Abstract
Objectives
Opioid use disorder is associated with high rates of mortality and has become an escalating global health issue. Opioid agonist treatment (OAT) with oral methadone or daily sublingual buprenorphine hydrochloride, either administered separately or in combination with naloxone hydrochloride (SL-BPN, SL-BPN/NX), is supervised by a healthcare professional experienced in treating opioid use disorder to ensure proper dosing and prevent misuse. For that reason, there may be substantial direct and indirect costs associated with OAT. Recently, weekly and monthly subcutaneous depot formulations of buprenorphine (SC-BPN) have been approved. This study aimed to estimate management and patient-incurred costs associated with the most commonly used OATs compared to the cost of weekly and monthly SC-BPN.
Methods
We conducted a cost-minimisation analysis comparing the monthly costs of OAT treatment with oral formulations, i.e. oral methadone, SL-BPN, SL-BPN/NX and SC-BPN. The analysis assessed treatment acquisition costs and costs associated with management, supervision and administration of therapy, patients’ transportation costs and the indirect costs associated with patients’ time-use. The model was set up to reflect the Norwegian medically assisted rehabilitation system and considered the costs of a stable maintenance OAT regimen given continuously to patients already initiated and titrated on the therapy.
Results
OAT management with monthly formulation of SC-BPN was associated with a reduction in monthly costs of €605, €586, and €411 per month compared to SL-BPN, SL-BPN/NX and oral methadone, respectively. Similar results were estimated when comparing to the weekly formulation of SC-BPN.
Conclusion
The analysis showed that the monthly formulation of SC-BPN was the cost-minimising alternative, followed by the weekly formulation, when considering all cost components.
Introduction
Opioid use disorder is a chronic, debilitating and relapsing disease. It is associated with high rates of mortality and has become an escalating global health issueCitation1,Citation2. It is well established that opioid use disorder is associated with adverse psychosocial consequences, including criminal activity and incarcerationCitation3,Citation4, as well as both mental health and somatic comorbiditiesCitation5. According to the European Monitoring Centre for Drugs and Drug Addiction, there are approximately 9000 high-risk opioid users in NorwayCitation6.
Pharmacological opioid agonist treatment (OAT), also known as LAR (legemiddelassistert rehabilitering) in Norway, has been demonstrated to be the most effective therapy available for the treatment of opioid use disorderCitation7,Citation8. Replacing illicit opioids with a regular substitute dose of synthetic opioids, which acts slowly in the body, works to prevent withdrawal and can reduce patients’ cravings. For that reason, it has been associated with substantial reductions in illicit opioid use, criminal activity, deaths and transmission of blood-borne viruses, including human immunodeficiency virus (HIV) and hepatitis CCitation4,Citation9,Citation10.
In Norway, the OAT treatment system has successfully reached a large proportion of the population of the country’s high-risk opioid users, 7762 by the end of 2018, which corresponds to more than 86%Citation11. OAT is part of the interdisciplinary specialised health service, which comprises primary healthcare, specialist healthcare, the municipal social administration and the patient’s general practitioner (GP). Patients are prescribed OAT by their GP or the social administration. The treatment is initiated and titrated in outpatient centres located in all municipalities, and maintenance treatment tends to be managed at community pharmaciesCitation12.
OAT is organised and administered through a national programme encompassing a range of services such as assessment, detoxification, stabilisation, short- and long-term residential treatment and medication-assisted treatment. All services are offered in all outpatient centres. Patient preferences are a strong component of the shared decision-making process between the treatment system and the patient.
The most common OATs are daily sublingual buprenorphine hydrochloride, either as monotherapy (SL-BPN) or in a combination tablet containing naloxone hydrochloride dihydrate (SL-BPN/NX) and oral methadone (various products, generic and branded).
Buprenorphine is a partial agonist/antagonist that acts on μ- and κ-opioid receptors in the central nervous system. The drug is far more potent than morphine in low doses, but unlike what is the case for full agonists such as morphine, methadone and heroin, at larger doses, a maximum effect – a so-called ceiling effect – is achieved, which is somewhat lower.
Internationally, methadone is the most common OAT, as it has been shown to be a cost-effective treatment choiceCitation13. In 2018, 38% of patients receiving OAT in Norway received methadone, whereas 39% of patients were treated with SL-BPN, and 20% of patients received SL-BPN/NXCitation14. The high utilisation of methadone in Norway is ascribed to patients’ own indication of preference for methadone as OAT.
