Abstract
Objective
Anemia is a common complication of chronic kidney disease (CKD). The aim of this study was to evaluate hemoglobin levels at the initiation of erythropoiesis stimulating agent (ESA) therapy in patients with non-dialysis-dependent CKD (NDD-CKD) and anemia using a large-scale administrative database in Japan.
Methods
The longitudinal data of adult patients who initiated ESA therapy between April 2008 and December 2018 were extracted from a hospital-based administrative database. The primary outcome was hemoglobin level at the initiation of ESA therapy, whereas the exploratory outcome was hemoglobin level recorded 6 months after the onset of the ESA therapy.
Results
A total of 4939 patients were included in the primary analysis. The mean hemoglobin level at the initiation of ESA therapy was 9.1 g/dL, which was lower than the level (11 g/dL) recommended for the initiation of treatment by the current Japanese treatment guidelines. Moreover, 42.1% and 15.0% of the patients had hemoglobin levels <9.0 and <8.0 g/dL, respectively, at the initiation of ESA therapy. In 2964 patients for whom hemoglobin levels at 6 months after the initiation of ESA therapy were available, the mean hemoglobin level increased to 10.3 g/dL, and 61.9% and 31.1% of these patients had hemoglobin levels ≥10.0 and ≥11.0 g/dL, respectively.
Conclusion
This real-world database study revealed that hemoglobin levels at the initiation of ESA therapy in new users of ESA were lower than those recommended by treatment guidelines in Japan.
Introduction
The prevalence and severity of anemia, which is a common complication of chronic kidney disease (CKD)Citation1–3, increases as kidney function decreases in patients with non-dialysis-dependent CKD (NDD-CKD)Citation4. Comorbid anemia in CKD is associated with not only a lower quality of life, but also a higher risk of cardiovascular disease and further decline in kidney functionCitation5. Currently, erythropoiesis stimulating agents (ESAs) are the standard care for the treatment of anemia in patients with CKD.
Many countries refer to the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelineCitation6, which states that, in the treatment of renal anemia in patients with NDD-CKD, the decision to initiate ESA therapy should be made when hemoglobin concentration falls below 10 g/dL, after considering the condition of each individual patient. In Japan, the hemoglobin treatment criteria are different from those of KDIGO-guideline. In a previous clinical study of the treatment of Japanese patients with anemia and NDD-CKD, the risk of renal composite outcomes was significantly lower at a target hemoglobin level of 11–13 g/dL compared with that observed at a target hemoglobin level of 9–11 g/dLCitation7. Therefore, the 2008 and 2015 Japanese Society for Dialysis Therapy (JSDT) guidelines propose the initiation of ESA therapy in patients with NDD-CKD with a hemoglobin level <11 g/dL and a target hemoglobin level of 11–13 g/dLCitation8,Citation9. In addition, the Japanese Society of Nephrology (JSN), which initially proposed target hemoglobin levels of 10–12 g/dL for ESA therapy in patients with CKD in 2012, revised the guideline to raise the target levels to 11–13 g/dL for anemia in patients with NDD-CKD in 2018Citation10,Citation11.
The actual clinical condition of the treatment of anemia in Japanese patients with NDD-CKD has been investigated in several studiesCitation12–16. For example, a prospective cohort study of patients with NDD-CKD reported that up to 70% of patients with hemoglobin levels <11 g/dL were not on ESA therapy, and suggested that an insufficient awareness of anemia accounts for the lack of treatment of this condition in the early stages of CKDCitation12. However, to the best of our knowledge, no real-world database studies have focused on hemoglobin levels at the initiation of ESA therapy, and adherence to these guidelines in clinical practice remains uncertain. Therefore, using a large-scale, hospital-based administrative database in Japan, the present study aimed to determine the hemoglobin levels at the initiation of ESA therapy in anemic patients with NDD-CKD who were newly started on ESAs.
Methods
Data sources
This was a retrospective real-world database study using longitudinal data extracted from a hospital-based administrative claims database provided by Medical Data Vision Co. Ltd. (MDV, Tokyo, Japan). The database included electronic administrative health insurance claims and diagnosis-procedure combination (DPC) data from approximately 400 acute medical care hospitals representing approximately one-fifth of hospitals with DPC-based bundles payment systems in Japan. Data were collected for demographic and clinical characteristics, drug prescription information, and laboratory tests for anemia in patients with NDD-CKD, including those who were 65 years or older, during the study period between 1 April 2008 and 31 December 2018 (referred to as the “study period”) ().
