lifestyle interventions and metformin have been studied most widely, with the lifestyle intervention being more effective in the majority of subjects. The application of therapeutic interventions at the time of prediabetes to preserve long-term outcomes has been controversial, however, due to a lack of evidence relating to the pathogenic effects of prediabetes and the effectiveness of interventions to produce a long-term clinical benefit. Recent studies have confirmed that prediabetes, however defined, is associated with a significantly increased risk of macrovascular and microvascular complications essentially identical to those of diabetes, and also with subclinical derangements of the function of microvasculature and neurons that likely signify increased risk of compilations in future. Normoglycaemia, prediabetes and type 2 diabetes appear to be part of a continuum of increased risk of adverse outcomes. Long-term (25–30 years) post-trial follow up of two major diabetes prevention trials have shown that short-term interventions to prevent diabetes lead to long-term reductions in the risk of complications. These findings support the concept of therapeutic intervention to preserve long-term health in people with prediabetes before type 2 diabetes becomes established.
Introduction
Hyperglycaemia is associated with many acute and chronic complications. Chronic hyperglycaemia leads to micro- and macrovascular complications, and, inevitably, a reduced health- and lifespan in people living with diabetesCitation1,Citation2. In an attempt to mitigate the increased mortality and morbidity in type 2 diabetes (T2D), current guidelines are aimed at the optimal management of T2D and include complex algorithms as the clinical course of the disease progresses. Considering the high burden of cardiovascular disease (CVD) and the risk of adverse outcome in people with T2D who develop CVD, the T2D guidelines prioritize cardiovascular outcomesCitation3,Citation4. Many clinicians, however, are advocating for earlier intervention to mitigate CV risk more effectively, even before the onset of T2D. Both T2D and prediabetes are both characterized by abnormal glucose homeostasis, albeit individuals with prediabetes have intermediate indices of hyperglycaemia. Prediabetes therefore positions between normal glucose tolerance and overt diabetesCitation5.
Timely intervention in individuals with prediabetes, using intensive lifestyle intervention and/or with pharmacologic antidiabetic agents, have the potential to delay or prevent the progression from prediabetes to T2D and its complicationsCitation5,Citation6. The benefit of early intervention in the prediabetic population has however not received the same attention as secondary and tertiary prevention of CVD in T2D. Here, we review the current best evidence on the impact of prediabetes on long-term clinical outcomes and consider the potential benefits and harms that may arise from therapeutic intervention in this high-risk population.
What is prediabetes?
Diagnosis of prediabetes and type 2 diabetes
Prediabetes refers to a state of intermediate hyperglycaemia with glucose levels higher than “normal” (fasting and/or postprandial plasma glucose (PPG) values) but not exceeding diagnostic thresholds for T2D (fasting plasma glucose (FPG) values ≥7 mmol/L and/or 2-hour post load glucose (2hPG) ≥11.1 mmol/L and/or HbA1c ≥6.5%)Citation4. Impairments of FPG (impaired fasting glucose [IFG]) and/or 2hPG post-load glucose (impaired glucose tolerance [IGT]) may occur simultaneously. An individual with FPG ≥5.6–6.9 mmol/L has IFG, and if the 2-hour post load glucose (2hPG) is ≥7.8–11 mmol/L during a standard 75 g oral glucose tolerance test (OGTT), he/she has IGT. Note that the World Health Organization (WHO), and American Diabetes Association (ADA), use different FPG values to diagnose IFG (WHO: FPG 6.1–6.9 mmol/L and ADA: 5.6–6.9 mmol/L), and the WHO does not currently endorse the use of HbA1c for the diagnosis of prediabetesCitation7. More recently, glycated haemoglobin (HbA1c) ≥5.7–6.4% has been incorporated as a convenient alternative test. Considering the ethnic disparities in HbA1c, it is however important that the ideal HbA1c cut off is further corroborated by performing an OGTT in certain populations.
The diagnoses of prediabetes or T2D are categorical in nature. Thus, a subject with an FPG of 6.9 mmol/L and HbA1c of 6.4% has prediabetes, provided the 2hPG is not diagnostic of diabetes. Given the limitations in terms of reproducibility of the OGTT, any equivocal results should be scrutinized, and testing repeated. The FPG cut-off for T2D, interestingly, was proposed by the ADA in 1997, based on the association between FPG and the incidence of certain microvascular complications, particularly retinopathyCitation1. The HbA1c cut off of 6.5% was proposed by the ADA in 2010, following an expert review published in the previous yearCitation8. It is important to note that the robustness of the diagnostic cut-offs has been questioned and the relationship of other complications to the HbA1c level may differ from that of retinopathyCitation9–11. The relationships between hyperglycaemia (HbA1c or blood glucose level) and the risk of macrovascular complications is continuous in T2D, with no clear inception point where risk increases in parallel with glycaemiaCitation12,Citation13. There is no doubt that the cut-offs used for diagnosing diabetes have been a great advance in facilitating provision of care for people at risk of the devastating adverse long-term consequences of T2D. Nevertheless, these cut-offs do not define the presence vs. absence of risk of complications nor adverse outcome, and there appears to be a continuum of (especially microvascular) risk as the prevailing level of glycaemia increasesCitation10.
Prediabetes and clinical outcomes
Incident type 2 diabetes
The impact of prediabetes on the subsequent risk of developing T2D has been well described and will be addressed here only briefly, as the main focus of our review is on the relationships between prediabetes and clinical vascular outcomes. The landmark early diabetes prevention trials, the Diabetes Prevention Program (DPP; USA)Citation14 and the Diabetes prevention Study (DPS; Finland)Citation15 were conducted in populations with IGT, following observations in the “Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe” (DECODE) study and elsewhere that post-load hyperglycaemia was a much stronger predictor of adverse long-term clinical outcomes than FPGCitation16. The rates of incident diabetes in the control groups of these trials were high (110/1,000 person-years in the DPPCitation14 and 78 cases/1000 person years (DPS)Citation15. An early meta-analysis of observational studies published in 1997 that contributed to the design of the DPP indicated lower incidence rates of T2D with IGT (57 cases/1,000-patient-years) compared with the control groups in the DPP and FDPSCitation17. This highlights the fact that the randomised intervention trials described above did not in fact include a truly normoglycaemic control group.
These studies included subjects with FPG 5.3–6.9 mmol/L (DPP) or <7.8 mmol/L (DPS), with mean baseline FPG in control groups of 5.9 mmol/L and 6.1 mmol/L, respectively. Thus, most participants in these trials had plasma glucose levels that were broadly consistent with co-occurring IFG and IGT, and it would be interesting to see an analysis where the populations were stratified more rigorously for commonly-used definitions of prediabetes/intermediate hyperglycaemia.