To administer these OAT regimens, a healthcare professional specialising in treating opioid use disorder typically conducts an individual patient assessment to decide whether the treatment needs to be supervisedCitation8. This is often deemed necessary to ensure proper management of the patient and to prevent the medicinal products from being ingested in violation of the doctor’s requisition or being made available to others. A recent survey on the status of OAT in Norway revealed that almost all OAT in Norway is carried out under the supervision of a healthcare professional experienced in the management of opioid use disorderCitation14.
Daily supervision of medication consumption is time-consuming, both for the patient and the healthcare professional, and this is one of the factors which likely contribute to the low rates of adherence observed on SL-BPN/NXCitation15. A vast body of literature has documented substantial non-adherence to OAT treatment as high as 33% after six weeks and increasing to 74.4% after six monthsCitation16–20. Non-adherence has been found to cause a more than threefold increase in the risk of overdosingCitation19; therefore, increasing the rate of adherence to OAT therapy would significantly impact the opioid crisis by potentially reducing the risk of relapse and increasing overall success outcomes. Weekly and monthly subcutaneous buprenorphine depot formulations (SC-BPN) represent an alternative to currently recommended first-line OATs in Norway. SC-BPN is a ‘hybrid medicine’ which delivers a steady dose of BPN via an extended-release formulation based on nanoscale drug delivery (FluidCrystal technologyCitation21; Camurus AB). In a recent multicentre, double-blind, double-dummy, randomised clinical trial, SC-BPN demonstrated superior efficacy compared to SL-BPN/NX, as measured by the proportions of opioid-negative urine samples recorded among participantsCitation22. Weekly and monthly formulations in multiple strengths were developed for titration purposes and to allow for a transition from frequent supervised administration of OAT to gradually fewer administrations and even directly transitioning to monthly administration.
Since 2019, SC-BPN has been recommended for use by the Norwegian Medicines Agency (NOMA) as an alternative to SL-BPN/NX formulations because of its efficacy and cost-effectiveness. However, no detailed analysis of the costs associated with depot- versus oral-based buprenorphine therapy in a Norwegian healthcare setting has been published.
This study aimed to compare the total costs of SC-BPN and oral OATs in Norway in the context of their typical use within the Norwegian healthcare system.
Method
Cost-minimisation analysis
The main analysis was based on a cost-minimisation approach (CMA). The aim of a CMA is to find the least costly treatment when considering all costs related to treatment. In the case of therapeutic equivalence between the evaluated comparators, total costs of the treatments are the only components one needs to analyse in order to find the most cost-effective treatment choice.
There is evidence of superior treatment effects in terms of reduced illicit opioid useCitation22 and improved treatment satisfaction and quality of lifeCitation23 when patients are treated with SC-BPN. For simplicity and to ensure a conservative analytical approach to modelling costs, it is assumed that SC-BPN is equally as effective in reducing illicit opioid use as SL-BPN-based OAT and has a comparable safety profile, and therefore, a cost-minimisation analysis was considered sufficient.
The cost-minimisation analysis compared weekly and monthly SC-BPN with the current standard of care medicines:
SL-BPN;
SL-BPN/NX; and
oral methadone.
All therapies were assumed to be given in a stable maintenance setting to patients already initiated and titrated on therapy. The analysis analysed monthly treatment acquisition costs, costs associated with management, including the cost of prescriptions, supervision and administration of the therapy, patients’ transportation costs, and indirect costs associated with patient time-use.
Applied cost estimates
Drug costs
Drug acquisition costs were based on the lowest available pharmacy purchasing prices in Norway at the time the analysis was conducted, 19 November 2021. The Norwegian survey of patients enrolled in OAT found that on average, patients initiated on OAT with methadone received 92 mg per day. Patients initiated on OAT with SL-BPN received 15 mg, whereas patients receiving SL-BPN/NX received 13 mg per dayCitation14. For patients receiving SC-BPN, we assumed one administration of SC-BPN if they received the monthly formulation, or 4.35 administrations per month if they received the weekly formulation.
