Abstract
Objective
Evaluate systemic therapy utilization patterns and outcomes by line of therapy among patients with advanced/recurrent endometrial cancer (EC) treated in the United States.
Methods
This retrospective observational study used the Optum Clinformatics Extended Data Mart Date of Death database (1 January 2004–31 December 2019) and included de-identified data from adult patients with advanced/recurrent EC who were treated with first-line (1L) platinum-based chemotherapy and initiated second-line (2L) anti-neoplastic therapy. The index date was the date of 1L therapy initiation. The number and sequence of treatments received and the proportion of patients who received each type of treatment for each line of therapy were evaluated. To account for new drug approvals, patients first treated in 2018 or 2019 were also assessed separately.
Results
Among the 1317 patients who met all eligibility criteria, 520 (39.5%) and 235 (17.8%) patients received 3 or 4+ lines of treatment, respectively, during a median total follow-up time of 25.2 months (range, 2.5–173.3 months) following the index date. Chemotherapy, including platinum- and non-platinum-based regimens, was the most common treatment across all lines of therapy: 2L, 80.0%; 3L, 66.2%; 4L+, 80.4%. Overall, 2.5%, 2.3%, and 8.9% of 2L, 3L, and 4L + patients, respectively, received anti-program death 1 (anti-PD-1) immunotherapies. In patients first treated in 2018 and 2019 (n = 163), 9.8% of patients received anti-PD-1 immunotherapy in the 2L. In the overall population, median time to next treatment (TTNT) was 19.3, 10.5, and 8.1 months for patients undergoing 2L, 3L, and 4L treatment, respectively.
Conclusions
Among patients with advanced/recurrent EC treated with 1L platinum-based therapy in clinical practice, chemotherapy was the most common treatment choice across all lines of therapy. Immunotherapy use was low overall but increased in patients who started treatment in 2018 or 2019. Overall, median TTNT decreased as lines of therapy increased.
Introduction
Endometrial cancer is one of the most common gynecologic cancers worldwide and the fourth most commonly diagnosed cancer in women in the United StatesCitation1,Citation2. In the United States, it is estimated that there will be 66,570 new diagnoses and 12,940 deaths from endometrial cancer in 2021 aloneCitation2. In contrast to other common cancers, the incidence and mortality rates for endometrial cancer have increased in recent years, with the incidence increasing at a rate of approximately 1% per year and mortality increasing from 0.3% between 1997 and 2008 to 1.9% between 2008 and 2018Citation2,Citation3. Although most patients with endometrial cancer are diagnosed with early-stage disease and are successfully treated with surgery with or without adjuvant radiation, approximately 10–15% of women will present with advanced-stage disease at diagnosis, which is associated with a worse prognosis, an increased likelihood of recurrence, and limited treatment optionsCitation3. Regardless of systemic treatment regimen, the median overall survival for patients with advanced or recurrent endometrial cancer treated in clinical trials is approximately 1 yearCitation3.
Patients with advanced or recurrent endometrial cancer are most commonly treated with chemotherapy, with platinum-based regimens being preferred for first-line treatmentCitation3. Recent advances in understanding the genetic and molecular biology of endometrial cancer have expanded the therapeutic landscape to include novel targeted therapies, including everolimus, bevacizumab, and trastuzumabCitation3,Citation4. Immune checkpoint inhibitors that target the programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) pathway in patients with mismatch repair-deficient tumors have demonstrated efficacy alone and in combination with antiangiogenic treatments such as lenvatinibCitation3,Citation5,Citation6. Following pembrolizumab’s 2017 approval for patients with unresectable or metastatic microsatellite instability-high or mismatch repair-deficient solid tumors (including endometrial cancer), the first endometrial cancer-specific approval for a PD-1 inhibitor occurred in 2019 with the approval of pembrolizumab in combination with lenvatinibCitation7. In addition, trials evaluating the efficacy of poly (ADP ribose) polymerase (PARP) inhibitors alone and in combination with immunotherapy are ongoing and may further change the treatment landscapeCitation3,Citation6.
Although the availability of biomarker-directed systemic therapies holds promise for improving outcomes in women with advanced or recurrent endometrial cancer, limited information is available regarding their use and impact in real-world clinical practice. Therefore, we conducted a retrospective observational study using real-world data to assess treatment patterns and outcomes by line of therapy in patients with advanced or recurrent endometrial cancer who were treated with a first-line platinum-based chemotherapy in the United States.