A meta-analysis of prospective cohort studies demonstrated a 5–8-fold increase in the relative risk of developing T2D in people with IGT or IFG vs. NGT. When IGT and IFG co-occurred, a 12-fold increase was noted vs. NGTCitation18. A Cochrane systematic review and meta-analysis presented pooled hazard ratios (HR, vs. NGT) for developing T2D using ADA IFG criteria (FPG ≥5.6 mmol/L) with reported HRs of 4.3 (95% CI 2.6, 7.1)) for IFG only, 3.6 (2.31, 5.64) for IGT only and 6.9 (4.2, 11.5) for combined IFG and IGTCitation19. This analysis also confirmed the increased risk to develop T2D with HbA1c in the ADA prediabetes range (HR of 5.6 (2.8, 11.1) vs. NGT). A subsequent systematic review reported a steep increase in the incidence of T2D in people with HbA1c 5.5% to 6.5% (which corresponds roughly to the current ADA criteria for diagnosing type 2 diabetes using HbA1c []), compared with lower HbA1c valuesCitation20. A recent cohort study that included 3,492 subjects with ADA-defined IFG demonstrated a lifetime risk of developing diabetes of 46% (men) and 58% (women), at age 45 yearsCitation21.
Macrovascular complications
Prediabetes is associated with a significantly increased risk of CV events and mortalityCitation1,Citation2. summarizes the main findings of studies of the effects of prediabetes or glycaemia in the prediabetic range on macrovascular outcomes. Mendelian randomization studies report a strong association between hyperglycaemia in the prediabetes range and genetic variants that predict an increased risk of CVD linearly, over and above the severity of prediabetesCitation22. The AusDiab study reported that IFG and a prior diagnosis of T2D were significant independent predictors of all-cause and CV death after multivariable adjustment for other risk factors (IGT significantly predicted all-cause death, but not CV death)Citation23. Similar findings were presented from a cohort in Taiwan with IFG (WHO criteria)Citation24, from the Baltimore Health in Aging study, where IFG (WHO) and IGT predicted adverse CV outcomes (especially when IFG and IGT cooccurred)Citation25, and from the National Health and Nutrition Examination Survey (NHANES) cohortCitation26. A further large cohort study found no significant association between prediabetes and adverse cardiovascular outcomes; a low incidence of T2D and short follow-up after the development of T2D may partly explain this findingCitation27. Finally, the presence of prediabetes and diabetes increased the risk of a composite cardiovascular endpoint in a population of patients with CKD (renal outcomes from this study are summarized in a separate section, below)Citation28.
Table 2. Overview of key studies on the relationship between prediabetes or glycaemia in the prediabetic range and macrovascular complications.
Prediabetes, and T2D are increasingly noted to be heterogenous states of abnormal glucose homeostasis, and it therefore does not come as a surprise that the various measures of hyperglycaemia in prediabetes leads to a range of clinical outcomes, not disregarding the other CV risk factors that is often also present. For example, in one study, prediabetes diagnosed using HbA1c (5.7–6.4% [ADA criteria] or 6.0–6.4% [International Expert Committee criteria]) predicted higher rates of adverse clinical outcomes (CVD or death) vs. prediabetes diagnosed using measures of blood glucoseCitation29. However, clustering of additional CV risk factors appeared to account for most of the difference. A report from the United Kingdom (UK) Biobank supported the importance of comorbid classical CV risk factors in driving adverse CV outcomes in the setting of prediabetesCitation30. Interestingly, higher vs. lower blood glucose 30 min into an OGTT predicted mortality more accurately than the FPG or 2-hPG in another study, highlighting the shortcomings of the OGTT timepoints with regards to predicting adverse outcomesCitation31.
Increased carotid intima-media thickness (cIMT) is a marker of increased overall burden of atherosclerosis and is a strong independent predictor of future major adverse CV outcomesCitation32. Average measures of cIMT were higher in people with prediabetes vs. NGT and were higher still in people with diabetes (60 subjects in each group)Citation33. Data from the US Dallas heart Study also demonstrated increased cIMT and higher coronary calcium scores (a marker of coronary atherosclerosis) in people with vs. without prediabetesCitation34. Accordingly, cIMT may be useful for further CV risk stratification in people with prediabetes.
Data on prediabetes and CV risk are conflicting. A systematic review and meta-analysis by Mutie et al. (average follow-up 10 years) concluded that prediabetes is causally related to the onset of coronary artery disease, but not to other diabetes complicationsCitation35. Other systematic reviews report that both IFG and IGT are associated with increased risk of CV and all-cause death, CV events and adverse cardiac eventsCitation36,Citation37. summarizes the results of the more recent of these analyses (2021)Citation36.
Figure 1. An example of associations between prediabetic states and cardiovascular disease events, coronary heart disease events, or mortality from a recent systematic review. ADA, American Diabetes Association; IFG, impaired fasting glucose; IFG, impaired glucose tolerance; WHO, World Health organization. See for details of diagnostic criteria. Reference values were glycaemic categories below the lower cut-off value for each prediabetes condition. Drawn from data presented in reference 36.
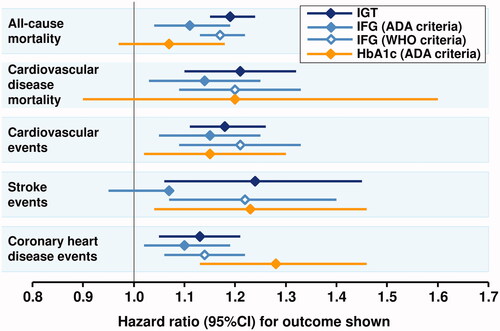
Figure 2. Association between prediabetes and heart failure from a systematic review of 15 studies. ADA, American Diabetes Association; IFG, impaired fasting glucose; IFG, impaired glucose tolerance; WHO, World Health organization. See for details of diagnostic criteria. The reference value was normoglycaemia in each case. Drawn from data presented in reference 40.
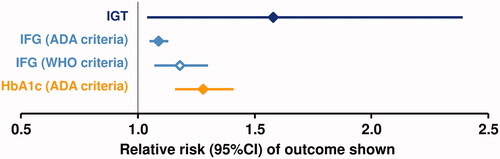
Heart failure (HF) is recognized increasingly as a common complication of T2D with a high burden of morbidity and premature mortalityCitation38. Individual studies of the association of prediabetes with HF have also produced conflicting resultsCitation27,Citation36,Citation39. A recent (2021) meta-analysis of 15 studies that included 9,827,430 individuals found significant associations between prediabetes vs. normoglycaemia and incident HF ()Citation40. Several reports have associated prediabetes (sometimes previously undiagnosed) with worsened outcomes in patients with pre-existing HFCitation41–43.