Figure 1. Study design. Briefly, Hb levels of new ESA users extracted from a hospital-based administrative database were analyzed at the initiation of ESA therapy for the primary outcome. Secondary outcomes were patient and disease characteristics at the initiation of ESA, and exploratory outcome was the mean hemoglobin level after six months. Abbreviations. ESA, Erythropoiesis stimulating agent; Hb, Hemoglobin; MDV, Medical Data Vision.
Study cohort
For the main cohort, patients fulfilling the following criteria were selected from the MDV database: (1) at least one ESA prescription (Anatomical Therapeutic Chemical [ATC] code B03C0) within the study period, with the first date of ESA therapy designated as the index date; (2) at least one recorded hemoglobin value within 30 days before the index date; (3) at least six months of look-back period before the index date; (4) at least two hospital visits during the look-back period; and (5) age ≥18 years at the index date. The inclusion criterion “at least two hospital visits during the look-back period” was used to selectively collect data on ESA-naïve patients. Patients meeting any of the following criteria were excluded from the analyses: (1) diagnosis of cancer or disseminated intravascular coagulation or history of radiation therapy, kidney transplantation, or dialysis at the index date or during the look-back period before the index date; (2) record of autologous blood storage at or 14 days after the index date; or (3) diagnosis of sepsis during the look-back period.
Patients with available hemoglobin levels six months after the index date who did not meet the exclusion criteria were included as a sub-cohort for exploratory analysis regardless of their continuation with ESA therapy after the index date. For the analysis of ESA dosing and frequency, patients who switched from one ESA to another during the follow-up period were excluded. Details of the codes used in the present study are provided in Supplemental Tables S1, S2 and S3.
Outcomes
The primary outcome was hemoglobin level at the index date. Secondary outcomes were patient and disease characteristics at the index date, including diagnostic rate of kidney diseases, type of ESA, concomitant medications and laboratory test values. The diagnosis was determined based on the International Classification of Diseases 10 (ICD-10) code in the month containing the index date. The concomitant medications were captured using the ATC classification system code within 90 days before or at the index date. For laboratory test values including hemoglobin, the latest value within 30 days before or at the index date was obtained. Index hemoglobin levels were stratified by sex, estimated glomerular filtration rate (eGFR) category (≥60, 45 to <60, 30 to <45, 15 to <30, <15 mL/min/1.73 m2), patient age (<65, 65 to <75, ≥75 years), presence of concomitant antidiabetic drugs and the type of hospital visits (outpatients or inpatients). Inpatients were judged by the information as of the index date. Blood transfusions within 7 days before the index date were captured using a procedure code.
Maintenance hemoglobin levels 180 (±45) days after the index date were analyzed exploratorily for only patients with available hemoglobin levels six months after the index date. Previous Japanese clinical studies on ESAsCitation17,Citation18 have shown that hemoglobin levels increased and plateaued in less than 24 weeks in anemic NDD-CKD patients. Therefore, the six-month follow-up period in this study was considered adequate for the evaluation of maintenance hemoglobin levels. ESA dose and frequency of prescription during the follow-up period were also evaluated.
Statistical analysis
This study is descriptive and has not been tested in any statistical tests. Descriptive analyses were performed using numbers and percentages for categorical values, and quantitative data were presented as means ± standard deviation (SD) and medians. All data analyses were performed using SAS statistical software version 9.2 (SAS Inc., Cary, NC, USA).
Ethics
The study protocol was approved by the Ethics Review Committee of Mitsubishi Tanabe Pharma Corporation and informed consent was waived by the committee (Approval number H-19-015). Because this is an observational study using an anonymized medical database, informed consent is not required for research purposes, according to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Results
Study population and patient characteristics
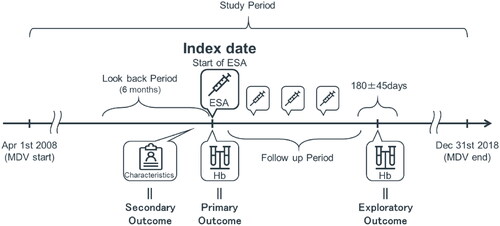
As shown in , a total of 21872 patients who were prescribed ESAs and had at least one recorded hemoglobin value within 30 days before the index date were extracted from the MDV database. Of these, 4939 patients who met the selection criteria were included in the primary analysis (main cohort). The characteristics of the main cohort are summarized in . The mean age was 72.8 years at the initiation of ESA therapy. Long-acting ESAs were chosen as the first treatment in most patients, with darbepoetin alfa (52.3%) as the most common ESA, followed by epoetin beta pegol (40.6%). Most patients had a diagnosis associated with kidney disease on the index date. The diagnosis rates for chronic kidney disease (ICD-10 code N18) or unspecified kidney failure (ICD-10 code N19), including the disease code for anemia in CKD, were 75.0% and 80.9%, respectively. Antihypertensive (84.6%), antihyperlipidemic (40.1%) and antidiabetic (39.0%) agents were recorded as concomitant medications. In addition, 17.8% of patients received iron supplementation. At the index date, the mean eGFR was 19.8 mL/min/1.73 m2, and 82.7% of the patients had an eGFR of <30 mL/min/1.73 m2. Other laboratory test results for the main cohort are shown in Supplemental Table S4, and 25.6% of these patients had ferritin level data, with a mean value of 220.7 ng/mL.