Methods
Study design and analysis database
This retrospective observational study was designed to assess treatment patterns by line of therapy in patients with advanced/recurrent endometrial cancer who were treated with a first-line platinum-based chemotherapy and initiated second-line anti-neoplastic therapy. It was conducted using deidentified patient-level administrative claims data from Optum’s deidentified Clinformatics Data Mart Database (1 January 2004–31 December 2019), which includes both commercial and Medicare Advantage health plan data from over 60 million unique lives spanning all 50 states. The database includes verified, adjudicated, adjusted, and de-identified administrative claims submitted for payment by providers and pharmacies; clinical data not essential for billing or compensation are not captured.
Patient population
Adult patients aged ≥18 years with a diagnosis of endometrial cancer were identified by International Classification of Diseases Ninth Revision, Clinical Modification (ICD-9-CM) codes 179.x and 182.x and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes C54 and C55. To ensure that patients with EC were properly identified, patients were required to have ≥2 diagnoses for endometrial cancer >30 days apart, including 1 diagnosis before the date of initiation of first-line therapy with a platinum-based anti-neoplastic agent (index date).
All patients were required to have documented first-line treatment with a platinum-based regimen (i.e. carboplatin, cisplatin, or oxaliplatin) and initiated second-line treatment with chemotherapy, immunotherapy, or a targeted therapy agent (see Supporting Information Table S1 for a list of generic product identifier [GPI] and Healthcare Common Procedure Coding System [HCPCS] codes for anti-neoplastic agents). Patients who received surgery alone (i.e. without ≥2 lines of anti-neoplastic therapy) were excluded. Given the absence of disease stage or recurrence information in claims data, the initiation of a second line of therapy was used as a proxy to identify patients with advanced or recurrent endometrial cancer. To ensure that the anti-neoplastic therapy did not occur in response to indications other than endometrial cancer, all patients were required to have ≥12 months of continuous enrollment without any use of anti-neoplastic agents (except hormonal agents) before the start of first-line treatment. Patients were also required to have ≥1 claim for an endometrial cancer-related surgery before the index date (i.e. hysterectomy [subtotal, total, radical, or unspecified], salpingo-oophorectomy, or total hysterectomy plus bilateral salpingo-oophorectomy plus omentectomy with or without lymphadenectomy including sentinel lymph node dissection; see Supporting Information Table S2 for a list of all ICD-9-CM, ICD-10-CM, Common Procedural Terminology [CPT], and HCPCS codes). At least 30 days of continuous eligibility after the initiation of the last observed line of treatment were also required.
Treatment-line identification
The first claim for a platinum-based anti-neoplastic agent was identified as the initiation of first-line therapy. The index date was defined as the date of initiation of first-line therapy with a platinum-based anti-neoplastic agent. Both monotherapy and combination therapy regimens were considered. To identify combination therapy, all anti-neoplastic agents, with the exception of hormonal agents, received in a window of 30 days from the initiation of first-line therapy were included in the first-line therapy regimen. Hormonal agents were excluded from the identification of lines of therapy because they were not considered distinct lines of therapy (see Supporting Information Table S3 for a list of GPI and HCPCS codes). The end of the first-line therapy and the initiation of the second and subsequent lines of therapy was defined as (1) the day prior to the initiation of a new anti-neoplastic agent (excluding hormonal agents) that was not part of the first-line regimen or (2) the resumption of the same treatment regimen after a gap of more than 120 days (re-treatment).
The date of initiation of the second and subsequent lines of therapy (either in monotherapy or combination therapy) was defined as the date of initiation of a new anti-neoplastic agent (excluding hormonal agents) that was not part of the prior line of therapy or at the resumption of the same treatment regimen after a gap of more than 120 days (re-treatment). Similar to first-line therapy, a window of 30 days was used to identify all anti-neoplastic agents used as part of the line of therapy regimen. For all treatment-line determinations, counting for the 120-day gap started on the first day after the last day of supply of the therapy regimen. If another line of therapy was not observed in the data, the end of the second-line episode was defined as the end of continuous eligibility, death, loss to follow-up, or end of data availability, whichever occurred first.
Study exposure
The study exposure was the first claim for a platinum-based anti-neoplastic agent identified using HCPCS codes for medical claims or National Drug Codes (NDCs) for pharmacy claims (see Supporting Information Table S1). The observation period was defined as the period from the index date until the end of continuous eligibility, death, loss to follow-up, or end of data availability, whichever occurred first.