Coronary flow reserve (CFR), a measure of cardiac microvascular function, was lower in people with prediabetes vs. NGT in a cross-sectional study, with a larger defect in people with T2DCitation44. It has been reported however, that changes in CFR only become clinically significant when T2D is establishedCitation45. Impaired endothelial function may be an early event in the pathogenesis of microvascular dysfunction in people with prediabetes or T2DCitation46. In addition, cardiac troponin-T, a sensitive marker of myocardial damage, has been shown to be elevated in people with prediabetes as well as T2DCitation47. Together, these reports suggest that the presence of subclinical cardiovascular impairment in people with prediabetes may position these subjects at increased risk of established diabetes-like complications in future, and that overall glycaemic exposure over time contributes.
Microvascular complications
The eye
Participants in the DPP who had elevated blood glucose, but no history of diabetes, demonstrated a prevalence of retinopathy at baseline of 7.9%Citation48. Further data from the same study found that the prevalence of retinopathy was higher among people who developed T2D during the randomised phase of the trial (12.6%), compared with those who did not (7.9%, p = .03 for the difference)Citation48. Retinal arteriolar dilatation in response to metabolic stress (induced by flickering light), was used to measure retinal vascular function in the community-based Maastricht Study cohortCitation49. Mean arteriolar dilatation was 3.4% in 1,269 people with NGT, 3.0% in 335 people with prediabetes, and 2.3% in 609 people with diagnosed T2DCitation49. A more recent study from this cohort found no significant correlation between prediabetes and arteriolar risk, but demonstrated a linear trend between the blood glucose level and retinal microvascular width across the range of prediabetic and diabetic dysglycaemiaCitation50. The results of a study using similar methodology in a different cohort supported the possibility of an association between prediabetes and reduced retinal arteriolar diameterCitation51. Macular thinning has also been observed as subclinical manifestations of retinal damage in the Maastricht cohort, again with a severity intermediate between NGT and T2DCitation52.
Other studies have demonstrated reduced contrast-sensitivity function and photo stress-recovery time in people with prediabetes vs. NGTCitation53 or ultrastructural derangements of retinal micro vessels in the absence of loss of retinal structure (normal cone cell density)Citation54. These studies represent further demonstrations of a subclinical deterioration in retinal vascular function in the setting of prediabetes. Subjects in a primary care database in the UK with newly-diagnosed T2D had a higher incidence of retinopathy if they had vs. had not received a diagnosis of prediabetes beforehand (adjusted odds ratio 1.76 [1.69–1.85]), consistent with increased risk of complications from a higher prior exposure to hyperglycaemiaCitation55. Impaired retinal function has not been observed in the setting of prediabetes in all studies, howeverCitation56.
Nerves and brain
In the NHANES cohort (1999–2004), the relative risk of peripheral neuropathy was 1.1 (0.9, 1.3) for prediabetes and 1.7 (1.5, 2.0) for diabetes, each vs. NGT, although the 95%CI for the RR associated with prediabetes crossed zero in this caseCitation57. A study from the Cooperative Health Research in the Region of Augsburg (KORA) cohort in Germany found a similar prevalence of distal sensorimotor polyneuropathy (DSPN) in people with combined IGT + IFG (24%) and T2D (22%); associations between isolated IFG or IGT and DSPN did not reach statistical significance, howeverCitation58. A systematic review suggested an increased incidence of neuropathy associated with prediabetes, but wide variation between studies precluded a meta-analytic approachCitation59. In contrast, other studies have associated type 2 diabetes – but not prediabetes – with forms of neuropathy, but these are highly dependent on the sensitivity of the diagnostic tools used to diagnose DSPNCitation60,Citation61.
The Maastricht cohort included a measure of peripheral neural function (heat-induced hyperaemia in skin); with results that paralleled those in the retina, indicating a significant defect in skin microvascular function in prediabetes, and a larger defect in T2D, vs. NGTCitation49. The authors concluded that prediabetes was associated with “generalized microvascular dysfunction”.
Reduced length and density of corneal nerves provided another manifestation of early neuropathy in prediabetesCitation62,Citation63. Cardiac autonomic neuropathy also appears to be already present in people with prediabetesCitation64,Citation65. Sensory nerve superexcitability, a marker of subclinical impairment of nerve conduction, was demonstrated to occur to a greater extent in 40 prediabetic subjects vs. 20 normoglycaemic subjects; again the defect was more pronounced in people with T2DCitation66.
Data from Sweden associated prediabetes significantly with loss of brain volume (especially white matter) and progression of cognitive impairment over the span of 9 yearsCitation67. Similarly, the Maastricht study participants with prediabetes were found to have more cerebral lacunar infarcts, more white matter lesions and more loss of brain volume (driven by loss of white matter) compared with NGT subjects; these deficits were more severe in participants with T2DCitation68. Increasing levels of hyperglycaemia drove deficits in executive function in the NHANES 2011–2014 cohortCitation69.
The kidney
Data from the NHANES 1999–2006 cohort indicated the prevalence of chronic kidney disease (CKD) was intermediate in subjects with prediabetes (17%; ADA criteria), compared with diagnosed diabetes (33%) or NGT (12%), after adjustment for race/ethnicity, age and genderCitation70. Nine years of prospective follow-up in a population in Korea identified prediabetes (IGT or based on HbA1c) as an independent predictor of incident CKDCitation71. Cross sectional data from the KORA registry, adjusted for fasting glucose, 2hPG, HbA1c, fasting insulin and insulin resistance, associated isolated IFG with increased risk of microalbuminuria or CKD (∼50% increase in risk), with 2.6-fold increase in the risk of reduced kidney function with IFG + IGTCitation72.
The Chronic Renal Insufficiency Cohort followed a population of 3,701 subjects with chronic kidney disease (CKD) for an average of 7.5 yCitation28. The main composite renal outcome included end-stage renal disease (renal transplantation or dialysis), a 50% decline in eGFR from baseline to ≤5 mL/min/1.73 m2, or doubling of urine protein:creatinine ratio to ≥0.22 g/g creatinine. Subjects with prediabetes (based on ADA criteria for HbA1c or FPG) were not at greater risk of the composite renal outcome vs. NGT (adjusted HR 1.13 [95%CI, 0.96–1.32]), although diabetes increased the risk of this outcome significantly (adjusted HR 1.47 [95% CI, 1.27–1.70]). However, there was a 23% increase in the adjusted risk of progression of proteinuria (p = .002) associated with prediabetes vs. no prediabetes, vs. a risk increase of 50% for T2D (p < .001). Thus, the presence of prediabetes did not affect a broad renal composite outcome significantly, although there was a sign of early renal injury signified by exacerbation of proteinuria.
