Abstract
Objective
Congenital hemophilia B is a rare bleeding disorder caused by defects in the gene encoding factor IX (FIX) leading to coagulation deficiency. Recurrent bleeds may cause chronic pain, disability, and reduced quality of life. Phase 2 b and 3 single-arm, open-label, single-dose trials assessing etranacogene dezaparvovec gene therapy for hemophilia B have demonstrated sustained FIX activity levels over observed periods, but long-term durability of the treatment effect has not been established. Using statistical modeling, we estimate long-term durability of FIX activity levels after receiving etranacogene dezaparvovec.
Methods
Participants from Phase 2 b (N = 3; NCT03489291) and 3 studies (N = 52; NCT03569891) were included. Two participants who did not respond to treatment were excluded. FIX activity was assessed by one-stage activated partial thromboplastin time assay. FIX activity levels at Month 6 post-treatment were considered baseline. Bayesian and Frequentist linear mixed models predicted FIX activity levels up to 25.5 years at an individual and population level with pre-treatment adeno-associated virus 5 (AAV5) neutralizing antibody (NAb) status as primary covariate.
Results
Bayesian and Frequentist linear mixed models predicted no more than 6/55 (10.91%) observed participants would have FIX activity levels <2% up to 25.5 years post-infusion. Bayesian model-based predictions of future participants suggest >80% would be free from prophylactic FIX replacement products 25.5 years post-infusion. Both models predicted FIX activity levels were not significantly influenced by pre-treatment AAV5 NAb status.
Conclusions
People with hemophilia B receiving etranacogene dezaparvovec would likely achieve durable FIX activity levels and remain free of prophylactic FIX replacement products for up to 25.5 years following single administration. The long-term factor IX durability predictions are based on statistical methods and results in vivo may differ.
PLAIN LANGUAGE SUMMARY
Hemophilia B is a rare bleeding condition where blood does not clot properly, causing excessive bleeding. It is caused by a change or mutation to a gene, leading to lower-than-normal levels of a clotting factor, called factor IX. Standard treatment involves replacing missing factor IX through lifelong, regular treatment with factor replacement products. Etranacogene dezaparvovec is a gene therapy developed to replace the faulty gene and increase factor IX activity levels in the blood, thereby reducing bleeding, after one treatment. In clinical trials of etranacogene dezaparvovec, people with severe or moderately severe hemophilia B had stable and long-lasting increases in factor IX activity levels that reached near to the normal range seen in people without hemophilia B.
The purpose of this study was to predict whether these increases in factor IX activity levels will last over an extended period of time after receiving etranacogene dezaparvovec.
Mathematical predictions showed less than 11% of clinical trial participants would have unacceptable factor IX activity levels (less than 2%) up to 25.5 years after receiving etranacogene dezaparvovec. Further predictions of potential future people with hemophilia B showed that over 80% would not need treatment with factor replacement products 25.5 years after receiving etranacogene dezaparvovec.
The goal of treatment is to increase factor IX activity levels into the near-to-normal range in people with hemophilia B and therefore decrease or eliminate bleeding. These results suggest people with hemophilia B receiving etranacogene dezaparvovec would have long-lasting factor IX activity levels and would not need regular factor replacement products for up to 25.5 years following a single treatment of etranacogene dezaparvovec.
Introduction
Congenital hemophilia B is a rare bleeding disorder characterized by an increased bleeding tendency due to either a partial or complete deficiency of the essential blood coagulation factor, factor IX. The clinical phenotype of the disease is dependent on the level of factor IX activity. In its most severe form (conventionally defined by circulating factor IX activity levels of <1%), symptoms can become apparent early in lifeCitation1. Individuals with plasma levels >1%, although defined as having a moderate deficiency by laboratory assay, may in some cases demonstrate a phenotype of severe musculoskeletal and other hemorrhages. Bleeding, including spontaneous bleeding in the absence of recognized trauma, is the main symptom of the disease, and this usually increases when the infant becomes mobile. Recurrent bleeds may lead to severe arthropathy, joint contractures and pseudotumors, resulting in chronic pain, disability, and reduced quality of lifeCitation1,Citation2. Current standard of care for hemophilia B with a severe bleeding phenotype (and severe bleeding risk) is intravenous infusion of replacement factor IX concentrate at regular intervals. The primary goals are prevention of bleeding episodes and the rapid and definitive treatment of bleeds that may occur even with regular prophylaxisCitation1. While the occurrence of bleeds is not completely predictable, factor IX activity levels have generally been used as a surrogate to estimate the risk of bleeding. The current guidelines indicate that while a trough factor level of 1% was previously deemed an adequate goal, converting a person with a severe phenotype into a moderate or mild phenotype, factor levels of 1–3% are insufficient to totally prevent bleeds and the risk of bleeding remains, therefore, targeting higher trough levels (>3–5%, or higher) has become preferableCitation1.