Outcomes
The primary endpoint of the analysis was to evaluate treatment patterns as defined by the number of lines of treatment that patients received, the sequence of treatments received, and the proportion of patients who received each type of treatment for each line of therapy. Treatment patterns were reported overall and among the subset of patients with an index date in 2018 or 2019.
Secondary endpoints included evaluation of the duration of treatment for each line of therapy, the duration of the platinum-free interval, the duration of the treatment-free interval, and the time to next treatment for each line of therapy. The duration of a line of therapy was defined as the period from the date of initiation of the line of therapy until the initiation of the next line of therapy or the end of continuous eligibility, death, loss to follow-up, or end of data availability, whichever occurred first; as such, each line of therapy included the period during which the patient was on treatment and the treatment-free interval. Substitution of paclitaxel or docetaxel for protein-bound forms (or vice versa) or substitution among carboplatin, cisplatin, and oxaliplatin was not considered a switch to the next line of therapy.
The duration of the treatment-free interval (or the platinum-free interval for platinum-based lines of therapy) was defined as the time from the last day of supply of the medications used as part of the treatment regimen to the date of initiation of the next line of therapy. For secondary endpoints, all outcomes were reported by line of therapy (i.e. first-, second-, third-, fourth-line, and subsequent lines of therapy). Overall survival following initiation of second-line treatment was also assessed.
Statistical analysis
All analyses were conducted using SAS Enterprise Guide, Version 7.15, or its latest version (SAS Institute, Cary, NC). A fixed 12-month period prior to the index date was used to assess baseline comorbidities, and a ≥ 12-month period of variable length was used to assess treatments previously received (i.e. previously received surgery, radiotherapy, or hormonal therapy) and time from first observed endometrial cancer diagnosis to the index date. For subsequent lines of therapy, treatments previously received and comorbidities were assessed both during a fixed 12-month period prior to the initiation of the line of therapy and between the start of the previous line of therapy and the initiation of the current line of therapy.
The number of lines of therapy was evaluated during the entire observation period. Treatment sequencing was described using a sunburst chart and descriptive table to report the type of treatment regimen received, stratified by line of therapy. The durations of active treatment and platinum-free/treatment-free intervals were evaluated descriptively for each line of therapy. Means, standard deviations, and medians were reported for continuous variables, and counts and percentages were reported for categorical variables. To account for censoring as well as the number and timing of patients who experienced progression to the next line of treatment, Kaplan–Meier (KM) survival curves (i.e. time to event analyses) were used to describe the KM rates of time to next treatment by line of therapy. For patients with a next line of therapy (i.e. patients with an event), time to next treatment was calculated as the time from the initiation of a line of therapy to the date of initiation of the next line of therapy. Patients without a next line of therapy were censored. For these patients, time to censoring was calculated as the time from the initiation of a line of therapy until the end of continuous eligibility, death, loss to follow-up, or end of data availability, whichever occurred first.
Overall survival following initiation of second-line treatment was defined as the time between the initiation of second-line treatment therapy and the date of death. Patients alive at the end of the observation period were censored. Because only the month and year of death were available in the Death Master File, the day of death was imputed as the first day of the month of death. To account for potentially incomplete death data, a sensitivity analysis was conducted whereby overall survival was calculated by excluding patients for whom there was no record of death but who may have been lost to follow-up (i.e. no claims in the last 3 or 6 months of the patient’s continuous eligibility period).
Study conduct
This study complied with all applicable laws regarding patient privacy, and no direct participant contact or primary collection of individual human participant data occurred. Informed consent, ethics committee, and institutional review board approval were not required because all data omitted patient identification information.
Results
Study population
In total, 1317 patients with advanced or recurrent endometrial cancer who were treated with a first-line platinum-based chemotherapy, initiated second-line anti-neoplastic therapy, and met all selection criteria were included in the analysis (). The mean age at first-line treatment initiation (index date) was 65.9 years (). The highest proportions of patients were treated in the South (38.9%, 512/1317) and the Midwest (27.6%, 364/1317). The median time between the first endometrial cancer diagnosis and initiation of first-line therapy was 2.4 months. At any time prior to first-line treatment initiation, 14.4% of patients received radiotherapy, with brachytherapy being most common. In total, 8.0% of patients received any type of hormonal treatment prior to initiation of first-line therapy, and use of hormone treatment increased with each line of therapy; megestrol acetate and aromatase inhibitors were the most commonly used hormonal agents across all lines of therapy. In the overall population, the median total follow-up time was 25.2 months following the index date. During this time, 39.5% (520/1317) of patients received three lines of treatment, and 17.8% (235/1317) of patients received four or more lines of treatment.