To calculate the average drug costs, we applied the exact dosing for patients receiving methadone. To compute an average dosing of 15 mg per day for patients receiving SL-BPN from the available dosages, we assumed that 12.5% of patients receive 8 mg per day, and 87.5% of patients receive a dosage of 16 mg per day. Similarly, we assumed a treatment distribution for patients treated with SL-BPN/NX of 37.5% receiving 8 mg BPN + 2 mg NX and 62.5% receiving 16 mg BPN + 4 mg NX. Unit costs and treatment distributions are presented in .
Table 1. Drug costs and treatment distribution.
Drug management
The unit costs associated with management of the medications considered in the present analysis are presented in . In Norway, OAT is commonly initiated and titrated in clinics, but long-term maintenance treatment tends to be managed at community pharmacies. In June 2016, the Norwegian Ministry of Health introduced a reimbursement scheme for medical providers performing different activities associated with OATCitation33. Under this scheme, pharmacies receive a monthly administration fee of €25.47 per patient for tasks associated with patient follow-up and medical record-keeping in addition to medication preparation and dosing. To deter misuse, and due to the high street value of OAT medications, dosing at the pharmacy must be supervised by an experienced healthcare professional. For this service, pharmacies are reimbursed €3.92 per supervised intake of oral methadone and €10.45 per supervised intake of buprenorphine. Finally, pharmacies are reimbursed for partial prescriptions of OAT medication with €3.92 per partial prescriptionCitation30.
Table 2. Unit costs of drug management.
The model assumes that administration of SC-BPN is typically performed at the patient’s GP, either by the GP herself or by another qualified healthcare professional, meaning that neither the pharmacy administration costs nor partial prescription fees apply. Furthermore, treatment with SC-BPN does not require supervised intake. Currently, there is no negotiated reimbursement fee associated with the administration of SC-BPN. To set a comparable estimate, we set the administration cost of SC-BPN at €12.01Citation31, which is equivalent to the reimbursement rate for a blood sample performed by a GP.
The cost of three annual prescriptions is included for oral methadone, SL-BPN, and SL-BPN/NX. This is based on the assumption that one annual renewal is mandatory, and at least two additional renewals will be required in relation to holiday breaks or irregular attendance. Similarly, two annual prescriptions are assumed to be necessary for SC-BPN based on the expectation that, on average, one break or renewal is required.
Patient unit costs
Patient-incurred costs include the value of the patient’s time and the travel costs associated with treatment at a pharmacy or at the GP clinic. The unit cost of an hour of patient timeFootnotei is set to €23.91, based on the average hourly salary after tax published by the Norwegian Medicines Agency for use in health technology appraisalsCitation32. Following the same source, travel costs associated with each treatment are based on a standard rate of €10.09. This is based on the assumption that the average travel distances from patients’ homes to GP clinics and pharmacies are similar.
In its 2018 report, the Norwegian Centre for Addiction Research concluded that the majority of patients receive OAT supervision at a community pharmacy (which provides no treatment other than OAT)Citation14. For that reason, we assume that all maintenance for oral therapy happens in pharmacies (consistent with 2018 report), and that local GP clinics carry out the injections for SC-BPN.
Resource use
The assumptions regarding resource use for each of the given treatments are based on estimates from Waal et al. (2018), who described the current situation of medically-assisted rehabilitation treatments in Norway via a survey of 7,122 patients currently enrolled in OATCitation14. The report found that, on average, 4.1 of patients’ seven weekly administrations were supervised, which implies a total of 17.83 monthly supervisions. In this analysis, patient time and travel costs are applied at this same weekly frequency implying that each of the 17.83 supervisions are associated with a ‘supervision fee’, one hour of patient time-use, and one patient travel to the clinic. The Waal 2018 survey did not distinguish between OAT types, so in our analysis, the average of 4.1 is applied to both oral methadone, SL-BPN and SL-BPN/NX formulations.
For the baseline analysis, we assumed that one hour of patient time is required for a supervised dose of either SL-BPN/NX, SL-BPN or oral methadone administered at the pharmacy. Similarly, the patient time required for a GP-administered treatment with SC-BPN is assumed to be one hour in total. In both instances, the one hour is assumed to cover all patient time required to travel to and from treatment, wait to see the GP, complete paperwork and receive the medication.