Gene therapy offers the potential for a new treatment paradigm for people with hemophilia. The cells transduced with the liver-directed vector are able to produce coagulation factor at a steady level, shifting the clinical picture of the disease from a severe to a milder (or even normal) bleeding phenotypeCitation3.
Etranacogene dezaparvovec is a gene therapy developed for the treatment of hemophilia B. It comprises a recombinant adeno-associated viral (AAV) vector serotype 5 (rAAV5) capsid containing the coding sequence for the Padua variant of the human coagulation factor IX, which is a highly active variant of factor IX resulting in activity levels 5–8 times higher compared with wild-type factor IXCitation4. Etranacogene dezaparvovec is a derivative of AMT-060Citation4,Citation5. Each of these gene therapy products are the same other than AMT-060 contains the wild-type F9 gene, whereas a change of only two nucleotides in the therapeutic gene expression cassette results in the expression of the Padua F9 transgene from etranacogene dezaparvovec. The resultant wild-type and Padua coagulation factor IX differ by only one amino acidCitation4,Citation5. AMT-060 has been studied in a Phase 1/2 clinical trial in people with severe or moderately severe hemophilia B (factor IX activity levels of ≤2%)Citation5. Etranacogene dezaparvovec has been assessed in the same target participant population in Phase 2bCitation4,Citation6 and Phase 3 clinical trialsCitation7. Study participants had a history of a severe bleeding phenotype at this level of factor IX deficiency, shown by the clinical requirement for routine prophylactic infusions of factor IX replacement products.
The Phase 2 b and Phase 3 clinical trials assessing etranacogene dezaparvovec are open-label, single-dose, single-arm, multicenter trials. Both trials have completed the initial efficacy endpoint evaluation and the longer-term follow-up is currently ongoingCitation4,Citation6,Citation7. While both trials have demonstrated the durability of sustained factor IX activity levels over the observed periods, durability over an extended period of time remains unknown. Other hemophilia B gene therapy programs have shown sustained factor IX activity levels over several yearsCitation8,Citation9. It is not known to what extent the long-term durability of gene expression may be affected by the presence of pre-existing antibody-mediated or cellular-mediated immune responses that recognize the AAV vector or by the anticipated normal slow turnover of end-differentiated hepatocytes.
Using statistical modeling, we present an analysis that estimates the long-term durability of factor IX activity levels after receiving etranacogene dezaparvovec, using data from the Phase 2 b and Phase 3 clinical trials. Statistical approaches are commonly used to make such predictions and, given the small database, linear mixed models are a good option as they allow for information sharing across subgroupsCitation10. The aim of this model and analysis was to estimate the durability of factor IX activity levels in clinical trial participants over an extended period of time after receiving etranacogene dezaparvovec. The proportion of 1000 future participants that would have factor IX activity levels <2% after receiving etranacogene dezaparvovec was also predicted. Both Bayesian and Frequentist linear mixed models were used to predict factor IX activity levels over the extended time period at both an individual and population level for a comprehensive analysis.
Methods
Data and population
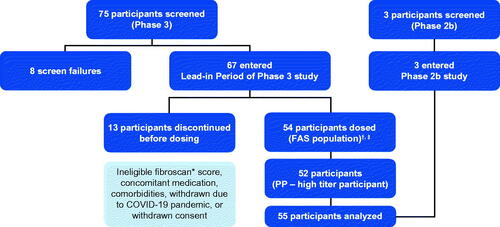
The population used in this analysis consists of all participants treated with etranacogene dezaparvovec in clinical trials including the Phase 2 b (N = 3; NCT03489291)Citation4,Citation6 and Phase 3 (N = 52; NCT03569891) studiesCitation7, with the exception of two participants who did not respond to treatment and continued to receive prophylactic factor IX replacement products (one participant received only a partial dose of etranacogene dezaparvovec, the other participant had a notably high AAV5 neutralizing antibody [NAb] titer of 3212)Citation7. Full inclusion/exclusion criteria for both trials are listed in the supplementary material (Table S1; Table S2). The two study populations were naively pooled and the combined set of 55 participants was used for statistical modeling and is referred to as the analysis population (). Details on constructing the analysis data are provided in the supplementary material. Regarding the two clinical trials, ethical approval has been obtained from the appropriate local Independent Ethics Committee (IEC) or Institutional Review Board (IRB) and informed consent has been obtained from all participants. All IECs and/or IRBs reviewed and approved the protocols prior to study initiation. All protocol amendments, informed consent form documents, relevant supporting information, and written study materials were also approved prior to their use. These trials are conducted in accordance with current applicable regulations, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) (E6[R2]), European Union (EU) Directive 2001/20/EC, EU Directive 2005/28/EC, Food and Drug Administration (FDA) of the United States (US) guidelines, Advanced Therapy Medicinal Products (ATMP) guidelines and its updates (ENTR/F/2/SF/dn D(2009) 35810), and local ethical and legal requirements.