Figure 2. Patient selection diagram from a hospital-based administrative database. Of a total of 21872 patients extracted from the database, 4939 patients were included in the primary analysis (main cohort) and 2964 patients were included in the exploratory analysis (sub-cohort). Abbreviations. DIC, Disseminated intravascular coagulation; ESA, Erythropoiesis stimulating agent; Hb, Hemoglobin; ICD-10, International Classification of Diseases 10th edition; RT, Radiation therapy.
Table 1. Demographic characteristics of patients in the main cohort and sub-cohort at the index date and during look-back period.
Hemoglobin levels six months after the index date were available for 2964 patients (60.0% of the main cohort) who were included in the sub-cohort. The characteristics of the patients were similar in the main cohort and sub-cohort, as shown in .
Hemoglobin level at the initiation of erythropoiesis stimulating agent therapy
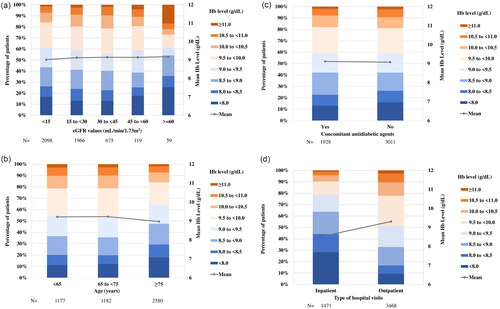
In the main cohort, the hemoglobin (mean ± SD) level at the index date was 9.1 ± 1.1 g/dL (, ). The proportion of patients with hemoglobin levels of <9.0 and <8.0 g/dL at the index date were 42.1% and 15.0%, respectively. Although there was no apparent difference in mean hemoglobin levels by sex (9.2 ± 1.1 g/dL in males and 9.0 ± 1.1 g/dL in females), the proportion of patients with hemoglobin levels of <9.0 g/dL were more commonly observed in females (48.3%) than in males (37.7%). Histograms of hemoglobin levels at the index date stratified by eGFR, age category, presence of concomitant antidiabetic drugs and the type of hospital visits (outpatients, inpatients) are shown in ). No apparent differences in mean hemoglobin levels or distribution patterns of hemoglobin were noted between subgroups except for the type of hospital visits, although no statistical tests were performed. Approximately 30% of patients were inpatients at the index date. Hemoglobin levels tended to be lower in inpatients (8.6 g/dL) than in outpatients (9.3 g/dL) (). Reasons for hospitalization at the index date were congestive heart failure (12.8% of the inpatients), chronic kidney failure (7.3%), chronic kidney disease stage G5 (4.6%), end-stage kidney disease (4.5%) and chronic congestive heart failure (3.3%). The proportion of patients who received blood transfusions within 7 days before the index date was 8.2% for inpatients and 0.5% for outpatients.
Figure 3. Histogram of Hb levels at the index date in the main cohort. The proportion of patients in each Hb category and mean Hb levels are presented for all patients, males, and females. Abbreviations. Hb, Hemoglobin; N, Number; SD, Standard deviation.
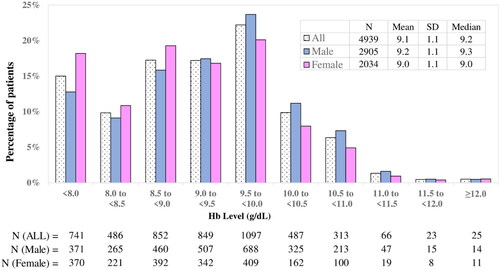
Figure 4. Histogram of Hb levels at the index date by subgroups. The distribution of Hb and the mean Hb levels at the index date were stratified by eGFR categories (a), patient age categories (b), the presence or absence of concomitant antidiabetic agents (c) and the type of hospital visits (d). Abbreviations. Hb, Hemoglobin; eGFR, Estimated glomerular filtration rate; N, Number.