A prospective, 2-year cohort study from Japan demonstrated that prediabetes was associated with an increased risk of proteinuria, but not with decline of eGFRCitation73. Data from the study in UK primary care patients with new type 2 diabetes (3 years of retrospective follow-up) demonstrated a significant association of a prior diagnosis of prediabetes with nephropathy (OR 1.14 [1.10–1.19])Citation54. Increased renal cortical stiffness has been identified in people with both prediabetes and T2D, compared with NGT, in a cross-sectional studyCitation74. Increased glomerular hyperfiltration may be an early (and reversible) step in the pathogenesis of CKD associated with prediabetes or diabetesCitation75–78. Prediabetes and CKD interacted to increase night time systolic blood pressure in a community-dwelling elderly populationCitation79.
Meta-analyses support these observations: one associated the presence vs. absence of prediabetes with a “modest” increase in the risk of CKD (RR 1.12 [1.02, 1.21])Citation80. Another meta-analysis significantly associated increased risk of CKD with IFG and elevated, non-diabetic HbA1c levels (both ADA criteria), but not with IGTCitation35.
Pathogenetic mechanisms
Cardiovascular risk factors and CKD are prevalent among people with diabetes, and in the USA interventions to mitigate CV risk has been applied less often in the prediabetes population than for people with T2DCitation81. In one observational study, findings of increased markers of atherosclerosis (and CKD) in people with vs. without prediabetes were attenuated by adjustment for other CV risk factors, suggesting that these were responsible for much or all of the increased atherosclerotic risk, providing a rationale for intensive comprehensive multifactorial intervention to mitigate themCitation33. However, IFG and IGT were independent predictors of all-cause and/or CV death in the AusDiab cohortCitation23, as was IFG in the cohort from TaiwanCitation24. The early (1999) study from the DECODE group suggested that only elevated post-load (not fasting) glucose predicted adverse outcomes; however, both IFG and IGT (and especially both together) predicted averse outcomes in the more recent studies described aboveCitation82.
Hyperglycaemia and prediabetes, in particular, share common pathogenetic mechanisms. People with prediabetes are at least twice as likely to be obese, compared with people with normal glucose regulationCitation83, and the prevalence of prediabetes increases in line with the severity of obesityCitation84. More than 8 individuals in every 10 with prediabetes in the USA is overweight or obeseCitation85. Accordingly, obesity is recognized as a major risk factor for developing diabetesCitation86. Visceral adipocytes in the setting of abdominal obesity secrete a range of inflammatory cytokines that impair insulin sensitivity and pancreatic β-cell functionCitation87. The accumulation of ectopic fat in key organs responsible for metabolic regulation is a well recognized consequence of obesityCitation88. The deposition of ectopic fat promotes hyperglycaemia and insulin resistance in liver and skeletal muscle, oxidative stress, endothelial dysfunction and accelerated atherosclerosis in the vasculature and remodelling of the heart that increases the risk of heart failure and atrial fibrillationCitation89. These mechanisms are likely to accelerate the progression of the dysglycaemic continuum, and to contribute to the pathogenetic mechanisms linking prediabetes and diabetes with vascular disease. Other mechanisms linked to obesity, such as increased sympathetic drive, may also promote adverse outcomes such as neuropathy independently of dysglycaemia, to some degreeCitation90. However, it is difficult to separate such mechanisms, as sympathetic overdrive is also associated with insulin resistanceCitation91. It is important to note that many of the studies of associations between prediabetes and vascular outcomes summarized above adjusted for the effects of individual cardiometabolic risk factors. Accordingly, prediabetes per se appears to be associated with an increased risk of adverse vascular outcomes.
People with prediabetes are at higher long-term exposure to elevated blood glucose than people with NGT, which likely accounts for much of the observed risk of microvascular complications. Consistent with this view, the microvascular dysfunction in the Maastricht cohort (described above) was driven mainly by hyperglycaemiaCitation45. Increased levels of methyglyoxal, a toxic, inflammatory intermediate in the generation of advanced glycation end products in the setting of hyperglycaemia, were associated with microvascular, but not macrovascular complications in this cohortCitation92. The generation of extracellular vesicles containing specific microRNAs has been proposed as a novel pathogenetic mechanism for the development of vascular complications in prediabetes and diabetesCitation93. Increased mitochondrial production of superoxide free radicals has been proposed as a common early step in pathways that cause damage to cells in the setting of hyperglycaemiaCitation94. Obesity, insulin resistance and hypertension drive cerebral microvascular dysfunction in prediabetes, providing a pathogenetic link to the genesis of macrovascular complicationsCitation95.
Other medical issues may impact further on long-term outcomes in people with prediabetes in some areas. For example, in South Africa an epidemiological transition is underway from infectious diseases (e.g. tuberculosis and human immunodeficiency virus [HIV]) to non-communicable diseases including prediabetes and T2DCitation96,Citation97. While improved healthcare has reduced the impact of infections diseases across the population, these continue coexist with non-communicable diseases at a high prevalenceCitation98. Specific interactions between infectious and non-communicable disease, e.g. the exacerbation of CV risk factors by HIV, and other potential consequences of this high combined disease burden, e.g. pressure on healthcare availability and delivery, may impact on the evolution of dysglycaemia in countries such as South Africa in the future.
Does intervention in prediabetes reduce the long-term risk of complications?
Intensive lifestyle intervention and metformin are the most widely studied interventions for the prevention or delay of T2D in subjects with prediabetes. The DPP showed that lifestyle intervention was more effective than metformin for diabetes prevention in certain subgroups, although other smaller randomized diabetes prevention trials produced variable resultsCitation6,Citation99. National and international guidelines recommend lifestyle intervention for all who are able to undertake it, with advice on improved health behaviours, such as diet, physical activity, and smoking cessation; addressing all of these factors will improve clinical outcomes. Some guidelines provide recommendations on the use of metformin for specific subpopulations, particularly women with prior gestational diabetes, based largely on the results of the DPP (reviewed elsewhere)Citation6,Citation99.
The (cluster) randomized phase of the DaQing diabetes prevention study compared an intensive lifestyle intervention with a control group who received standard care; the randomized phase was followed by a long-term epidemiological study, with no attempt to maintain the prior randomized treatmentsCitation100. Subjects who were previously randomized to the lifestyle intervention benefitted from reductions in the risk of cardiovascular events (HR 0.74 [0.59, 0.92], p = .006), microvascular complications (HR 0.65 [0.45, 0.95]; p = .025), cardiovascular deaths (HR 0.67 [0.48, 0.94], p = .022), and death from any cause (HR 0.74 [0.61, 0.89], p = .0015) during a total of 30 years of follow-upCitation100.