Figure 1. Analysis population. *Or equivalent scan (magnetic resonance elastography, shear wave elastography); †FAS (N = 54) included participants who enrolled, entered the lead-in period, were dosed with etranacogene dezaparvovec and provided ≥1 efficacy endpoint assessment; ‡PP population (N = 53) included all participants from the FAS who adhered to a stable and adequate prophylaxis use during the lead-in period, completed ≥18 months of efficacy assessments, and had no major protocol deviations that impacted the interpretation of efficacy. In the Phase 3 clinical trial, one participant 75 years of age, died at 464 days (∼15 months) following etranacogene dezaparvovec infusion from cardiogenic shock, preceded by a urinary tract infection, which was considered unrelated to treatment. Abbreviations. FAS, full analysis set; PP, per protocol.
Assumption of baseline factor IX activity
Factor IX activity was observed by Week 3 following etranacogene dezaparvovec infusion in the 55 participants in whom continuous factor IX prophylaxis treatment was discontinuedCitation4,Citation6,Citation7. During the first ∼3–12 weeks after etranacogene dezaparvovec infusion, establishing stable factor IX expression may have differing kinetic profiles in individual participants. This may be impacted by the emergence of liver inflammation and associated supportive care with corticosteroids in some participants that may impact transgene expression. All participants in this analysis achieved relatively stable factor IX activity and discontinued any use of prophylactic factor IX replacement products and/or corticosteroids by Month 6 following etranacogene dezaparvovec infusionCitation4,Citation6,Citation7. Therefore, in this analysis, we only include factor IX activity levels measured at Month 6 post-infusion and beyond. Factor IX activity levels at Month 6 post-infusion are considered as the baseline measurement and will be referred to as the analysis baseline.
Factor IX activity levels <2% were assumed to correlate with a severe bleeding phenotype needing regular prophylactic treatment with factor IX replacement products, in alignment with the inclusion criteria for the Phase 2 b and Phase 3 studiesCitation4,Citation6,Citation7.
Assessment of factor IX activity
Factor IX activity was assessed by the one-stage activated partial thromboplastin time (aPTT) assay measured by central laboratory. Specific methodology in the central laboratory utilized ILS Hemosil reagents (Instrumentation Laboratory Company, Lexington, MA, USA) HemosIL SynthasIL, HemosIL factor IX-deficient plasma and HemosIL calibrator, with assays performed on an ACL TOP 300/500 instrument. Factor IX activity levels reported at each time point for the analysis population is the outcome variable of interest. Particularly, values that were measured more than five half-lives after the most recent administration of prophylactic factor IX replacement products (reflecting factor IX activity derived from etranacogene dezaparvovec only and not contaminated by the infused factor IX replacement products) were included in the durability analysis.
Covariates considered
While the process of transduction into the target cells following gene therapy administration is complex, one key determinant is the presence of NAbs against the AAV capsidCitation11. The capsid of the vector for etranacogene dezaparvovec is derived from AAV5 and therefore, prior exposure to the virus (including environmental exposure to AAV serotypes other than AAV5) may result in the presence of antibodies that can neutralize the infectivity of AAV5Citation12. Pre-existing AAV5 NAbs, especially in high titers, may prevent efficient transduction of hepatocytes and subsequently result in lower factor IX activity level, reducing the effectiveness of the gene therapyCitation11.
Due to the possible impact of pre-existing AAV5 NAbs, the pre-infusion AAV5 NAb status was considered an important covariateCitation13. NAbs are detectable, and a participant is classified as positive, when the NAbs titer is over the limit of detection (1:7); otherwise, a participant would be classified as AAV5 NAb negativeCitation14.
