 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Factor VIII (FVIII) replacement and emicizumab have demonstrated efficacy for prevention of bleeds among patients with hemophilia A (PwHA) compared to on-demand (OD) use. Evidence investigating clinical outcomes and healthcare costs of non-inhibitor PwHA switching from prophylaxis with FVIII concentrates to emicizumab has not been well-established within large real-world datasets. This study aimed to investigate billed annualized bleed rates (ABRb) and total cost of care (TCC) among non-inhibitor PwHA switching from FVIII-prophylaxis to emicizumab-prophylaxis.
Methods
This retrospective, observational study was conducted using IQVIA PharMetrics Plus, a US administrative claims database. The date of first claim for emicizumab was defined as the index date. OD patients and inhibitor patients were excluded. Bleeds were identified using a list of 535 diagnosis codes. Bayesian models were developed to estimate the probability ABRb worsens and TCC increases after switching to emicizumab. Wilcoxon rank-sum tests were used to test statistical significance of changes in ABRb and TCC after switch.
Results
Among the 121 identified patients, the difference in mean ABRb between FVIII-prophylaxis (0.68 [SD = 1.28]) and emicizumab (0.55 [SD = 1.48]) was insignificant (p = .142). The mean annual TCC significantly increased for patients switching from FVIII-prophylaxis ($518,151 [SD = $289,934]) to emicizumab ($652,679 [SD = $340,126]; p < .0001). The Bayesian models estimated a 21.0% probability of the ABRb worsening and a 99.9% probability of increasing TCC after switch.
Conclusions
This study found that in male non-inhibitor PwHA, switching from FVIII prophylaxis to emicizumab incurs substantial cost increase with no significant benefit in ABRb. This evidence may help guide providers, payers, and patients in shared decision-making conversations around best treatment options.
Introduction
Hemophilia A (HA), a rare, recessive, X-linked bleeding disorder, is caused by a deficiency of clotting factor VIII (FVIII) occurring in approximately 1 in 5000 males born in the United States (US)Citation1. The national age-adjusted incidence for HA was 12.0/100,000 person-years during 2012–2018 among males born between 1995 and 2014Citation2. The severity of HA is categorized based on biochemical phenotypes as mild, moderate, or severe depending on the quantitative level of FVIII present, as well as phenotypically by the severity and frequency of bleeding patients experienceCitation3. Spontaneous bleeding episodes characteristically occur in joints, with ankles, knees, and elbows being the most frequent sites. Control and prevention of bleeds are paramount to minimizing acute and chronic damage and essential for reducing long-term sequelae, decreasing healthcare resource utilization, and improving quality of lifeCitation3,Citation4.
FVIII products are approved for either prophylactic or episodic care (on-demand [OD] therapy)Citation3. Typical FVIII prophylaxis regimens for patients with moderate-severe HA is ∼2–3 intravenous infusions weekly with the goal of increasing FVIII trough levels to eliminate spontaneous bleeding events and the subsequent acute and chronic joint damage. Current FVIII replacement therapies include plasma-derived and recombinant products. Among recombinant FVIII products, varying half-lives may have an impact on infusion frequency (standard half-life [SHL] or extended half-life [EHL]). The half-life of EHL products is approximately 1.5 times longer than SHL products, thereby potentially lowering treatment burden through fewer infusions and less time with sub-therapeutic FVIII levelsCitation4,Citation5. Approximately, 30% of patients with HA (PwHA) on prophylaxis will develop neutralizing alloantibodies or inhibitors to exogenous FVIIICitation6, placing them at increased risk of fatal bleeds, prolonged bleeding episodes, mortalityCitation7, lower quality of lifeCitation8, and compromised physical functioningCitation9. The development of inhibitors introduces new levels of complexity to treatment options, such as bypassing agents and immune tolerance induction which requires more frequent administration and has decreased efficacy in bleed controlCitation10.
An alternative treatment regimen, emicizumab, has been recently approved to address the unmet need among PwHA. Emicizumab, a recombinant humanized bispecific monoclonal antibody that mimics FVIII by bringing together FIX and FXCitation11, was approved in 2018 and is currently indicated for routine prophylaxis for both inhibitor and non-inhibitor PwHA. Emicizumab is administered subcutaneously every week, every 2 weeks, or every 4 weeks. Long-term follow-up of patients enrolled in Phase III clinical trials (HAVEN 1-4), demonstrated efficacy of emicizumab for prevention of bleeding episodes while being well-toleratedCitation12–14.