Figure 1. Patient attrition chart for the selection of patients with advanced/recurrent endometrial cancer who were treated with a first-line platinum-based chemotherapy and initiated second-line anti-neoplastic therapy. aPatients must not have any record of treatment with an anti-neoplastic agent (excepting hormonal agent) in the 12 months before 1L treatment initiation. bPlatinum-based regimens included carboplatin, cisplatin, and oxaliplatin. cInitiation of 2L treatment with a chemotherapy, immunotherapy, or targeted therapy agent. dEndometrial cancer-related surgery included hysterectomy (subtotal, total, radical, or unspecified), salpingo-oophorectomy, or total hysterectomy + bilateral salpingo-oophorectomy + omentectomy with or without lymphadenectomy including sentinel lymph node dissection. Abbreviations. 1L, first-line; 2L, second-line; ICD-9-CM/ICD-10-CM: International Classification of Disease, Ninth/Tenth Revision, Clinical Modification.
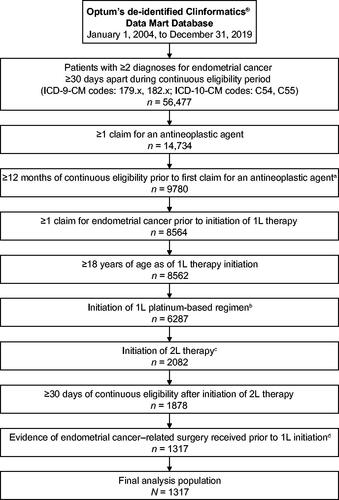
Table 1. Demographic and clinical characteristics prior to the initiation of each line of therapy.
Treatment patterns, overall population
For the overall population, the percentages of patients who received each type of treatment and each agent are detailed in , and displays the order of the types of treatments received. All patients received first-line platinum-based chemotherapy, with 83.8% (1103/1317), 14.5% (191/1317), and 1.7% (23/1317) of patients treated with carboplatin-, cisplatin-, and oxaliplatin-based regimens, respectively. The median first-line treatment duration was 9.5 months, which included a median of 4.4 months of treatment and 4.5 months of a platinum-free interval.
Figure 2. Treatment sequence for recurrent, metastatic, or high-risk endometrial cancer in (A) the overall population (N = 1317) and (B) patients who were indexed in 2018 or 2019 (N = 163). Each ring represents one line (L) of therapy, starting with first-line treatment in the innermost ring. Abbreviations. 1L, first-line; 2L, second-line; 3L, third-line; 4L, fourth-line; PD-1, programmed death 1; PD-L1, programmed death ligand 1.
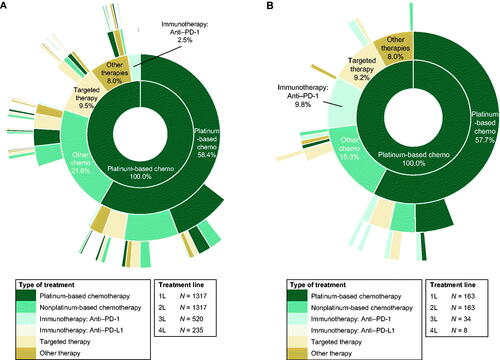
Table 2. Duration and type of treatment for advanced or recurrent endometrial cancer, overall population.
Per the study design, all patients were required to initiate second-line treatment to be included in the analysis. The most common second-line treatment was chemotherapy, with 58.4% (769/1317) of patients being re-treated with a platinum-based regimen and 21.6% (284/1317) of patients receiving a non-platinum-based regimen. For second-line therapy, the remaining patients were treated with targeted therapies (125/1317, 9.5%), other therapies (106/1317, 8.0%), anti-PD-1 immunotherapies (33/1317, 2.5%), or anti-PD-L1 immunotherapies (1/1317, 0.1%; ). Within the targeted therapies category, bevacizumab-based treatment was the most common (104 of 125 patients); pembrolizumab was the most common anti-PD-1 immunotherapy (31 of 33 patients). The median second-line treatment duration was 7.6 months, which included a median duration of treatment of 3.1 months and a treatment-free interval of 2.8 months. The treatment-free interval duration varied based on treatment regimen, with patients re-treated with a platinum-based therapy experiencing a median platinum-free interval duration of 5.5 months compared with a treatment-free interval duration of 0.9 months for patients treated with non-platinum-based regimens in the second line ().