An epidemiologic follow-up study was also conducted after the randomized phase of the DPP (the DPP Outcomes study [DPPOS])Citation101. Again, no attempt was made to enforce prior randomized treatment, although all who wished to continue on (unmasked) metformin could do so; placebo was discontinued, former recipients of the intensive lifestyle intervention received additional lifestyle support, and all groups received group-based lifestyle supportCitation101. A significant degree of diabetes prevention persisted throughout 25 years of follow-up, with the difference in effectiveness of the lifestyle intervention and metformin narrowing over timeCitation102. Prior randomisation to neither the intensive lifestyle intervention nor metformin was associated with a significant reduction in the risk of a composite microvascular outcome in the total cohort after 15 yearsCitation103 or 25 yearsCitation102,Citation104 of follow-up in the DPPOS, although a recent report from this study has not demonstrated a significant effect on the incidence of retinopathyCitation105. However, prevention of diabetes per se was associated with a significant microvascular benefit compared with those who did develop diabetes. Regression from prediabetes to IGT was also associated with a lower risk of microvascular disease in the DPPOS, which was attributed to a lower level of long-term exposure to hyperglycaemiaCitation106. Follow-up in the DPPOS continues, and future analyses containing more cardiovascular events may shed more light on the effectiveness of the initial interventions for improving long-term clinical outcomes.
Other antidiabetes drugs have been shown to prevent or delay the onset of type 2 diabetes in at-risk subjects, including acarbose, and thiazolidinediones, although tolerability issues have limited their use on practice (reviewed elsewhereCitation107). A prespecified analysis of the STOP-NIDDM trial with acarbose reported a reduction in the risk of cardiovascular eventsCitation108, although the number of events was small and methodological flaws with the analysis have been reportedCitation109; this finding therefore requires confirmation.
For the future, members of the newer classes of SGLT2 inhibitorsCitation110 and GLP-1 receptor agonistsCitation111 have been shown to reduce the risk of new-onset type 2 diabetes in subjects at risk. Long-term follow-up in these populations will be needed to establish whether these agents reduce the risk of adverse cardiovascular outcomes in people with prediabetes.
Summary and conclusions
Professor John S Yudkin of University College London, UK, wrote in 2014 that “Pre-diabetes is an artificial category with virtually zero clinical relevance”Citation112. This was a reasonable standpoint based on the data available at that time: the evidence base associating the prediabetic state with an increased risk of diabetes-like vascular complications was sparse and conflicting, with no data on the impact on outcomes of intervening therapeutically at this time, other than a reduced risk of developing clinical T2D. Moreover, successive revisions to the glycaemic cut-offs for diagnosing T2D led to a substantial increase the proportion of the population diagnosed with prediabetes. For example, the US Centers for Disease Control estimated in 2020 that 88 million adult Americans – more than one third of the US population (34.5%) have prediabetes according to current diagnostic criteria.Citation113 This represents a potentially huge burden on the public health, which should clearly not be accepted lightly.
The (mostly recent) data summarized above demonstrate significant associations of prediabetes with macrovascular and, increasingly, microvascular complications that are essentially identical to those seen in T2D. A number of studies demonstrated clinically significant increases in the prevalence of complications in people with prediabetes that were intermediate in magnitude between the prevalence values seen in people with NGT and those in people with established clinical type 2 diabetes. Moreover, several studies not only cited evidence for subclinical disturbances of the function of microvascular vessels and nerves that likely put the subject at risk of developing established diabetes-like complications in the future. Overall, these findings support the concept of prediabetes lying within a continuum of risk that increases steadily as patients’ glycaemia climbs above the normoglycaemic range, through the prediabetic range and into levels that are diagnostic for T2D, that will become increasingly relevant as life expectancy increases.
These findings identify prediabetes as a pathologic state associated with increased risk of adverse long-term vascular and renal outcomes. We know that preventing diabetes via intensive lifestyle intervention (DaQing studyCitation100), or by lifestyle intervention or metformin (DPP/DPPOSCitation99,Citation102,Citation103) has been associated with improved clinical outcomes during post-trial follow-up periods that long outlasted the duration of the original randomized interventions. We await further data from the DPPOS on the effects of the individual interventions, supported by more clinical events.
For the future, we will need better ways to predict who will and will not progress from prediabetes to clinical diabetes. The OGTT has the advantage of being able to diagnose both IFG and IGT, but it is relatively little used in many countries, and its reproducibility is poorCitation114. In addition, a substantial proportion of people with prediabetes will never develop diabetes, especially older personsCitation115. Standardization of methodology to diagnose prediabetes would be helpful, as this would facilitate quantitative evaluation of the risk of adverse clinical outcomes associated with different manifestations of prediabetes. It is worth noting, however, that all forms of prediabetes have been associated with increased vascular risk, as described above. This is perhaps unsurprising, as prediabetes is a state of intermediate hyperglycaemia, which would be detected by any of the criteria in use.
Early, intensive intervention in the dysglycaemic continuum has been shown to provide long-term “legacy benefits” of reduced risk of adverse cardiovascular outcomes and mortality long after the randomized intervention had endedCitation116. This was seen following the DaQing study (with lifestyle intervention in people with IGTCitation91) and the UK Prospective Diabetes StudyCitation117 and the Diabetes Control and Complications TrialCitation118 (in people with newly-diagnosed type 2 and type 1 diabetes, respectively). By contrast, such legacy benefits were not seen in large, randomized evaluations of more vs. less intensive glycaemic control in populations with advanced, long-standing diabetesCitation119. Given the ever-strengthening relationship between prediabetes and adverse vascular outcomes, therapeutic intervention at the stage of prediabetes, however diagnosed, is a rational outcome to support long-term cardiovascular, microvascular and renal health.
Supporting information
About this review
Search strategy
This is a narrative review, based on the results of structured PubMed searches: (prediabetes [ti] OR nondiabetic hyperglycemia [ti] OR intermediate hyperglycemia [ti]) AND (microvascular OR nephropathy OR retinopathy OR CKD), and (prediabetes [ti] OR nondiabetic hyperglycemia [ti] OR intermediate hyperglycemia [ti]) AND (macrovascular [ti] OR mortality [ti] OR cardiovascular [ti]). Reference lists were another source of information.
Prediabetes, non-diabetic hyperglycaemia, or intermediate hyperglycaemia?
The term, “prediabetes” is controversial: by no means all people with prediabetic hyperglycaemia will ever develop type 2 diabetes, and applying the label of “prediabetes” to this population risks stigma, anxiety, and economic consequences, e.g. related to health insuranceCitation120. Some expert societies including the World Health Organization and the International Diabetes Federation have moved away from this term, in favour of some of the alternative terms described above; others, including the European Association for the Study of Diabetes, the American Diabetes Association and the European Society of Cardiology retain the term in their guidelines. In the absence of a consensus on terminology, we have used the term, “prediabetes” here for its conciseness and wide use in the literature.