Changes in hemoglobin levels and erythropoiesis stimulating agent dosage after the initiation of erythropoiesis stimulating agent therapy
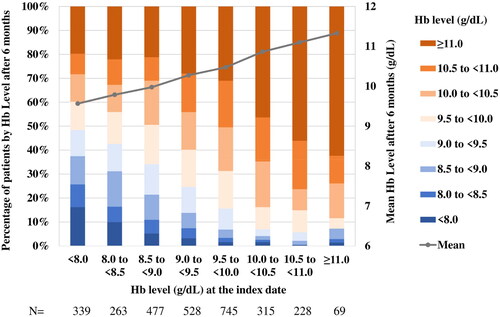
In the sub-cohort, the mean hemoglobin level increased from 9.2 ± 1.0 g/dL at the index date to 10.3 ± 1.4 g/dL after six months. The percentage of patients with hemoglobin levels of ≥10.0 and ≥11.0 g/dL were 61.9% and 31.1%, respectively, but only 20.6% and 2.3%, respectively, at the index date. The proportion of patients on ESA therapy was 76.4% 6 months after the index date (). During the follow-up period, the mean doses of darbepoetin alfa and epoetin beta pegol were 11.0 and 11.6 μg/week, respectively, with the average of 5.2 and 4.7 dosing frequency, respectively, over the six-month period (). In the sub-cohort, 17.6% of patients were on iron supplementation at the index date; after 6 months, the proportion of patients receiving iron therapy was 5.6% ().
Table 2. Hemoglobin levels and the number of patients on ESA or iron in the sub-cohort population at the index date and six months after the index date.
Table 3. Doses and frequencies of ESAs prescribed in the sub-cohort population during the follow-up period.
A trend for higher hemoglobin levels at six months was observed in patients with higher hemoglobin levels at the index date. Approximately half of the patients with hemoglobin levels of ≥10 g/dL at the index date achieved hemoglobin levels of ≥11 g/dL at the end of the six-month follow-up period ().
Discussion
We used the MDV database, which is one of the largest real-world medical databases available in Japan, to demonstrate for the first time that hemoglobin levels at the initiation of ESA therapy in new users of ESA with anemia secondary to NDD-CKD were lower than those proposed in the treatment guidelines. Because it was not known in advance whether the assignment of diagnostic codes for CKD or renal anemia was accurate, these codes were not used to identify patients with renal anemia in the present study. Rather, we first selected patients who had started ESA therapy and then excluded patients who had received ESAs for cancers or autologous blood donation to identify patients who received ESA for the purpose of treatment of renal anemia. The sample size identified in the present study was nearly 5000 patients, which was comparable to that of the post marketing surveillance (PMS) studies of long-acting ESAs in anemic patients with NDD-CKDCitation14,Citation15. Interestingly, the characteristics of the extracted patients were very similar to those of the PMS studies in terms of age, eGFR, male rate and use of concomitant medications. Therefore, the MDV database has been suitable for this purpose, and the findings of the present study can be considered as being representative of the real-world clinical experience in Japan.
The mean hemoglobin level at the onset of ESA therapy was 9.1 g/dL, with 42.1% of patients having a hemoglobin level <9.0 g/dL. These hemoglobin levels were lower than the treatment initiation value of 11.0 g/dL recommended by the JSDT guidelines. Although hemoglobin levels reportedly vary according to age, sexCitation9,Citation12 and the complication of diabetes mellitusCitation19,Citation20, there were no apparent differences in hemoglobin levels at the index date according to sex, age, eGFR category or concomitant antidiabetic agents in the study. Therefore, these factors are unlikely to be relevant to the large gap in the hemoglobin levels recorded at the initiation of ESA between clinical practice situation and guidelines. Hemoglobin levels of outpatients tended to be higher than those of inpatients, but the levels were both low, around 9 g/dL. A small proportion of inpatients received blood transfusions before starting ESA therapy, and this is unlikely to affect the present finding of low hemoglobin levels at the start of treatment for renal anemia in clinical practice. The risk of renal events has been reported to be significantly lower in an early intervention group initiated with ESA therapy at hemoglobin levels of ≥10 to <11 g/dL than in a late intervention group at hemoglobin levels of <9 g/dL in patients with NDD-CKDCitation21. Although it is desirable to intervene in cases of anemia before hemoglobin levels decrease to <9 g/dL, the lack of subjective symptoms of anemia and the burden of outpatient visits for ESA injections may delay the initiation of ESA therapy in patients with NDD-CKD.