HA is a clinically and economically burdensome disease with significant psychological and functional consequences, persistent treatment requirements, and lifelong healthcare needs and costs. New non-factor replacement therapies, such as emicizumab entering the market merit understanding the depth of clinical benefits relative to the economic burden placed on the healthcare system, payers, providers, and patients. While the benefits of emicizumab for inhibitor PwHA are well established, some uncertainty surrounds the clinical and economic outcomes among non-inhibitor PwHA in real-world settingsCitation15. With limited ability to compare therapeutic treatment options head-to-head, assessing real-world use of different treatment options is imperative. This study aimed to estimate and compare clinical outcomes, defined as billed annualized bleed rates (ABRb) and total cost of care (TCC) for non-inhibitor PwHA, before and after switching prophylaxis from FVIII concentrates to emicizumab.
Methods
Data source
This retrospective, pre-post cohort study queried the IQVIA PharMetrics Plus database (a longitudinal US commercial health plan database with >190 million members) from 2015 to 2020. Because all patient data were de-identified, institutional review board approval was not required.
Patient population
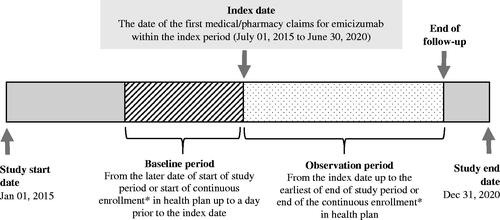
Patients who met the inclusion criteria were followed from the beginning of the study period or start of continuous enrollment until the end of the study period or end of continuous enrollment (). Male patients with ≥1 outpatient or pharmacy claim for emicizumab (first claim defined as the index date), ≥6 months of continuous enrollment before and after the index date, and prophylaxis with FVIII concentrates before switching to emicizumab met the inclusion criteria. Continuous enrollment was defined as no lapse in insurance coverage >60 days. Patients with a history of FVIII inhibitors or ≥2 claims at least 30 days apart for diagnoses of von Willebrand disease, hemophilia B, acquired HA or acquired coagulation factor deficiency were excluded.
Figure 1. Study design. *Continuous enrollment is defined as no lapse in insurance coverage for >60 days.
International Classification of Diseases (ICD) codes, National Drug Codes (NDCs), and Healthcare Common Procedure Coding System (HCPCS) codes were used to identify diagnoses, therapies, and procedures. Operational definitions were created to identify FVIII prophylaxis use, bleed events, and inhibitor status.
Identifying patients with FVIII prophylaxis
Patients with an annualized FVIII utilization of ≥2340 international units (IU)/kilogram (kg) (i.e. ≥45 IU/kg/weekCitation3) before the index date met the criteria for continuous FVIII prophylaxis. Annualized FVIII utilization was calculated as:
(1)
(1)
All FVIII claims associated with a bleed event (i.e. occurred up to 7-day prior to a bleed diagnosis) were eliminated from the calculation of the total annual dosage. This method identified bleed events that were evaluated or treated by the healthcare system and does not capture bleeds that were self-treated at home. Patient weight was not available in the data and was imputed using US national averages based on ageCitation16–18.
Identifying patients with inhibitors
To ensure PwHA with active inhibitors were excluded from the study, they were operationally defined based on having at least one of the following:
≥1 claim(s) for bypassing agent (Feiba-Activated prothrombin complex concentrate [aPCC] or recombinant activated factor VII [rFVIIa]) or (Novoseven RT);
OR
≥1 claim(s) for rituximab
OR
Immune tolerance induction therapy, identified using the following criteria:
^ ≥1 claim(s) for Bethesda/Nijmegen assays in the study period;
AND
^ High dose of FVIII dispensed in the complete baseline period, was defined as >3 times of the median IU factors dispensed for patients in the same age-group for more than 5 consecutive 28-day intervals, allowing one interval gapCitation19.