To obtain the total cost for each drug, we multiply the healthcare resource use as presented in by the unit costs for each cost category as presented in and add the drug costs.
Table 3. Monthly healthcare resource use associated with OAT.
Sensitivity analyses
Patient time-use associated with engagement in OAT constitutes important cost components in the analysis. However, several factors, including supervision practices, clinic procedures and the patients’ distance to the treatment facility, could impact the time spent attending OAT treatment at the pharmacy or clinic. For that reason, we carried out a sensitivity analysis varying the patient time-use associated with the treatments from 30 to 90 min. In addition to this, we conducted sensitivity analyses varying the average number of supervisions per week for patients receiving SL-BPN/NX or oral methadone.
Results
Baseline results
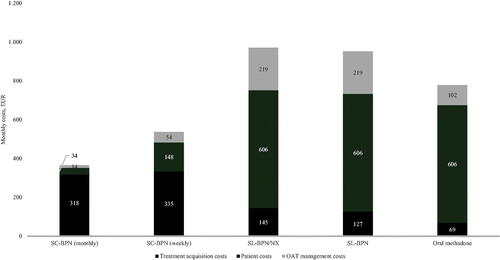
presents the estimated monthly per-patient cost of each OAT, separated into four cost categories: treatment acquisition costs, drug management cost, transportation costs and the indirect costs of patient time-use associated with treatment.
Table 4. Monthly administration and patient costs (EUR).
There were substantial differences between the drug management costs of all OATs. The management costs associated with monthly and weekly formulations of SC-BPN amounted to €13.86 and €54.05 per month, respectively, whereas the drug management costs of OAT with SL-BPN/NX or SL-BPN amounted to €218.84 and €102.38 per month, respectively, for patients receiving oral methadone. Cost differences were mainly driven by the costs associated with reimbursement payments to pharmacies for supervised intake of SL-BPN/NX, SL-BPN and oral methadone; this cost component accounted for 91% of the total difference in drug management costs between SL-BPN/NX and the monthly formulation of SC-BPN.
The requirement for supervised dosing four times per week for currently available OATs also drives a markedly larger cost for the patient compared to SC-BPN, both in terms of transportation costs (€179.86 per month versus €10.09 and €43.87 for monthly and weekly SC-BPN, respectively) and in terms of indirect costs due to patient time-use. In the baseline analysis, the value of patient time-use constitutes the single largest patient cost component. Patients enrolled in OAT who receive currently available therapies are estimated to spend 17.8 h receiving supervised treatments each month with an implied value of €426.26. By comparison, patients receiving OAT with the monthly formulation of SC-BPN spend only one hour receiving treatment each month with an implied cost of €23.91.
Adding the treatment acquisition costs to this, the total monthly costs associated with OAT treatment with the monthly and weekly formulation of SC-BPN were estimated to €365.92 and €536.44, respectively. By contrast, the total monthly costs of OAT with SL-BPN/NX, SL-BPN and oral methadone were estimated to €970.44, €951.98 and €777.75, respectively. This implies an incremental cost of €604.52 and €434.01 for SL-BPN/NX, €586.06 and €415.54 for SL-BPN, and €411.83 and €241.31 for oral methadone when compared to the monthly and weekly formulation of SC-BPN, respectively.
The results from the baseline analysis are graphically presented in .
Figure 1. Monthly management and patient costs (EUR). Note: The figure presents the total costs for one month of treatment with monthly and weekly formulation of SC-BPN as well as SL-BPN/NX and Oral methadone by cost category. OAT management costs include drug management, dispensing, prescriptions, supervised dosing, and injection costs. Patient costs include the cost of patient time and travel costs.
Sensitivity analyses
presents the results from the sensitivity analysis. Row 1 presents the results from the base case as comparison, and the number in brackets presents the incremental cost of treatment relative to treatment with the monthly formulation of SC-BPN and the weekly formulation of SC-BPN.
Table 5. Sensitivity analysis: varying patient time required for treatment and number of weekly supervisions.
The table also presents the results of varying the average number of supervisions per week for patients receiving SL-BPN/NX, SL-BPN or oral methadone from three to seven days per week. The lower value is relevant because some patients treated with SL-BPN/NX and SL-BPN receive a double dose (i.e. twice the individually titrated daily dose) three times a week after stabilisation is achieved. The higher value is relevant to assess the cost of patients who are under intensive management, fully adherent to the OAT regimen and require all administrations to be observed.