A total of 520 patients subsequently received third-line treatment. The most common third-line treatment was chemotherapy, with 33.7% (175/520) and 32.5% (169/520) of patients receiving platinum- and non-platinum-based regimens, respectively. The remaining patients were treated with targeted therapies (95/520, 18.3%), other therapies (69/520, 13.3%), anti-PD-1 immunotherapies (12/520, 2.3%), or anti-PD-L1 immunotherapies (1/520, 0.2%; ). For targeted therapies, bevacizumab-based treatment was the most common (84 of 95 patients); for anti-PD-1 immunotherapies, pembrolizumab was the most common agent (11 of 12 patients). The median third-line treatment duration was 5.1 months, which included a median of 3.3 months of treatment and 0.5 months of a treatment-free interval. For patients treated with a platinum-based chemotherapy regimen in the third line, the median platinum-free interval duration was 1.3 months; for patients treated with non-platinum-based regimens in the third line, the treatment-free interval duration was 0.3 months ().
A total of 235 patients received 4 or more lines treatment. For patients who received additional lines of treatment beyond fourth line, each line of treatment was counted once. The most common fourth-line treatment was chemotherapy, with 48.1% (113/235) and 32.3% (76/235) of patients receiving non-platinum- and platinum-based regimens, respectively. The remaining patients were treated with targeted therapies (76/235, 32.3%), other therapies (67/235, 28.5%), and anti-PD-1 immunotherapies (21/235, 8.9%); no patients received anti-PD-L1 immunotherapies. In patients who received targeted therapies, bevacizumab-based treatment was the most common (62 of 76 patients); in patients who received anti-PD-1 immunotherapy, pembrolizumab was the most common treatment (18 of 21 patients). The median fourth-line treatment duration was 4.5 months, which included a median of 3.1 months of treatment and 0.5 months of a treatment-free interval. For patients treated with a platinum-based chemotherapy regimen in the fourth line or beyond, the median platinum-free interval duration was 0.2 months; for patients treated with non-platinum-based regimens in the fourth line or beyond, the median treatment-free interval duration was 0.5 months ().
Treatment patterns, patients who initiated first-line treatment in 2018 or 2019
To account for changes in the treatment landscape for patients with advanced or recurrent endometrial cancer, treatment patterns were also assessed in the 163 patients who initiated first-line treatment in 2018 or 2019. In these patients, the overall median follow-up time was 15.2 months. During this time, 20.9% (34/163) received 3 lines of treatment and 4.9% (8/163) of patients received 4 or more lines of treatment. Details on treatment type and specific agent by line of therapy are presented in , and displays the order of the treatments received.
Table 3. Duration and type of treatment for advanced or recurrent endometrial cancer, patients with index date in 2018 or 2019.
In the subset of patients who initiated first-line treatment in 2018 or 2019, 78.5% (128/163), 19.0% (31/163), and 2.5% (4/163) of patients were treated with carboplatin-, cisplatin-, and oxaliplatin-based regimens as first-line treatment, respectively (). The most common second-line treatment was chemotherapy, with 57.7% (94/163) of patients being re-treated with a platinum-based regimen and 15.3% (25/163) of patients receiving a non-platinum-based regimen. For second-line therapy, the remaining patients were treated with anti-PD-1 immunotherapies (16/163, 9.8%), targeted therapies (15/163, 9.2%), other therapies (13/163, 8.0%), or anti-PD-L1 immunotherapies (1/163, 0.6%). Among the 34 patients with an index date in 2018 or 2019 who received third-line treatment, the most common third-line treatment was chemotherapy, with 29.4% (10/34) and 23.5% (8/34) of patients receiving platinum- and non-platinum-based regimens, respectively. The remaining patients were treated with targeted therapies (9/34, 26.5%), anti-PD-1 immunotherapies (5/34, 14.7%), or other therapies (2/34, 5.9%); no patients were treated with anti-PD-L1 immunotherapies. In patients who initiated first-line treatment in 2018 or 2019, bevacizumab-based treatment was the most common targeted therapy across all therapy lines examined. For anti-PD-1 immunotherapies, pembrolizumab-based treatment was the most common across therapy lines.