Outcomes
Billed annualized bleed rates
Clinical outcomes were assessed as billed ABR and included bleeding episodes that resulted in a claim for evaluation, treatment, or procedure. A clinical review of diagnosis codes and previous literatureCitation16 resulted in a list of 535 ICD-10 codes used to identify HA-related bleeding episodes (e.g. hemarthrosis) in the primary diagnosis position. Bleeds treated at home or those that did not trigger any medical claims were not captured. ABRb was categorized as either a spontaneous or traumatic bleed using specific diagnosis codes finalized during the clinical review. All bleed events caused by trauma, or a medical procedure was classified as traumatic bleeds and the remaining as spontaneous bleeds. A sensitivity analysis for ABRb was conducted using additional ICD-9/10 bleed codes not included in the base case list. We also investigated the difference in ABRb, irrespective of treatment before the COVID-19 pandemic (March 2020) and during the lockdown to identify potential confounding caused by systematic differences in reporting or delivery of care.
Total cost of care
Total costs included all hemophilia and non-hemophilia healthcare costs paid by the payer, expressed as the mean annualized cost per patient per year (PPPY) and adjusted to 2020 US dollars. TCC was further stratified by cost accrued from breakthrough/traumatic bleeds (FVIII costs accrued during ±7 day of a bleed event), prophylaxis treatment (remaining FVIII-related costs pre-switch and emicizumab-related costs post-switch), FVIII transitioning (first 4 weeks after switching), and concomitant use (after 4 weeks of switching). FVIII concomitant use after the expected transition period may represent FVIII dispenses for on-hand breakthrough/traumatic bleeds, additional prophylaxis, or maintaining chronic exposure to FVIII to minimize risk or complications of inhibitor development.
Subgroups
A sub-group analysis tested the impact of high FVIII utilization in the pre-switch period on mean change in ABRb and TCC by stratifying patients by the top 10% FVIII utilization (high-utilizers) and the remaining 90% (low-utilizers). Another sub-group analysis stratified patients into age categories.
Statistical methods
The analysis generated descriptive statistics to characterize the cohort, as well as summarized pre- and post-switch ABRb and TCC. Bayesian models were developed to generate inferences on the probability of a mean change in ABRb and TCC before and after switching from FVIII prophylaxis to emicizumab. Gaussian model for ABRb and gamma regression model for TCC difference were the best fitting models (Appendices). The models were run for 100,000 iterations to improve robustness. Inferences were conducted by computing posterior probabilities for hypotheses and summarized with 95% credible intervals (CrIs). The a-priori defined Bayesian inferences included: 1) what is the probability ABRb worsens after switching to emicizumab? and 2) what is the probability the TCC increases after switching to emicizumab? Wilcoxon signed-rank tests (WSRT) were performed as secondary endpoints.
Results
A total of 121 male, non-inhibitor, PwHA met criteria for inclusion. The mean (median) age was 25.9 (25) years and ranged from 2 to 63 years. The mean observation time in the pre-switch and post-switch periods was 2.5 and 1.1 years, respectively. The mean (standard deviation [SD]) baseline Charlson Comorbidity Index score was 1.08 (2.49). Prevalence of joint pain and soft tissue disorders in the pre-switch period were 46 and 28%, respectively. Prevalence of blood-borne viral infections was 14.1% for hepatitis B/C and 9.1% for Human Immunodeficiency Virus (HIV). During the pre-switch period, the mean (median) FVIII replacement dose was 82.8 (70.2) IU/kg/week ().
Table 1. Distribution of demographic and clinical characteristics among non-inhibitor hemophilia A patients.
The Bayesian model estimated a mean change in ABRb of −0.128 [95% CrI: −0.441–0.184] and predicted the ABRb will worsen 21.0% of the times after switching to emicizumab (). The mean change in ABRb was not statistically significant after switching (pre-switch: 0.68 [SD = 1.28] vs. post-switch: 0.55 [SD = 1.48]; WSRT p = .142). The Bayesian model estimated a less than 1% probability of ≥0.5 reduction in ABRb after switching ().
Table 2. Bayesian posterior summaries of difference in ABRb and TCC before and after switch from FVIII prophylaxis to emicizumab.