Overall, the results presented in support the conclusions from the baseline analysis. Even in a scenario where it is assumed that the patient on average only requires 30 min for a supervised treatment (including waiting time and transportation), the analysis still found that a monthly injection with SC-BPN is associated with a reduction in costs of €408, €390, and €216, compared to SL-BPN/NX and oral methadone, respectively.
Discussion and limitations
In this study, we documented the substantial direct and indirect costs associated with the currently recommended first-line OATs in Norway. We found that drug acquisition costs constitute 87 and 62% of the total monthly cost of treatment with the monthly and weekly formulation of SC-BPN, respectively. However, the results show that drug acquisition costs constitute 15, 13 and 9% of the total monthly cost of treatment with SL-BPN/NX, SL-BPN, and Oral methadone, respectively. For these three treatments, we identified supervision of administration and the cost of patient time as the primary cost drivers. The results are based on a Norwegian setting and might not apply to countries without universal healthcare.
The model considers only short-term treatment costs. Thus, it excludes cost reductions associated with potential future health gains from treatments. As previously pointed out, SC-BPN has demonstrated superior efficacy to SL-BPN/NX in a 28-week, randomised, double-blind, double-dummy clinical trialCitation22. The study found that treatment with SC-BPN was associated with a significant reduction in use of illicit opioids, as measured by urine tests. As such, if SC-BPN proves to be more effective in rehabilitating patients, the long-term savings associated with OAT using the monthly formulation of SC-BPN might be underestimated. For instance, rehabilitated patients result in savings related to premature death, comorbidities, criminal justice and productivity loss (e.g. sick time, disabilities, unemployment), all of which put a strain on societyCitation35. Besides the cost-saving elements, being rehabilitated would have positive implications for the individual’s physical and mental health, social relationships and financial opportunitiesCitation36.
Only patient time and travel for OAT are included in the model, and these are further included as incremental costs to the patient. Thus, the model excludes patient costs associated with receiving other treatments within the OAT framework (e.g. psychosocial or social interventions), since these were assumed to remain similar across treatments. At the same time, in cases where patients receive more than one treatment during a visit to the OAT treatment centre, the model would likely overestimate the incremental transportation costs and the costs associated with patient time-use.
Conclusion
We developed a cost-minimisation model which estimated the monthly treatment costs including treatment acquisition costs, costs of drug management and patient costs associated with available formulations of OATs. We found substantial differences both in drug management and in patient-incurred costs. The baseline results suggested that OAT with the monthly formulation of SC-BPN generated a reduction in the monthly costs associated with treatment of €605 compared to treatment with SL-BPN/NX, which is currently recommended as the first-line OAT in Norway, corresponding to a 62% reduction.
Furthermore, the study identified the requirement for daily supervised consumption of currently available OATs as the main driver of an overall cost difference. This requirement was also highlighted in the NoMA assessment of SC-BPN.
Transparency
Declaration of funding
This work was supported by Camurus AB.
Declaration of financial/other relationships
Buvidal buprenorphine injections are a product of Camurus AB. Rasmus Jensen is an employee at Camurus AB. Incentive is a paid vendor of Camurus AB. Anne Danø and Mikkel H. Pedersen are employees at Incentive.
Carl Gibbons was an employee at Camurus Ltd from 2017 to 2019 and did not receive any payment for work on this manuscript.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Mikkel Pedersen, Anne Danø and Rasmus Jensen contributed to the study design, development of the economic model, interpretation of the results, and drafting of the manuscript. Carl Gibbons contributed to the interpretation of the results, revision of the manuscript, and provided clinical expert knowledge. All authors have approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Acknowledgements
None.
Notes
i The value of patient time is equal to the value of leisure time, in accordance with the Norwegian Medicines Agency (NoMA) guidelinesCitation27,Citation34.
References
- Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta‐analysis of cohort studies. Addiction. 2011;106(1):32–51.
- Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- Bukten A, Skurtveit S, Gossop M, et al. Engagement with opioid maintenance treatment and reductions in crime: a longitudinal national cohort study. Addiction. 2012;107(2):393–399.