Time to next treatment and overall survival
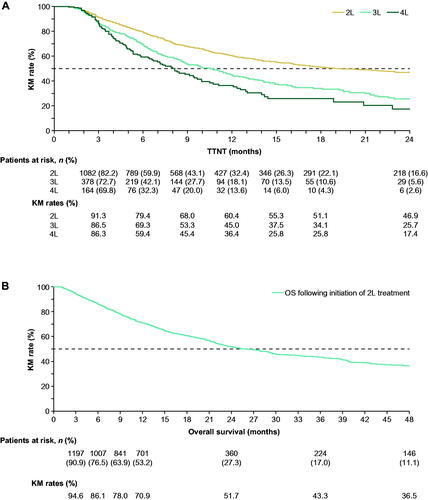
In the overall population, when accounting for censoring as well as the number and timing of patients who experienced progression to the next line of treatment, the KM-estimated median time to next treatment was 19.3 months from second- to third-line treatment, 10.5 months from third- to fourth-line treatment, and 8.1 months from fourth- to fifth-line treatment (). KM rates of overall survival following initiation of second-line treatment at 1, 2, 3, and 4 years were 70.9%, 51.7%, 43.3%, and 36.5%, respectively, with a median overall survival of 26.0 months (). Sensitivity analyses conducted to account for potentially incomplete death data resulted in similar findings.
Figure 3. (A) TTNT and (B) overall survival in the overall population. TTNT was measured as the time from the initiation of a line of therapy to the date of initiation of the next line of therapy. Patients who did not initiate a next line of therapy were censored at the end of continuous eligibility, death, loss to follow-up, or end of data availability, whichever occurred first. Overall survival was defined as the time between the initiation of second-line treatment and the date of death. Patients alive at the end of their eligibility period were censored. Abbreviations. 2L, second-line; 3L, third-line; 4L, fourth-line; KM, Kaplan–Meier; TTNT, time to next treatment.
Discussion
Although the treatment landscape for endometrial cancer has changed significantly in recent years with the introduction of immunotherapy and targeted agents, chemotherapy remains a mainstay of treatment. In addition, in patients with advanced endometrial cancer who experience recurrence or disease progression following first-line platinum-based chemotherapy, there is currently no preferred standard of care for additional lines of treatmentCitation3. To better understand how these patients are being treated in clinical practice and how newer therapies are being incorporated across treatment lines, we evaluated treatment patterns and outcomes by line of therapy in patients with advanced or recurrent endometrial cancer treated in the United States, using deidentified data from Optum’s CDM database.
In the overall population, re-treatment with chemotherapy was the most common treatment approach to second-line therapy, accounting for over 80% of patients. In particular, almost 60% of patients were re-treated with a platinum-based chemotherapy regimen for second-line therapy. These results are consistent with a retrospective analysis of a Japanese health insurance database that found that over 60% of patients with endometrial cancer who received paclitaxel/carboplatin chemotherapy for first-line treatment were re-treated with a platinum-based chemotherapy regimen as second-line therapyCitation8. In our analysis, chemotherapy was also the most common treatment option in the third and fourth-line settings, although the use of non-platinum-based chemotherapy regimens increased with each subsequent line of treatment as the use of platinum-based regimens decreased.
The reliance on re-treatment with platinum-based chemotherapy is notable, however, when reviewed in the context of the platinum-free treatment interval. Similar to findings in ovarianCitation9,Citation10 and cervical cancerCitation11, studies in patients with recurrent endometrial cancer have indicated that the duration of the platinum-free treatment interval following first-line treatment is an important predictor of responsiveness to platinum-based re-treatmentCitation12–14. In one retrospective study of Japanese patients with recurrent endometrial cancer, the response rate to second-line re-treatment with platinum-based chemotherapy ranged from 25% in patients with a platinum-free interval of less than 6 months to 61% in patients with a platinum-free interval of more than 12 monthsCitation14. In this analysis, re-treatment with platinum-based chemotherapy was common in the second line, despite a median platinum-free interval of 4.5 months following first-line treatment. In addition to the short duration of the platinum-free interval between treatment lines, the limited efficacy of re-treatment with chemotherapy was reflected in the median time to next treatment, which decreased with each treatment line overall. However, it is important to note that these findings were for the overall population; individual responses were not available as part of the study.