In the present study, the mean maintenance hemoglobin level recorded at 6 months after the index date in the sub-cohort was 10.3 g/dL. Similar results were reported both in the PMS of darbepoetin alfa, where the maintenance hemoglobin level was 10–10.6 g/dLCitation14, and in a university hospital electronic medical record database (the Japan Chronic Kidney Disease Database [J-CKD-DB]), which showed that mean maintenance hemoglobin level in patients with CKD stages G4 and G5 patients receiving ESA was 10.1 g/dLCitation16. Only approximately 30% of the patients included in the present study achieved the lower limit of 11.0 g/dL, which is the target maintenance hemoglobin level proposed in the JSDT guidelines. However, the achievement rate was above 60% based on the criteria for target maintenance hemoglobin levels of 10.0 g/dL recommended by the previous JSN guidelineCitation9 or the current KDIGO clinical practice guidelineCitation6. The maintenance hemoglobin levels in clinical practice appear to be determined considering safety concerns associated with targeting higher hemoglobin levels with high doses of ESAsCitation22,Citation23 and taking into account guidelines that recommend lower maintenance hemoglobin levels compared with JSDT.
Our study had several limitations. First, the database used in this study contains patient data from acute medical care hospitals exclusively, but not those from clinics. The population of the present study was similar to that of the PMS study of long-acting ESAs, but may not be representative of all patients with anemia and CKD in clinical practice. Second, the database lacks laboratory data for transferrin saturation, and ferritin values were available in only a quarter of patients at the index date. Therefore, the relationship between iron status and treatment for anemia could not be analyzed in this study. Finally, the database could not provide individual treatment records from other institutions. Therefore, the dose of ESA during the follow-up period may have been underestimated.
Despite these limitations, the findings of the present study, which included approximately 5000 patients with NDD-CKD and anemia, are expected to reflect actual clinical practice and provide insights into the management of anemia. Target hemoglobin levels in clinical practice might have changed during the study period, because the JSN guideline for the treatment of anemia was revised in 2018Citation11, and the target hemoglobin level in patients with NDD-CKD has been increased to 11–13 g/dL, which agrees with the JSDT guideline; however, mean hemoglobin levels for treatment initiation remained around 9.1 g/dL over time, and no clear change trend was observed during the study period (data not shown). Therefore, it is important to continue to investigate whether the recommendations in these guidelines are adhered in clinical practice.
Conclusions
In conclusion, this large-scale, real-world database study revealed that hemoglobin levels at the initiation of ESA therapy in Japanese anemic patients with NDD-CKD were 9.1 g/dL, which were lower than those suggested in treatment guidelines.
Transparency
Declaration of funding
This study was funded by Mitsubishi Tanabe Pharma Corporation Ltd. The sponsor funded the medical writing for preparation of the manuscript.
Declaration of financial/other relationships
Y.K., M.Ish. and K.U. have disclosed that they are employed by Mitsubishi Tanabe Pharma Corporation. M.Iss. and S.D. have disclosed that they are employed by IQVIA Solutions Japan KK. H.Y., H.K. and H.M. have disclosed that they received consultant fees from Mitsubishi Tanabe Pharma Corporation for this research project and are affiliated with the Department of Healthcare Quality Assessment at the University of Tokyo, which is a social collaboration program supported by National Clinical Database, Johnson & Johnson KK and Nipro Corporation. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Y.K., M.Ish., K.U., H.Y., H.K., M.Iss., S.D. and H.M. were involved in the conception and design, or analysis and interpretation of the data; Y.K., M.Ish., K.U., H.Y., H.K., M.Iss., S.D. and H.M. in the drafting of the paper or revising it critically for intellectual content; and Y.K., M.Ish., K.U., H.Y., H.K., M.Iss., S.D. and H.M. in the final approval of the version to be published; and all authors agree to be accountable for all aspects of the work.