Transparency
Declaration of funding
Merck KGaA, Darmstadt, Germany funded Fast Track review, colour printed figures, and open access publication for this article. No payments were made for authorship of this article and no other funding applied.
Declaration of financial/other relationships
UH is an employee of Merck Healthcare KGaA, Darmstadt, Germany. MG has provided paid editorial consultancy services to Merck Healthcare KGaA, Darmstadt, Germany. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None.
References
- Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167.
- Barr EL, Boyko EJ, Zimmet PZ, et al. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian diabetes, obesity, and lifestyle (AusDiab) study. Diabetologia. 2009;52(3):415–424.
- Cosentino F, Grant PJ, Aboyans V, ESC Scientific Document Group, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
- American diabetes association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38.
- Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42:59–77.
- Hostalek U, Campbell I. Metformin for diabetes prevention: update of the evidence base. Curr Med Res Opin. 2021;37(10):1705–1717.
- World Health Organization, International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF consultation; 2021 [cited 2021 May]. Available from: https://www.who.int/diabetes/publications/Definitionanddiagnosisofdiabetes_new.pdf.
- International Expert Committee. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334.
- Karnchanasorn R, Huang J, Ou HY, et al. Comparison of the current diagnostic criterion of HbA1c with fasting and 2-hour plasma glucose concentration. J Diabetes Res. 2016;2016:1–11.
- Kumar R, Nandhini LP, Kamalanathan S, et al. Evidence for current diagnostic criteria of diabetes mellitus. World J Diabetes. 2016;7(17):396–405.
- López-Jaramillo P, Velandia-Carrillo C, Gómez-Arbeláez D, et al. Is the present cut-point to define type 2 diabetes appropriate in Latin-Americans? World J Diabetes. 2014;5(6):747–755.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412.
- Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Emerging Risk Factors Collaboration, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
- Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350.
- DECODE Study Group, on Behalf of the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality. Arch Intern Med. 2001;161:397–404.
- Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46(4):701–710.
- Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78(3):305–312.
- Richter B, Hemmingsen B, Metzendorf MI, et al. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:CD012661.
- Zhang X, Gregg EW, Williamson DF, et al. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010;33(7):1665–1673.
- van Herpt TTW, Ligthart S, Leening MJG, et al. Lifetime risk to progress from pre-diabetes to type 2 diabetes among women and men: comparison between American Diabetes Association and World Health Organization diagnostic criteria. BMJ Open Diab Res Care. 2020;8(2):e001529.
- Burgess S, Malik R, Liu B, et al. Dose-response relationship between genetically proxied average blood glucose levels and incident coronary heart disease in individuals without diabetes mellitus. Diabetologia. 2021;64(4):845–849.
- Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian diabetes, obesity, and lifestyle study (AusDiab). Circulation. 2007;116(2):151–157.
- Wen CP, Cheng TY, Tsai SP, et al. Increased mortality risks of pre-diabetes (impaired fasting glucose) in Taiwan. Diabetes Care. 2005;28(11):2756–2761.
- Sorkin JD, Muller DC, Fleg JL, et al. The relation of fasting and 2-h post challenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of aging with a critical review of the literature. Diabetes Care. 2005;28(11):2626–2632.
- Saydah SH, Loria CM, Eberhardt MS, et al. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care. 2001;24(3):447–453.
- Deedwania P, Patel K, Fonarow GC, et al. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int J Cardiol. 2013;168(4):3616–3622.
- Neves JS, Correa S, Baeta Baptista R, et al. Association of prediabetes with CKD progression and adverse cardiovascular outcomes: an analysis of the CRIC study. J Clin Endocrinol Metab. 2020;105(4):e1772–e1780.
- Vistisen D, Witte DR, Brunner EJ, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: the whitehall II study. Diabetes Care. 2018;41(4):899–906.
- Welsh C, Welsh P, Celis-Morales CA, et al. Glycated hemoglobin, prediabetes, and the links to cardiovascular disease: data from UK biobank. Diabetes Care. 2020;43(2):440–445.
- Hulman A, Vistisen D, Glümer C, et al. Glucose patterns during an oral glucose tolerance test and associations with future diabetes, cardiovascular disease and all-cause mortality rate. Diabetologia. 2018;61(1):101–107.
- Nezu T, Hosomi N, Aoki S, et al. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb. 2016;23(1):18–31.
- Bulut A, Avci B. Carotid intima-media thickness values are significantly higher in patients with prediabetes compared to normal glucose metabolism. Medicine. 2019;98(44):e17805.
- Xing FY, Neeland IJ, Gore MO, et al. Association of prediabetes by fasting glucose and/or haemoglobin A1c levels with subclinical atherosclerosis and impaired renal function: observations from the Dallas Heart Study. Diab Vasc Dis Res. 2014;11(1):11–18.
- Mutie PM, Pomares-Millan H, Atabaki-Pasdar N, et al. An investigation of causal relationships between prediabetes and vascular complications. Nat Commun. 2020;11(1):4592.
- Gujral UP, Jagannathan R, He S, et al. Association between varying cut-points of intermediate hyperglycemia and risk of mortality, cardiovascular events and chronic kidney disease: a systematic review and meta-analysis. BMJ Open Diab Res Care. 2021;9(1):e001776.
- Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55(13):1310–1317.
- Dunlay SM, Givertz MM, Aguilar D, American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and the Heart Failure Society of America, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294–e324.
- Sinha A, Ning H, Ahmad FS, et al. Association of fasting glucose with lifetime risk of incident heart failure: the lifetime risk pooling project. Cardiovasc Diabetol. 2021;20(1):66.
- Cai X, Liu X, Sun L, et al. Prediabetes and the risk of heart failure: a meta-analysis. Diabetes Obes Metab. 2021;23(8):1746–1753.
- Kristensen SL, Preiss D, Jhund PS, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. 2016;9:e002560.
- Mai L, Wen W, Qiu M, et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes Metab. 2021;23(11):2476–2483.
- Pavlović A, Polovina M, Ristić A, et al. Long-term mortality is increased in patients with undetected prediabetes and type-2 diabetes hospitalized for worsening heart failure and reduced ejection fraction. Eur J Prev Cardiol. 2019;26(1):72–82.
- Erdogan D, Yucel H, Uysal BA, et al. Effects of prediabetes and diabetes on left ventricular and coronary microvascular functions. Metabolism. 2013;62(8):1123–1130.