Table 3. Bayesian probability distribution of range of difference in ABRb and TCC before and after switch from FVIII prophylaxis to emicizumab.
Spontaneous bleeds comprised the majority of ABRb. Pediatric patients (0–12 years) had the greatest reduction in bleeds, however, adolescents aged 13–18 years experienced increased bleeds. Adults had a 0.05 reduction in ABRb. For patients aged >26 years, ABRb changed from 0.71 (SD = 1.12) to 0.68 (SD = 1.89). High-utilizers of FVIII had a 0.03 increase in ABRb after switching (). Sensitivity analyses of ABRb using additional diagnosis-based definitions for bleeds yielded similar results.
Table 4. Billed annualized bleed rate (ABRb) during treatment with FVIII prophylaxis (baseline) and treatment with emicizumab (follow-up).
The Bayesian model estimated an increase in TCC of $159,680 [95% CrI: $74,842–$247,841] PPPY after switching and predicted a 99.9% probability the TCC will increase after switch (). The mean annual TCC increased significantly from $518,151 [SD = $289,934] to $652,679 [SD = $340,126] PPPY after switching (WSRT p < .0001). The Bayesian model estimated an 89.9% probability TCC would increase by >$103,970 (>20%) PPPY (). Higher cost in the follow-up was accounted by increase in prophylaxis cost after starting emicizumab, followed by added costs of FVIII transitioning and concomitant use ().
Figure 2. Total cost of care (TCC) per patient per year (PPPY) before and after switch from FVIII prophylaxis to emicizumab. PPPY, Per patient per year; TCC, Total cost of care; USD, United Stated Dollars. Notes: 1. The total of all the cost components may not add-up exactly to the overall costs as some of the claim lines were captured under both FVIII-specific and emicizumab-specific costs while rolling up costs at visit-level. 2. All costs are adjusted to 2020 USD. 3. †Non-FVIII and non-emicizumab related costs. 4. ‡Mean cost for first 4 weeks after switching. 5. §PPPY cost accrued after 4 weeks of switching. 6. ¶FVIII-specific cost related to breakthrough or traumatic bleeds. 7. ¤Non-bleed related FVIII costs pre-switch and emicizumab-related cost post-switch.
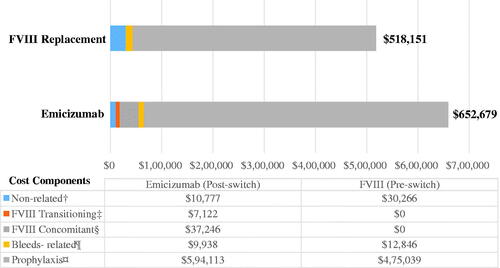
HA medication costs accounted for >94% of TCC in both pre- and post-switch periods, of which prophylaxis costs composed >90% of TCC in both periods. The mean TCC increased after the switch for all ages, with a larger increase among older age groups. For example, for the seven patients aged 3–6 years, the TCC increased from $121,227 to $172,084, whereas for 56 patients aged >26 years it increased from $684,072 to $853,187. The only stratum where the TCC decreased after switching was for the 13 high FVIII utilizers (from $1,033,222 to $885,533) ().
Table 5. Total cost of care (TCC) during treatment with FVIII prophylaxis (baseline) and treatment with emicizumab (follow-up).
Among the 104 patients with available data, the mean ABRb changed from 0.64 (SD = 1.79) prior to the COVID-19 pandemic (March 2020) to 0.53 (SD = 1.81) during the pandemic (results not shown in tables), irrespective of treatment (FVIII or emicizumab).