The improved understanding of the different genetic factors driving endometrial cancerCitation15 has expanded the treatment landscape for endometrial cancer beyond chemotherapy to include a number of targeted and immunotherapeutic treatment optionsCitation3,Citation4,Citation6. In this analysis, use of targeted therapies and anti-PD-1 immunotherapies increased with each line of treatment, but both were still used less frequently than chemotherapy across all treatment lines in the overall population. To account for the regulatory approval of biomarker-directed therapies that occurred during the study period, treatment patterns were also assessed in patients who received first-line treatment in 2018 or 2019. Platinum-based chemotherapy remained the most common second- and third-line treatment, but use of anti-PD-1 immunotherapies increased in both the second- and third-line settings compared with the overall population. The trend toward increased use of both anti-PD-1 immunotherapies and targeted therapies in the second-, third-, and fourth-line settings was consistent across both the overall population and in patients who received first-line treatment in 2018 or 2019. Currently, the literature assessing real-world treatment patterns in patients with advanced or recurrent endometrial cancer in the current treatment landscape with options beyond chemotherapy is limited. Available studies support a clinical benefit for immunotherapies and antiangiogenic treatments in specific patient populationsCitation16–18, but additional work is needed to ascertain how biomarker therapies are being used in current clinical practice. As more data become available and familiarity with targeted and immunotherapeutic treatment options increases, it will also be important to understand the factors driving treatment selection and the use of nonchemotherapy agents for patients with advanced or recurrent endometrial cancer.
Potential limitations of this study include the retrospective observational design and the limitations of the database itself. Database limitations include the inability to uniformly capture encounters outside the Optum network, inaccurate or missing coding data, and the lack of data from uninsured patients or patients with Medicaid or other types of health insurance, all of which may affect the generalizability of the results. Progression to subsequent lines of therapy was used as a proxy to identify patients with advanced or recurrent endometrial cancer; however, a platinum-based regimen may be used in other patient populations. The method used to identify patients with advanced or recurrent endometrial cancer may also have resulted in the selection of a specific patient population able to initiate second-line treatment because all patients who did not receive second-line treatment because of aggressive tumor biology or toxicity from first-line treatment were excluded. In addition, because the database was limited to administrative claims, relevant clinical information was not captured. The lack of data on histology, endometrial cancer stage, and clinical response to prior treatment may have impacted our findings as all are important factors in determining treatment approach. The lack of data on biomarkers is also notable as biomarker status can impact whether patients are candidates for specific targeted therapies and immunotherapies.
Accurate treatment line identification could have been undermined by the lack of visibility into clinical rationale for therapies received in patients because of the nature of administrative claims data. In addition, in patients who were in remission for an extended period, a second-line treatment could have mistakenly been identified as a first-line treatment despite the use of a 12-month washout period to identify the initiation of first-line therapy. The exclusion of hormone therapy as a distinct line of treatment may also have impacted the sequencing of treatments. In addition, it was not possible to confirm whether chemotherapy was used as adjuvant treatment because of the nature of the claims database. Underreporting of patient death data may also have affected overall survival results. Although the sensitivity analyses conducted to account for incomplete death data showed similar results, underreporting or misclassification of patients who died but were instead treated as having been lost to follow-up may have contributed to the longer than expected overall survival duration finding in our analysis. For the subgroup analysis of patients who initiated first-line treatment in 2018 or 2019, interpretation of the results is limited by the small number of patients, particularly those who went on to receive multiple treatment lines. Follow-up time in this subgroup was also shorter than in the overall population, which also could have influenced the number of patients with later lines of treatment. Given these limitations, extrapolation of our results to other patient populations should be approached with caution. Additional studies with more patients and longer follow-up times will be needed to confirm the treatment trends identified in these patients.
This real-world study highlights the lack of a standard of care; the worsening of outcomes, such as time to next treatment, for women with endometrial cancer that progresses following platinum-based therapy; and the limited use of promising emerging therapies, including immunotherapies. As new mono and combination therapies are developed and the understanding of genomics and its relationship to therapeutic options in the context of endometrial cancer evolve, there are likely to be opportunities to further individualize care and improve patient outcomes with fewer side effects in later lines of therapy.
Transparency
Declaration of funding
This study was supported by GSK.