R1_Supplementary_information_Kokado_et_al.docx
Download MS Word (33.7 KB)Acknowledgements
The authors thank Krishant Chand of IQVIA Solutions Japan KK for performing data analyses and manuscript preparation. We also thank International Medical Translation Service Inc. for manuscript writing and editorial assistance.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Koury MJ, Haase VH. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol. 2015;11(7):394–410.
- Mercadal L, Metzger M, Casadevall N, et al. Timing and determinants of erythropoietin deficiency in chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(1):35–42.
- Panjeta M, Tahirović I, Sofić E, et al. Interpretation of erythropoietin and haemoglobin levels in patients with various stages of chronic kidney disease. J Med Biochem. 2017;36(2):145–152.
- Astor BC, Muntner P, Levin A, et al. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002;162(12):1401–1408.
- Covic A, Jackson J, Hadfield A, et al. Real-world impact of cardiovascular disease and anemia on quality of life and productivity in patients with non-dialysis-dependent chronic kidney disease. Adv Ther. 2017;34(7):1662–1672.
- McMurray J, Parfrey P, Adamson JW, et al. Kidney disease: improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335.
- Tsubakihara Y, Akizawa T, Iwasaki M, et al. High hemoglobin levels maintained by an erythropoiesis-stimulating agent improve renal survival in patients with severe renal impairment. Ther Apher Dial. 2015;19(5):457–465.
- Japanese Society for Dialysis Therapy. 2008 JSDT “guideline for renal anemia in chronic kidney disease”. J Jpn Soc Dial Ther. 2008;41(10):661–716.
- Yamamoto H, Nishi S, Tomo T, et al. 2015 Japanese society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017;3(1):36.
- Japanese Society of Nephrology. Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Japan J Nephrol. 2012;54(8):1031–1191.
- Japanese Society of Nephrology. Essential points from evidence-based clinical practice guidelines for chronic kidney disease 2018. Clin Exp Nephrol. 2019;23(1):1–15.
- Akizawa T, Makino H, Matsuo S, et al. Management of anemia in chronic kidney disease patients: baseline findings from chronic kidney disease Japan cohort study. Clin Exp Nephrol. 2011;15(2):248–257.
- Yamamoto T, Miyazaki M, Nakayama M, et al. Impact of hemoglobin levels on renal and non-renal clinical outcomes differs by chronic kidney disease stages: the Gonryo study. Clin Exp Nephrol. 2016;20(4):595–602.
- Tanaka T, Nangaku M, Imai E, et al. Safety and effectiveness of long-term use of darbepoetin alfa in non-dialysis patients with chronic kidney disease: a post-marketing surveillance study in Japan. Clin Exp Nephrol. 2019;23(2):231–243.
- Hayashi T, Uemura Y, Kumagai M, et al. Effect of achieved hemoglobin level on renal outcome in non-dialysis chronic kidney disease (CKD) patients receiving epoetin beta pegol: MIRcerA clinical evidence on renal survival in CKD patients with renal anemia (MIRACLE-CKD study). Clin Exp Nephrol. 2019;23(3):349–361.
- Sofue T, Nakagawa N, Kanda E, et al. Prevalence of anemia in patients with chronic kidney disease in Japan: a nationwide, cross-sectional cohort study using data from the Japan chronic kidney disease database (J-CKD-DB). PLoS One. 2020;15(7):e0236132.
- Hayashi T, Wada A, Soma A, et al. Randomized therapeutic equivalence study of darbepoetin alfa with epoetin alfa for anemia treatment in chronic kidney disease patients not on dialysis. Kidney Dialy. 2010;68:931–945.
- Tsubakihara Y, Itami Y, Uda S, et al. Effect of haemoglobin (Hb) maintenance of C.E.R.A. administrated subcutaneously (SC) compared with rHuEPO in chronic kidney disease (CKD) patients not on dialysis. Kidney Dialy. 2011;70:953–963.
- Ravanan R, Spiro JR, Mathieson PW, et al. Impact of diabetes on haemoglobin levels in renal disease. Diabetologia. 2007;50(1):26–31.
- Li Vecchi M, Fuiano G, Francesco M, et al. Prevalence and severity of anaemia in patients with type 2 diabetic nephropathy and different degrees of chronic renal insufficiency. Nephron Clin Pract. 2007;105(2):c62–c67.
- Akizawa T, Tsubakihara Y, Hirakata H, et al. A prospective observational study of early intervention with erythropoietin therapy and renal survival in non-dialysis chronic kidney disease patients with anemia: JET-STREAM study. Clin Exp Nephrol. 2016;20(6):885–895.
- Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098.
- Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–2032.