Discussion
This study assessed the comparative effectiveness of billed ABR and costs associated with FVIII prophylaxis as compared to emicizumab prophylaxis among male non-inhibitor PwHA in the US. The analysis used a national claims database that is geographically diverse with a broad age range. The study also applied algorithms and clinical practice definitions to identify prophylaxis FVIII use, bleed events, and inhibitor statusCitation3,Citation16,Citation19. In addition to traditional statistical testing, the study also applied Bayesian statistical approaches, that provide a powerful framework to calculate the probability of an event or change in event over time for a given exposureCitation21. Treatment approach for bleed prevention and management differs between the inhibitor and non-inhibitor PwHA therefore, by removing the inhibitor population, the clinical characteristics of a non-inhibitor population become apparent and are not confounded by the more severe disease characteristics of an inhibitor population, or by the different treatment efficacies and costs in the inhibitor population. Our results demonstrate a significant increase in costs after switching therapies for an already expensive and clinically burdened disease, without a discernible improvement in ABRb among non-inhibitor PwHA. The Bayesian analysis suggests that the 21.0% potential worsening of the disease with emicizumab needs to be carefully weighed, especially for patients who are well-controlled on FVIII prophylaxis. The 99.9% probability of cost increase after switching to emicizumab is important to understand amidst the increasingly complex treatment landscape of this already very costly disease with restrictive payer environment. The substantial cost increase seen with switching to emicizumab is not offset by a clinical benefit.
Some studies investigating emicizumab with real-world data have generally followed patients to observe clinical outcomes, such as safety and efficacy of longitudinal use; correlation between laboratory monitoring and emicizumab’s hemostatic effect; association of spontaneous and traumatic bleeds with age; inhibitor status; and other outcomesCitation22–27. Other studies have assessed use of additional hemostatic agents for surgeryCitation28, treatment patterns, clinical, and economic outcomes among FVIII users by ageCitation29, or are simply descriptive analyses of bleed outcomesCitation27. There are two relevant studiesCitation30,Citation31 (to date) conducted by McCary et al. and Samelson-Jones et al. that pursued similar research questions using a similar study design to our study. Both studies extracted data from three hemophilia treatment centers in the US and followed a cohort of 93 patients. McCary et al. reported that the annualized rate of treated bleed dropped significantly from 1.6 to 0.4 (p = .0025) among non-inhibitor PwHA after switching to emicizumabCitation31. However, 86% of the patients were on prophylaxis before the switch, with a potential confounding impact from those patients who were on demand, and the population was 93.3% pediatric patients, limiting its generalizability. Additionally, this study suffered from threats to external validity namely, sampling and self-selection bias because the included patients switched to emicizumab of their own volition and may represent preferred candidates for a switch of therapy. Unlike this study, Samelson-Jones et al. estimated that the mean 6-month total costs before ($204,987) and after the switch ($180,571) did not statistically significantly changeCitation30. Differences in data sources and patient populations may explain divergent results. The study also calculated costs using wholesale acquisition costs (WAC) whereas, our study reports actual payer costs thereby providing superior estimates because WAC differs from the actual drug price due to discounting practices, particularly for products on market for a longer timeCitation32. Additionally, the differences between prescribed amount and actual consumed amount (adherence), are not captured.
In addition to testing efficacy, studies using HAVEN trial data have provided insight into long-term outcomesCitation14,Citation33. Klamroth et al.’s indirect comparison showed that individualized recombinant factor VIII-Fc fusion protein (rFVIIIFc) was more efficacious than emicizumab once every 4 weeks and reported similar efficacy when compared with more frequent emicizumab regimensCitation33. In Reyes et al., a network meta-analysis (NMA) of emicizumab use across varying dosages, reported a greater reduction in bleed rates compared to FVIII prophylaxisCitation14. However, due to stringent inclusion criteria, such as the requirement to have a measure of “treated bleeds”, which was not a common end-point before emicizumab, this NMA included only a limited number of FVIII trials which had notable heterogeneity in treated ABR measurement methods and could therefore be subject to information bias. Beyond clinical efficacy, existing literature in cost-effectiveness and other models were limited to patients with inhibitors, did not isolate non-inhibitor patients, or did not assess prophylactic and OD FVIII regimens separately, biasing the results toward the outlier inhibitor patients and leading to invalid comparisonsCitation34–38. On the contrary, the current analysis appropriately studied non-inhibitor PwHA switching from FVIII prophylaxis to emicizumab.