Declaration of financial/other relationships
BE reports consulting fees from Janssen Scientific Affairs, Novartis, Pfizer, and Pharmacyclics. MHL reports consulting fees from Janssen Scientific Affairs, Pfizer, and Pharmacyclics. PL reports consulting fees from Actelion, Janssen Scientific Affairs, Pfizer, Pharmacyclics, and Regeneron. IG reports consulting fees from Janssen Scientific Affairs, Novartis, and Regeneron. CW reports consulting fees from Janssen Scientific Affairs. PHT reports institutional grants from GSK and Merck; and personal fees from AstraZeneca, Celsion, GSK, Iovance, Novocure, and Seagen. BE, MHL, PL, and IG, and CW are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to GSK, which funded the development and conduct of this study and manuscript. JL and JAH are current employees of GSK. EMM is a former employee of GSK. CW is a former employee of Analysis Group, Inc. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
JL, EM, JAH, PHT conceived and designed the study. JL provided administrative support. BE, MHL, PL, IG, and CW analyzed the data; all authors interpreted the data. All authors participated in writing the manuscript, provided final approval of the manuscript, and are accountable for all aspects of the work.
Previous presentation
These data were originally presented at the American Society of Clinical Oncology Quality Care Symposium (ASCO-QCS) Annual Meeting, 24 and 25 September 2021.
Ethics statement
Optum’s Clinformatics Extended Data Mart is statistically de-identified under the Expert Determination method consistent with the Health Insurance Portability and Accountability Act (HIPAA) and managed according to Optum customer data use agreements.
TxPatternsECOptMs_CMRO_19Jan2022_Suppl_Materials.docx
Download MS Word (52.7 KB)Acknowledgements
Medical writing and editorial assistance, funded by GSK (Waltham, Massachusetts) and coordinated by Hasan H. Jamal, MSc, of GSK, were provided by Betsy C. Taylor, PhD, CMPP, and Jennifer Robertson, PhD, of Ashfield MedComms, an Inizio company (Middletown, Connecticut).
Data availability statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
- Brooks RA, Fleming GF, Lastra RR, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69(4):258–279.
- Di Tucci C, Capone C, Galati G, et al. Immunotherapy in endometrial cancer: new scenarios on the horizon. J Gynecol Oncol. 2019;30(3):e46.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38(26):2981–2992.
- Post CCB, Westermann AM, Bosse T, et al. PARP and PD-1/PD-L1 checkpoint inhibition in recurrent or metastatic endometrial cancer. Crit Rev Oncol Hematol. 2020;152:102973.
- Gravbrot N, Gilbert-Gard K, Mehta P, et al. Therapeutic monoclonal antibodies targeting immune checkpoints for the treatment of solid tumors. Antibodies (Basel). 2019;8(4):51.
- Akada K, Koyama N, Miura T, et al. Real-world database analysis of the characteristics and treatment patterns of patients with endometrial cancer in Japan. Curr Med Res Opin. 2021;37(7):1171–1178.
- Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9(3):389–393.
- Blackledge G, Lawton F, Redman C, et al. Response of patients in phase II studies of chemotherapy in ovarian cancer: implications for patient treatment and the design of phase II trials. Br J Cancer. 1989;59(4):650–653.
- Matoda M, Tanigawa T, Omatsu K, et al. Platinum-free interval in second-line chemotherapy for recurrent cervical cancer. Int J Gynecol Cancer. 2013;23(9):1670–1674.
- Matoda M, Omatsu K, Yamamoto A, et al. Importance of platinum-free interval in second-line chemotherapy for advanced or recurrent endometrial cancer. Eur J Gynaecol Oncol. 2014;35(3):224–229.
- Rubinstein M, Halpenny D, Makker V, et al. Retreatment with carboplatin and paclitaxel for recurrent endometrial cancer: a retrospective study of the Memorial Sloan Kettering Cancer Center experience. Gynecol Oncol Rep. 2019;28:120–123.
- Nagao S, Nishio S, Michimae H, et al. Applicability of the concept of "platinum sensitivity" to recurrent endometrial cancer: the SGSG-012/GOTIC-004/intergroup study. Gynecol Oncol. 2013;131(3):567–573.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
- Choi MC, Moon YW, Jung SG, et al. Real-world experience with pembrolizumab treatment in patients with heavily treated recurrent gynecologic malignancies. Yonsei Med J. 2020;61(10):844–850.
- Kuznicki ML, Bennett C, Yao M, et al. Predictors of response to immune checkpoint inhibition in a real world gynecologic cancer population. Gynecol Oncol Rep. 2020;34:100671.
- Rubinstein MM, Dickinson S, Narayan P, et al. Bevacizumab in advanced endometrial cancer. Gynecol Oncol. 2021;161(3):720–726.