- Schwartz RP, McKenzie M, Rich JD. Opioid addiction and incarceration: an overview. Med Health R I. 2007;90(5):157–158.
- Kelty E, Hulse G. Morbidity and mortality in opioid dependent patients after entering an opioid pharmacotherapy compared with a cohort of non-dependent controls. J Public Health. 2018;40(2):409–414.
- Norway Drug Report 2018. European Monitoring Centre for Drugs and Drug Addiction; 2018.
- Aas CF, Vold JH, Skurtveit S, for the INTRO-HCV Study Group, et al. Health-related quality of life of long-term patients receiving opioid agonist therapy: a nested prospective cohort study in Norway. Subst Abuse Treat Prev Policy. 2020;15(1):68.
- Helse- og omsorgsdepartementet. Forskrift om legemiddelassistert rehabilitering (LAR-forskriften). [Internet]. 2009. [cited 2021 May 31]. Available from: https://lovdata.no/dokument/SF/forskrift/2009-12-18-1641.
- Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59(10):1411–1419.
- Strathdee SA, Beyrer C. Threading the needle—how to stop the HIV outbreak in rural Indiana. N Engl J Med. 2015;373(5):397–399.
- Waal H, Bussesund K, Clausen T, et al. Statusrapport 2018: LAR i rusreformenes tid. SERAF; 2018.
- Lobmaier P, Skeie I, Lillevold P, et al. Statusrapport 2020: LAR behandling under første året med Covid-19 pandemi [Internet]; 2021. Available from: https://www.med.uio.no/klinmed/forskning/sentre/seraf/publikasjoner/rapporter/2021/seraf-rapport-nr-4-2021-statusrapport-2020.pdf.
- Onuoha EN, Leff JA, Schackman BR, et al. Economic evaluations of pharmacologic treatment for opioid use disorder: a systematic literature review. Value Health. 2021;24(7):1068–1083.
- Waal H, Bussesund K, Clausen T, et al. Statusrapport 2017: LAR 20 år Status, vurderinger og perspektiver. SERAF; 2018.
- Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315–326.
- Muruganandam P, Shukla L, Sharma P, et al. Too little dose – too early discontinuation?’—effect of buprenorphine dose on short term treatment adherence in opioid dependence. Asian J Psychiatr. 2019;44:58–60.
- Kumari S, Manalai P, Leong S, et al. Factors associated with non-adherence to buprenorphine-naloxone among opioid dependent African-Americans: a retrospective chart review: therapeutic adherence to buprenorphine-naloxone. Am J Addict. 2016;25(2):110–117.
- Bandawar M, Kandasamy A, Chand P, et al. Adherence to buprenorphine maintenance treatment in opioid dependence syndrome: a case control study. Indian J Psychol Med. 2015;37(3):330–332.
- Kinsky S, Houck PR, Mayes K, et al. A comparison of adherence, outcomes, and costs among opioid use disorder medicaid patients treated with buprenorphine and methadone: a view from the payer perspective. J Subst Abuse Treat. 2019;104:15–21.
- Pizzicato LN, Hom JK, Sun M, et al. Adherence to buprenorphine: an analysis of prescription drug monitoring program data. Drug Alcohol Depend. 2020;216:108317.
- Tiberg F, Johnsson M, Jankunec M, et al. Phase behavior, functions, and medical applications of soy phosphatidylcholine and diglyceride lipid compositions. Chem. Lett. 2012;41(10):1090–1092.
- Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder. JAMA Intern Med. 2018;178(6):764–773.
- Lintzeris N, Dunlop AJ, Haber PS, et al. Patient-Reported outcomes of treatment of opioid dependence With weekly and monthly subcutaneous depot vs. daily sublingual buprenorphine: a randomized clinical trial. JAMA Netw Open. 2021;4(5):e219041.
- Statens Legemiddelverk. Legemiddelvisning: Buvidal – 32 mg [Internet]. www.legemiddelsok.no. [cited 2021 Nov 19]. Available from: https://www.legemiddelsok.no/sider/Legemiddelvisning.aspx?pakningId=d5b39585-9b41-48fc-a3a7-83732f28ce9b&searchquery=buprenorphin&f=Han;MtI;Vir;ATC;Var;Mar;Mid;Avr;gen;par;&pane=1.