While future evidence is still needed, our study adds important real-world, comparative observations for non-inhibitor PwHA. The use of claims data presents an accurate estimation of costs to the healthcare system that is more informative than models or registries that require assumptions on price, adherence, and dose. The incorporation of billed bleeds allows for the comparison of an important clinical outcome for patients and providers. Our results further suggest that the discussion of treatment options for non-inhibitor PwHA needs to be more nuanced as clearly, every patient cannot be treated with emicizumab given potential for disease worsening and ongoing need for FVIII. Although some patients benefit by initiating emicizumab both clinically and economically, others will benefit more from the current standard of care. Personalization of care and identification of patients who receive the greatest clinical benefit for each therapy can optimize patient care. Payers and decision-makers should understand their patient population and the future treatment landscape to prepare for changes in costs as well as clinical outcomes. Treatment decisions should consider inhibitor status and the evidence from this study should only be used to inform disease management strategies among non-inhibitor PwHA. As additional real-world data becomes available for non-inhibitor PwHA, future research should compare different prophylaxis treatment options among diverse patient populations.
This study possesses limitations resulting from the inherent nature of claims databases, including lack of information about specific patient characteristics, medications administered during inpatient admissions, and variation in coding and billing practices. Furthermore, due to the absence of required valid information in administrative claims, some of the potential sub-group analyses, such as outcome assessments by frequency of emicizumab injection (e.g. biweekly vs. monthly) or by long-acting vs. short-acting FVIII replacement products were not able to be conducted. Moreover, because the sample was sourced from the commercially insured population, the cohort may not be representative of uninsured, Medicare, or Medicaid populations. Our study could not capture bleeds treated entirely at home or those that did not trigger any claim. Misclassification could arise from lack of disease-specific and clinical parameters in administrative claims, such as inhibitor status, HA severity, FVIII trough target, weight, or differentiation between prophylaxis and OD treatment. Although our study investigated direct costs and benefits in terms of bleed rates, other tangible benefits to patients or caregivers, such as convenience of subcutaneous infusion over the costs of maintaining venous lines, treatment schedules/frequencies, and other quality of life-related aspects were not studied. The study is susceptible to history and maturation bias because it included patients with prevalent HA, and changes in age or internal states between switching therapies may have affected the outcomes, which may have been further amplified due to large difference between the assessment time periods. Last, because the study period for this investigation coincided with the onset of the COVID-19 pandemic, it may have reduced the bleeding risk or willingness to seek bleed treatment, more so toward the end of the inclusion period.
Conclusion
Our findings illustrate the high probability of a substantial increase in cost when patients switch to emicizumab and the low probability of a clinically meaningful change in ABRb. Increased use of emicizumab may cause an increase in economic burden for payers without providing a substantial clinical benefit, substantively impacting their ability to manage the health of their patients. This evidence can aid providers and payers to better understand the economic impact of switching to emicizumab and meaningfully engage in shared decision-making process to improve patient care.
Transparency
Declaration of funding
Funding was provided by Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA.
Declaration of financial/other relationships
KB is a consultant to Complete HEOR Solutions, North Wales, PA, USA, and current holder of equity in publicly-traded companies. BGS, JC, and MB are employees of Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA, USA, and current holders of individual stock/stock options. CSH is a consultant to Complete HEOR Solutions. NA and SC are employees of Complete HEOR Solutions, which has received financial compensation for conducting the study analysis. The authors report no other relevant financial relationships or otherwise to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
KB, BGS, JC, and MB contributed to the conceptualization and design of the study. CSH, NA, and SC contributed to the study conduct, data analysis, and interpretation of results. All authors contributed to revising the article critically for intellectual content. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Acknowledgements
The authors thank Irene Doherty, PhD for medical writing assistance, and Niranjan Kathe, PhD for his supervision throughout the project. ID is a consultant to, and NK is an employee of Complete HEOR Solutions, North Wales. Funding for the manuscript writing was provided by Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA.
Data availability statement
The data that support the findings of this study are available from the IQVIA repository, https://www.iqvia.com/solutions/real-world-evidence, but restrictions apply to the availability of these data, which were used under license for this study and so are not publicly available.
References
- Soucie JM, Evatt B, Jackson D. Occurrence of hemophilia in the United States. Am J Hematol. 1998;59(4):288–294.
- Soucie JM, Miller CH, Dupervil B, et al. Occurrence rates of haemophilia among males in the United States based on surveillance conducted in specialized haemophilia treatment centres. Haemophilia. 2020;26(3):487–493.
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1-47–47.
- Simpson ML, Valentino LA. Management of joint bleeding in hemophilia. Expert Rev Hematol. 2012;5(4):459–468.
