Abstract
Whether lowering the hemoglobin A1c to <6.0% in patients with type 2 diabetes can reduce the risk of cardiovascular disease (CVD) remains under debate. The ACCORDION and the VADT studies both found reductions in the primary CVD composite associated with intensive glycemic control, though the difference is not statistically significant. However, the lack of significance is often overinterpreted as non-effective: a p-value >.05 only implies that the study “failed to reject” the null hypothesis (i.e. lowering the A1c level to <6.0% results in no CVD benefit), which is different from concluding the null hypothesis being true. In this study, we used Bayesian analysis to reanalyze results from the ACCORDION and VADT-15 trials. Our results suggest achieving an A1c goal of <6.0% as compared to moderate control could result in a moderate risk reduction in MACE.
Introduction
Whether lowering the hemoglobin A1c to <6.0% in patients with type 2 diabetes can reduce the risk of cardiovascular disease (CVD) remains under debate. The Action to Control Cardiovascular Risk in Diabetes Follow-On trial (ACCORDION(NCT00000620), A1c <6.0 vs. 7–8%) and the Veterans Affairs Diabetes Trial 15-year Follow-up (VADT-15 (NCT00032487), A1c <6.0 vs. 8–9%) both found reductions in the primary CVD composite (ACCORDION: Hazard Ratio (HR): 0.95, 95% Confidence Interval (CI):0.87–1.04; VADT-15: HR: 0.91, 95% CI: 0.78–1.06) associated with intensive glycemic control, though the difference is not statistically significantCitation1,Citation2. However, the lack of significance is often overinterpreted as non-effective: a p-value >.05 only implies that the study “failed to reject” the null hypothesis (i.e. lowering A1c level to <6.0% results in no CVD benefit), which is different from concluding the null hypothesis being true. Bayesian analysis can add insight to the interpretation of data collected in clinical trials by providing probabilities of treatment effects and by considering prior knowledge to provide posterior probabilitiesCitation3,Citation4. In this study, we used Bayesian analysis to reanalyze results from the ACCORDION and VADT-15 trials.
Methods
We extracted baseline characteristics of the study populations and progressions of their A1c, systolic blood pressure, low-density lipoprotein cholesterol levels, and body mass index from the study reports of the two trialsCitation1,Citation2. We used the Building, Relating, Assessing, and Validating Outcomes (BRAVO) diabetes modelCitation5, a validated and calibrated person-level diabetes microsimulation modelCitation6, to generate the distribution of major adverse cardiovascular events (MACE) risk reductions as a result of intensive glycemic control, based on variations in population characteristics, trajectories of biomarkers progression for both arms, and embedded risk equations in the BRAVO model (i.e. prior knowledge). We used the Gibbs sampler to estimate the posterior probability distribution of the MACE risk reduction based on the generated prior and the published distributions of the MACE risk reduction observed in the two original trials.
Results
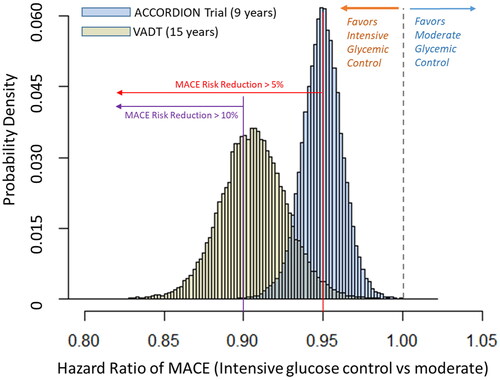
For the ACCORDION study, compared with a moderate A1c goal of 7−8%, probability of reducing MACE by any level, ≥5%, and ≥10%, from an intensive A1c goal of <6.0% were 99, 50, and 1% (blue bars, ), respectively. For the VADT-15 study, compared with a moderate A1c goal of 8−9%, the probability of reducing the risk of MACE by any level, ≥5%, and ≥10% from an intensive A1c goal of <6.0% was 99, 90 and 50%, respectively (orange bars).
Discussions
We used a Bayesian method to reanalyze the results from two large contemporary glycemic control trails to determine whether lowering patients’ A1c levels to <6.0% could lead to any CVD benefits. Our results suggest achieving an A1c goal of <6.0% as compared to moderate control could result in a moderate risk reduction in MACE.
Our study results do not change the results reported in the two trials, but rather are a complement to conventional frequentist statistics by providing direct estimates of the probability of the treatment being effective and the effect size being clinically meaningful (e.g. ≥5% risk reduction). Such analysis is especially useful for further understanding the benefit of the treatment when the benefit is more probable than not despite the absence of frequentist statistical significance. Note that our estimation is the average treatment effect of goal achievement, and variations of the treatment effect are likely to exist across the study population. Specifically, for the elderly population, especially those with multimorbidity, intensive A1c goals can potentially increase the risk of adverse events (e.g. hypoglycemia) and may not be recommended.
The limitation of our study, as most of the Bayesian-based analyses, is the influence of priors on study outcomes. The study results could depend on the priors selected. The prior we generated is consistent with current clinical knowledge.
Reducing A1c level to <6.0% in patients with type 2 diabetes could have a moderate effect on lowering the risk of MACE. Our findings are separate and complementary statistical inferences of intensive glycemic control in the ACCORD and VADT trials.
Transparency
Declaration of funding
This study is funded by NIDDK [1R01DK133465].
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Dr. Shao is the guarantor of this work and has full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. H.S. and J.G. analyzed data and prepared the results. H.S., J.G., and P.Z. wrote the manuscript. N.L., J.G., and V.F. provided clinical expertise, L.S, S.T., and P.Z. provided public health expertise. All authors contributed critically to the discussion and participated in the manuscript development.
Acknowledgements
None.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Reaven PD, Emanuele NV, Wiitala WL, VADT Investigators, et al. Intensive glucose control in patients with type 2 diabetes — 15-year follow-up. N Engl J Med. 2019;380(23):2215–2224.
- The ACCORD Study Group Nine-Year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39(5):701–708.
- Brophy JM. Bayesian interpretation of the EXCEL trial and other randomized clinical trials of left main coronary artery revascularization. JAMA Intern Med. 2020;180(7):986–992.
- Wijeysundera DN, Austin PC, Hux JE, et al. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol. 2009;62(1):13–21.e5.
- Shao H, Fonseca V, Stoecker C, et al. Novel risk engine for diabetes progression and mortality in USA: building, relating, assessing, and validating outcomes (BRAVO). Pharmacoeconomics. 2018;36(9):1125–1134.
- Shao H, Yang S, Stoecker C, et al. Addressing regional differences in diabetes progression: global calibration for diabetes simulation model. Value Health. 2019;22(12):1402–1409.