- Statens Legemiddelverk. Legemiddelvisning: Buvidal – 64 mg [Internet]; 2021 [cited 2021 Nov 19]. Available from: www.legemiddelsok.no. https://www.legemiddelsok.no/sider/Legemiddelvisning.aspx?pakningId=77430dba-82f5-4764-842b-2f20f1f72c11&searchquery=buprenorphin&f=Han;MtI;Vir;ATC;Var;Mar;Mid;Avr;gen;par;&pane=1.
- Statens Legemiddelverk. Legemiddelvisning: suboxone – 8 mg/2 mg [Internet]. www.legemiddelsok.no. [cited 2021 Nov 19]. Available from: https://www.legemiddelsok.no/sider/Legemiddelvisning.aspx?pakningId=29ac0b83-2a5b-4379-9993-25c3a597f10c&searchquery=buprenorphin&f=Han;MtI;Vir;ATC;Var;Mar;Mid;Avr;gen;par;&pane=1.
- Statens Legemiddelverk. Legemiddelvisning: Suboxone - 16 mg/4 mg [Internet]; 2021 [cited 2021 Nov 19]. Available from: http://www.legemiddelsok.no. https://www.legemiddelsok.no/sider/Legemiddelvisning.aspx?pakningId=cff531fa-58df-4424-ab03-686df8cbcbce&searchquery=buprenorphin&f=Han;MtI;Vir;ATC;Var;Mar;Mid;Avr;gen;par;&pane=1.
- Statens Legemiddelverk. Legemiddelvisning: Buprenorphine Sandoz - 8 mg [Internet]. www.legemiddelsok.no. [cited 2021 Nov 19]. Available from: https://www.legemiddelsok.no/sider/Legemiddelvisning.aspx?pakningId=16e8b6f7-9d1b-4378-98b8-58d9390e0fe9&searchquery=buprenorphin&f=Han;MtI;Vir;ATC;Var;Mar;Mid;Avr;gen;par;&pane=1.
- Statens Legemiddelverk. Legemiddelvisning: Metadon Martindale Pharma – 2 mg/ml [Internet]; 2021 [cited 2021 Nov 19]. www.legemiddelsok.no. Available from: https://www.legemiddelsok.no/sider/Legemiddelvisning.aspx?pakningId=f2bf4c87-3197-459a-9ed9-e61ffe4b3497&searchquery=metadon&f=Han;MtI;Vir;ATC;Var;Mar;Mid;Avr;gen;par;&pane=1.
- Apotekavtalen bilag 1: Avtale om opgjør for LAR-legemidler og LAR-tjenester i apotek Internet]. Aftale mellom De regionale helseforetak i Norg og Norges Apotekerforenin; 2019 [cited 2021 Jan 27]. Available from: http://www.apotek.no/Files/Apotekregelverk/Rundskriv/Andre/20190101%20LAR-avtalen%202%20av%204.pdf.
- Den norske legeforening. Normaltariff for fastleger og legevakt (2019–2020) [Internet]; 2019 [cited 2021 Jan 27]. Available from: https://normaltariffen.legeforeningen.no/asset/pdf/Fastlegetariffen-2019-2020.pdf.
- The Norwegian Medicines Agency. Enhetskostnadsdatabase [Unit cost database] [Internet]; 2021. Available from: https://legemiddelverket.no/offentlig-finansiering/dokumentasjon-for-metodevurdering/enhetskostnadsdatabase.
- Utlevering av LAR legemidler: Nasjonal godtgjørelse for utlevering i apotek. Helsedirektoratet; 2017.
- Norwegian Medicines Agency (NoMA). Guidelines for the submission of documentation for single technology assessment (STA) of pharmaceuticals [Internet]; 2021 [cited 2022 Jun 16]. Available from: https://legemiddelverket.no/Documents/English/Public%20funding%20and%20pricing/Documentation%20for%20STA/Guidelines%2018.10.2021.pdf.
- Oderda G, Lake J, Rudell K, et al. Economic burden of prescription opioid misuse and abuse: a systematic review. J Pain Palliat Care Pharmacother. 2015;29(4):388–400.
- Muller AE, Skurtveit S, Clausen T. Performance of the WHOQOL-BREF among norwegian substance use disorder patients. BMC Med Res Methodol. 2019;19(1):44.