- Chhabra A, Spurden D, Fogarty PF, et al. Real-world outcomes associated with standard half-life and extended half-life factor replacement products for treatment of haemophilia a and B. Blood Coagul Fibrinolysis. 2020;31(3):186–192.
- Santagostino E, Young G, Carcao M, et al. A contemporary look at FVIII inhibitor development: still a great influence on the evolution of hemophilia therapies. Expert Rev Hematol. 2018;11(2):87–97.
- Walsh CE, Soucie JM, Miller CH. Impact of inhibitors on hemophilia a mortality in the United States: Impact of inhibitors on hemophilia. Am. J. Hematol. 2015;90(5):400–405.
- Brown TM, Lee WC, Joshi AV, et al. Health-related quality of life and productivity impact in haemophilia patients with inhibitors. Haemophilia. 2009;15(4):911–917.
- Osooli M, Donfield SM, Carlsson KS, et al. Joint comorbidities among Swedish carriers of haemophilia: a register‐based cohort study over 22 years. Haemophilia. 2019;25(5):845–850.
- Ragni MV, Berntorp E, Carcao M, et al. Chapter 8 inhibitors to clotting factor. WFH guidelines for the management of hemophilia 3rd edition. Haemophilia. 2020:26;107–120.
- Gelbenegger G, Schoergenhofer C, Knoebl P, et al. Bridging the missing link with emicizumab: a bispecific antibody for treatment of hemophilia A. Thromb Haemost. 2020;120(10):1357–1370.
- Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia a without inhibitors. N Engl J Med. 2018;379(9):811–822.
- Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295-305–e305.
- Reyes A, Révil C, Niggli M, et al. Efficacy of emicizumab prophylaxis versus factor VIII prophylaxis for treatment of hemophilia a without inhibitors: network Meta-analysis and Sub-group analyses of the intra-patient comparison of the HAVEN 3 trial. Curr Med Res Opin. 2019;35(12):2079–2087.
- Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia a with inhibitors. N Engl J Med. 2017;377(9):809–818.
- Shrestha A, Eldar-Lissai A, Hou N, et al. Real-world resource use and costs of haemophilia A-related bleeding. Haemophilia. 2017;23(4):e267-75–e275.
- Fryar CD, Kruszon-Moran D, Gu Q, et al. Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999–2000 through 2015–2016. Natl Health Stat Rep. 2018;(122):1–16.
- Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2000;2002(246):1–190.
- Su J, Zhou J, Buckley B, et al. The immune tolerance induction factor utilizations and costs for the management of male hemophilia-a patients who developed inhibitors. Blood. 2016;128(22):4758–4758.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139.
- Seaman SR, Richardson S. Equivalence of prospective and retrospective models in the bayesian analysis of case-control studies. Biometrika. 2005;92(2):505–505.
- Levy-Mendelovich S, Brutman-Barazani T, Budnik I, et al. Real-World data on bleeding patterns of hemophilia a patients treated with emicizumab. J Clin Med. 2021;10(19):4303.
- Barg AA, Livnat T, Budnik I, et al. Emicizumab treatment and monitoring in a paediatric cohort: real‐world data. Br J Haematol. 2020;191(2):282–290.
- Barg AA, Budnik I, Avishai E, et al. Emicizumab prophylaxis: prospective longitudinal real‐world follow‐up and monitoring. Haemophilia. 2021;27(3):383–391.
- Barg AA, Avishai E, Budnik I, et al. Emicizumab prophylaxis among infants and toddlers with severe hemophilia a and inhibitors—a single‐center cohort. Pediatr Blood Cancer. 2019;66(11):e27886.
- Misgav M, Brutman‐Barazani T, Budnik I, et al. Emicizumab prophylaxis in haemophilia patients older than 50 years with cardiovascular risk factors: real‐world data. Haemophilia. 2021;27(2):253–260.
- Ebbert PT, Xavier F, Seaman CD, et al. Emicizumab prophylaxis in patients with haemophilia a with and without inhibitors. Haemophilia. 2020;26(1):41–46.