- Atar AI, Altuner TK, Bozbas H, et al. Coronary flow reserve in patients with diabetes mellitus and prediabetes. Echocardiography. 2012;29(6):634–640.
- Huemer MT, Huth C, Schederecker F, et al. Association of endothelial dysfunction with incident prediabetes, type 2 diabetes and related traits: the KORA F4/FF4 study. BMJ Open Diab Res Care. 2020;8(1):e001321.
- Selvin E, Lazo M, Chen Y, et al. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation. 2014;130(16):1374–1382.
- Diabetes prevention program research group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the diabetes prevention program. Diabet Med. 2007;24:137–144.
- Sörensen BM, Houben AJ, Berendschot TT, et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: the Maastricht Study. Circulation. 2016;134(18):1339–1352.
- Li W, Schram MT, Berendschot TTJM, et al. Type 2 diabetes and HbA1c are independently associated with wider retinal arterioles: the Maastricht Study. Diabetologia. 2020;63(7):1408–1417.
- Lott ME, Slocomb JE, Shivkumar V, et al. Impaired retinal vasodilator responses in prediabetes and type 2 diabetes. Acta Ophthalmol. 2013;91(6):e462–e469.
- De Clerck EEB, Schouten JSAG, Berendschot TTJM, et al. Macular thinning in prediabetes or type 2 diabetes without diabetic retinopathy: the Maastricht Study. Acta Ophthalmol. 2018;96(2):174–182.
- Chande PK, Raman R, John P, et al. Contrast-sensitivity function and photo stress-recovery time in prediabetes. OPTO. 2020;12:151–155.
- Zaleska-Żmijewska A, Piątkiewicz P, Śmigielska B, et al. Retinal photoreceptors and microvascular changes in prediabetes measured with adaptive optics (rtx1™): a case-control study. J Diabetes Res. 2017;2017:1–9.
- Palladino R, Tabak AG, Khunti K, et al. Association between pre-diabetes and microvascular and macrovascular disease in newly diagnosed type 2 diabetes. BMJ Open Diab Res Care. 2020;8(1):e001061.
- Li Rudvan AL, Can ME, Efe FK, et al. Evaluation of retinal microvascular changes in patients with prediabetes. Niger J Clin Pract. 2021;24(6):911–918.
- Katon JG, Reiber GE, Nelson KM. Peripheral neuropathy defined by monofilament insensitivity and diabetes status: NHANES 1999-2004. Diabetes Care. 2013;36(6):1604–1606.
- Bongaerts BW, Rathmann W, Kowall B, et al. Postchallenge hyperglycemia is positively associated with diabetic polyneuropathy: the KORA F4 study. Diabetes Care. 2012;35(9):1891–1893.
- Kirthi V, Perumbalath A, Brown E, et al. Prevalence of peripheral neuropathy in pre-diabetes: a systematic review. BMJ Open Diab Res Care. 2021;9(1):e002040.
- Dyck PJ, Clark VM, Overland CJ, et al. Impaired glycemia and diabetic polyneuropathy: the OC IG survey. Diabetes Care. 2012;35(3):584–591.
- Thaisetthawatkul P, Lyden E, Americo Fernandes J, Jr, et al. Prediabetes, diabetes, metabolic syndrome, and small fiber neuropathy. Muscle Nerve. 2020;61(4):475–479.
- De Clerck EEB, Schouten JSAG, Berendschot TTJM, et al. Reduced corneal nerve fibre length in prediabetes and type 2 diabetes: the Maastricht Study. Acta Ophthalmol. 2020;98(5):485–491.
- Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy identifies small-fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care. 2015;38(8):1502–1508.
- Coopmans C, Zhou TL, Henry RMA, et al. Both prediabetes and type 2 diabetes are associated with lower heart rate variability: the Maastricht Study. Diabetes Care. 2020;43(5):1126–1133.
- Ziegler D, Voss A, Rathmann W, KORA Study Group, et al. Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: the KORA S4 survey. Diabetologia. 2015;58(5):1118–1128.
- Lin YC, Lin CS, Chang TS, et al. Early sensory neurophysiological changes in prediabetes. J Diabetes Investig. 2020;11(2):458–465.
- Marseglia A, Fratiglioni L, Kalpouzos G, et al. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: a population-based cohort study. Alzheimers Dement. 2019;15(1):25–33.
- van Agtmaal MJM, Houben AJHM, de Wit V, et al. Prediabetes is associated with structural brain abnormalities: the maastricht study. Diabetes Care. 2018;41(12):2535–2543.
- Casagrande SS, Lee C, Stoeckel LE, et al. Cognitive function among older adults with diabetes and prediabetes, NHANES 2011–2014. Diabetes Res Clin Pract. 2021;178:108939.
- Plantinga LC, Crews DC, Coresh J, for the CDC CKD Surveillance Team, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. CJASN. 2010;5(4):673–682.
- Kim GS, Oh HH, Kim SH, et al. Association between prediabetes (defined by HbA1C, fasting plasma glucose, and impaired glucose tolerance) and the development of chronic kidney disease: a 9-year prospective cohort study. BMC Nephrol. 2019;20(1):130.
- Markus MRP, Ittermann T, Baumeister SE, et al. Prediabetes is associated with microalbuminuria, reduced kidney function and chronic kidney disease in the general population: the KORA (cooperative health research in the augsburg region) F4-Study. Nutr Metab Cardiovasc Dis. 2018;28(3):234–242.
- Furukawa M, Onoue T, Kato K, et al. Prediabetes is associated with proteinuria development but not with glomerular filtration rate decline: a longitudinal observational study. Diabet Med. 2021;38(8):e14607.
- Sumbul HE, Koc AS, Gülümsek E. Renal cortical stiffness is markedly increased in pre-diabetes mellitus and associated with albuminuria. Singapore Med J. 2020;61(8):435–442.
- Shilpasree AS, Patil VS, Revanasiddappa M, et al. Renal dysfunction in prediabetes: confirmed by glomerular hyperfiltration and albuminuria. J Lab Physicians. 2021;13(03):257–262.
- Rodriguez-Poncelas A, Coll-de-Tuero G, Blanch J, et al. Prediabetes is associated with glomerular hyperfiltration in a european mediterranean cohort study. J Nephrol. 2018;31(5):743–749.
- Rodríguez-Poncelas A, Franch-Nadal J, Coll-de Tuero G, et al. High levels of fasting glucose and glycosylated hemoglobin values are associated with hyperfiltration in a Spanish prediabetes cohort. The PREDAPS study. PLOS One. 2019;14(9):e0222848.
- Okada R, Wakai K, Naito M, et al. Renal hyperfiltration in prediabetes confirmed by fasting plasma glucose and hemoglobin A1c. Ren Fail. 2012;34(9):1084–1090.