- Lewandowska M, Randall N, Bakeer N, et al. Management of people with haemophilia A undergoing surgery while receiving emicizumab prophylaxis: real‐world experience from a large comprehensive treatment Centre in the US. Haemophilia. 2021;27(1):90–99.
- Caplan EO, Patel AM, DeClue RW, et al. Real-world treatment, clinical outcomes and healthcare resource utilization among persons with hemophilia a by age. J Comp Eff Res. 2021;10(15):1121–1131.
- Samelson‐Jones BJ, Guelcher C, Kuhn J, et al. Real‐world cost estimates of initiating emicizumab in US patients with haemophilia A. Haemophilia. 2021;27(4):591–598.
- McCary I, Guelcher C, Kuhn J, et al. Real-World use of emicizumab in patients with hemophilia with and without inhibitors. Blood. 2019;134(1):1137–1137.
- Kakani P, Chernew M, Chandra A. Rebates in the pharmaceutical industry: evidence from medicines sold in retail pharmacies in the US. No. w26846. National Bureau of Economic Research. 2020.
- Klamroth R, Wojciechowski P, Aballéa S, et al. Efficacy of rFVIIIFc versus emicizumab for the treatment of patients with hemophilia a without inhibitors: matching-adjusted indirect comparison of A-LONG and HAVEN trials. J Blood Med. 2021;12:115–122.
- Cortesi PA, Castaman G, Trifirò G, et al. Cost-effectiveness and budget impact of emicizumab prophylaxis in haemophilia A patients with inhibitors. Thromb Haemost. 2020;120(2):216–228.
- Lee H, Cho H, Han JW, et al. Cost‐utility analysis of emicizumab prophylaxis in haemophilia A patients with factor VIII inhibitors in Korea. Haemophilia. 2021;27(1):e12–e21.
- Sun SX, Wu Y, McDermott M, et al. Cost-Effectiveness model of recombinant FVIII versus emicizumab treatment of patients with severe hemophilia A without inhibitors. Blood. 2019;134(1):2102–2102.
- Stonebraker JS, Ducore JM. Modelling future usage and cost of factor and emicizumab to treat haemophilia a for the US Western states region IX haemophilia treatment centres. Haemophilia. 2021;27(1):e22–e29.
- Zhou ZY, Raimundo K, Patel AM, et al. Model of short- and Long-Term outcomes of emicizumab prophylaxis treatment for persons with hemophilia A. J Manag Care Spec Pharm. 2020;26(9):1109–1120.
Appendices
Development of Bayesian models for billed annualized bleed rates (ABRb) and total cost of care (TCC)
Bayesian models for ABRb
Several Bayesian models, such as Gaussian model of ABRb difference, binomial model of risk of bleed after switching, and independence of ABRb were developed. For the mean difference in ABRb, the Gaussian model was the best-fitted model, which estimated the probability of change in ABRb after a switch. The likelihood function was Gaussian for this model and the priors were Gaussian and inverse Gaussian. The data was modeled as follows:
Model parameters are μ and t. Test the hypothesis that μ > 0.
The sampler was extremely well-behaved with almost immediate convergence and no evidence of auto-correlations.
Bayesian models for TCC
Several Bayesian models, such as Gaussian model of annualized cost difference, gamma model of increase in annualized cost after switching, and independence annualized cost were developed. Bayesian gamma regression model was the best-fitted model. A gamma distribution is continuous and limited to the positive real line and can also be skewed, which is consistent with the properties of costs. We next estimated a gamma model of costs, where the shape and scale parameters were modeled hierarchically to build in a linear function of switching. The data was modeled as gamma distributed as follows:
where i = 1…, N indexes patients, ci is the annualized cost of patient i, and ti is an indicator of switching. The gamma distribution has a shape parameter, k, and a scale parameter, theta. The scale parameter contains a linear predictor. Inference centers on exp(β1) – exp(β0), which shows the incremental cost of switching to emicizumab from FVIII prophylaxis. The model was fit in WinBUGS, and posteriors obtained for model parameters a Metropolis–Hastings algorithm was used to simulate the posterior. Markov chain initially was not well behaved, and exhibited drift and trend, as well as high auto-correlations after 1000 iterations. However, after running the chain for a longer burn-in period and thinning every tenth draw, an excellent sample was obtained.