- Obayashi K, Saeki K, Kurumatani N. Nighttime BP in elderly individuals with prediabetes/diabetes with and without CKD: the HEIJO-KYO study. CJASN. 2016;11(5):867–874.
- Echouffo-Tcheugui JB, Narayan KM, Weisman D, et al. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta-analysis. Diabet Med. 2016;33(12):1615–1624.
- Ali MK, Bullard KM, Saydah S, et al. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988–2014. Lancet Diabetes Endocrinol. 2018;6(5):392–403.
- DECODE study group, European Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American diabetes association diagnostic criteria. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Lancet. 1999;354:617–621.
- Al-Zahrani JM, Aldiab A, Aldossari KK, et al. Prevalence of prediabetes, diabetes and its predictors among females in Alkharj, Saudi Arabia: a cross-sectional study. Ann Glob Health. 2019;85:109.
- Pedicelli S, Fintini D, Ravà L, et al. Prevalence of prediabetes in children and adolescents by class of obesity. Pediatr Obes. 2022;17:e12900.
- Perreault L. Goals for medical treatment in obesity and prediabetes: improving outcomes for both. Endocr Pract. 2018;24(12):1093–1098.
- La Sala L, Pontiroli AE. Prevention of diabetes and cardiovascular disease in obesity. IJMS. 2020;21(21):8178.
- Wondmkun YT. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:3611–3616.
- Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124(24):e837–e841.
- Berezin AE, Berezin AA, Lichtenauer M. Emerging role of adipocyte dysfunction in inducing heart failure among obese patients with prediabetes and known diabetes mellitus. Front Cardiovasc Med. 2020;7:583175.
- Haboubi N, Williams H, Al-Ansari A. Adiposity and neurological disorders: a review. EMJ Neurol. 2022. DOI:10.33590/emjneurol/21-00177
- Mancia G, Bousquet P, Elghozi JL, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25(5):909–920.
- Hanssen NMJ, Scheijen JLJM, Houben AJHM, et al. Fasting and post-oral-glucose-load levels of methylglyoxal are associated with microvascular, but not macrovascular, disease in individuals with and without (pre)diabetes: the Maastricht Study. Diabetes Metab. 2021;47(1):101148.
- Alexandru N, Procopciuc A, Vîlcu A, et al. Extracellular vesicles-incorporated microRNA signature as biomarker and diagnosis of prediabetes state and its complications. Rev Endocr Metab Disord. 2022;23(3):309–332.
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625.
- van Sloten TT, Sedaghat S, Carnethon MR, et al. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(4):325–336.
- Modjadji P. Communicable and non-communicable diseases coexisting in South Africa. Lancet Glob Health. 2021;9(7):e889–e890.
- Assaad Khalil SH, Abdelaziz SI, Al Shammary A, et al. Prediabetes management in the Middle East, Africa and Russia: current status and call for action. Diab Vasc Dis Res. 2019;16(3):213–226.
- Houle B, Clark SJ, Gómez-Olivé FX, et al. The unfolding counter-transition in rural South Africa: mortality and cause of death, 1994–2009. PLOS One. 2014;9(6):e100420.
- Hostalek U, Gwilt M, Hildemann S. Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs. 2015;75(10):1071–1094.
- Gong Q, Zhang P, Wang J, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing diabetes prevention outcome study. Lancet Diabetes Endocrinol. 2019;7(6):452–461.
- National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes prevention program outcomes study (DPPOS); 2021 [cited 2021 October]. Available from: https://repository.niddk.nih.gov/studies/dppos.
- Diabetes Prevention Program Group and Diabetes Prevention Program Outcome Study Research Group. New data on clinical outcomes from the Diabetes Prevention Program Outcomes Study. Presentation at the 80th Virtual Scientific Sessions of the American Diabetes Association, June 26 2020.
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the diabetes prevention program outcomes study. Lancet Diabetes Endocrinol. 2015;3:866–875.
- Busko M. DPPOS at 22 years: 'diabetes prevention is possible' long term. Medscape Diabetes & Endocrinology; 2021 [cited 2021 May]. Available from: https://www.medscape.com/viewarticle/932876.
- White NH, Qing P, for the Diabetes Prevention Program Research Group, et al. The effect of interventions to prevent type 2 diabetes on the development of diabetic retinopathy: the DPP/DPPOS experience. Diabetes Care. 2022;45(7):1640–1646.
- Perreault L, Pan Q, Schroeder EB, Diabetes Prevention Program Research Group, et al. Regression from prediabetes to normal glucose regulation and prevalence of microvascular disease in the diabetes prevention program outcomes study (DPPOS). Diabetes Care. 2019;42:1809–1815.
- Naik V, Dave R, Stephens JW, et al. Evidence based prevention of type 2 diabetes: role of lifestyle intervention as compared to pharmacological agents. Int J Diabetes Clin Res. 2015;2:6.
- Chiasson JL, Josse RG, Gomis R, STOP-NIDDM Trial Research Group, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–494.
- Kaiser T, Sawicki PT. Acarbose for prevention of diabetes, hypertension and cardiovascular events? A critical analysis of the STOP-NIDDM data. Diabetologia. 2004;47(3):575–580.
- Inzucchi SE, Docherty KF, Køber L, et al. Dapagliflozin and the incidence of type 2 diabetes in patients with heart failure and reduced ejection fraction: an exploratory analysis from DAPA-HF. Diabetes Care. 2021;44(2):586–594.
- le Roux CW, Astrup A, Fujioka K, et al. 3 Years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–1409.
- University College London. Press release: pre-diabetes label 'unhelpful and unnecessary; 16 July 2014 [cited 2021 October). Available from: https://www.ucl.ac.uk/news/2014/jul/pre-diabetes-label-unhelpful-and-unnecessary.
- Centers for Disease Control and Prevention. National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2020 [cited 2020 October]. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html.
- Libman IM, Barinas-Mitchell E, Bartucci A, et al. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93(11):4231–4237.
- Shang Y, Marseglia A, Fratiglioni L, et al. Natural history of prediabetes in older adults from a population-based longitudinal study. J Intern Med. 2019;286(3):326–340.
- Murray P, Chune GW, Raghavan VA. Legacy effects from DCCT and UKPDS: what they mean and implications for future diabetes trials. Curr Atheroscler Rep. 2010;12(6):432–439.
- UK Prospective Diabetes Study Group. Effect of intensive blood glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–865.
- Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-Year follow-up. Diabetes Care. 2016;39:686–693.
- Lipska KJ, Laiteerapong N. Lack of glycemic legacy effects in the veterans affairs diabetes trial. N Engl J Med. 2019;380(23):2266–2267.
- Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. BMJ. 2014;349:g4485.