Alanine transaminase (ALT) elevations (or transaminitis) may be observed in participants receiving liver-targeted gene therapy. ALT elevations are usually detected within ∼3–12 weeks after infusion and are a marker of liver inflammation or liver cell injuryCitation15–19. It is hypothesized that in most cases, ALT elevations result from a T lymphocyte-mediated immune response against the liver cells that have been transduced by the AAV vectorCitation15,Citation18,Citation20. The targeted elimination of these cells may be associated with the partial or complete loss of gene expression that may be observed coincident with ALT elevationsCitation15,Citation18,Citation20. While the presence of pre-existing AAV NAbs prior to dosing has been implicated in reduced gene delivery and expression, there has been no association of pre-existing AAV5-directed cell-mediated immunity (or T lymphocyte immune response) and clinical outcomes in previous trials of gene therapy in hemophiliaCitation19. Furthermore, no demographic feature or biomarker has been identified that predicts the risk of ALT elevation following AAV-based gene therapy. Although ALT elevations have been reported at later time points, these are generally sporadic and transient (except in scenarios where prophylactic immune suppression has been used) and there is little evidence that these elevations arise from the same mechanism as the ALT elevations observed within the initial 90 days after AAV vector administrationCitation21.
This analysis assessed whether the level and the durability of factor IX activity was impacted by ALT elevations. ALT elevations were included as an exploratory endpoint in the clinical trials of etranacogene dezaparvovec, defined as either one of the following two events occurring within the initial 90 days post-infusion:
ALT > upper limit of normal when baseline ALT is below upper limit of normal, or
ALT > 2 ×baseline ALT
AAV5 NAb status was used as the primary covariate for this analysis as this variable is available pre-treatment (measured by using a sensitive luciferase-based NAb assay in the Phase 2 b clinical trial and a custom-developed, cell-based in vitro AAV5 transduction inhibition assay in the Phase 3 clinical trial) and can thus influence decision making. ALT elevations, on the other hand, are observed post-treatment, and it cannot be predicted who will develop ALT elevations, therefore, it is not considered a primary covariate. We present the results of the model with AAV5 NAb status only in the main text and the model with both AAV5 NAb status and ALT elevations in the supplementary materials.
Statistical methods
For modeling, factor IX levels were transformed from their original scale to log scale. This serves three main purposes. Firstly, the factor IX activity levels on the original scale are presumed to have a log-normal distribution, the model fits the observed data well on the log scale. Secondly, the log-transformed outcome is robust to outliers and measurement error, thus reducing bias in predicting long-term factor IX activity. Finally, the model estimates and predictions of factor IX activity are always positive when transformed back to the original scale, thus remaining biologically plausible.
Parameter estimates, as well as predictions of the long-term factor IX activities, were made in logarithmic scale and back transformed to the original scale for factor IX activity levels.
Linear mixed modeling approaches were implemented to predict long-term factor IX activity levels up to 25 years past the analysis baseline (which is defined as 6 months post-etranacogene dezaparvovec infusion) i.e. 25.5 years after receiving the etranacogene dezaparvovec infusion.
Time since analysis baseline (6 months post-infusion) and pre-infusion AAV5 NAb status (negative or positive) are used as covariates in the model. Each participant is allowed to have their own intercept and slope.
It is assumed that the unexplained modeling error is attributed to the aPTT assay measurement variability. However, only the underlying factor IX activity without measurement error is of clinical interest. When predicting factor IX activity, the estimate is made without residual error.
Two modeling approaches were used: Bayesian and Frequentist. Since reliable historical data was unavailable on factor IX activity levels after receiving gene therapy, the model parameters for the Bayesian modeling approach were assigned non-informative prior distributions. Underlying factor IX activity levels for the analysis population (N = 55) were then estimated and long-term durability was predicted through extrapolation. The parameters for the Frequentist model were estimated from the 55 participants included in the analysis, and then the long-term factor IX activity levels for these 55 participants were predicted using the estimated parameter values. Model selection criteria (AIC, BIC) were used to determine a final model.
Underlying factor IX activity levels of 1000 future participants were predicted from the Bayesian model conditional on analysis data; 60% (600) were assigned to the pre-infusion AAV5 NAb-positive group and 40% (400) to the pre-infusion AAV5 NAb-negative group; these proportions were the same as observed in the analysis population. Finally, to assess long-term durability of factor IX activity levels and the risk of bleeding, the proportion of predicted future participants with factor IX activity levels <2% was calculatedCitation22.
Results
The baseline demographics and other important variables for the analysis population are shown in . All participants were male with a mean age (standard deviation [SD]) of 41.4 years (15.2) and a median age (range) of 37.0 (19.0–75.0) years. The majority of participants (n = 44; 80%) had severe hemophilia B at the time of diagnosis. The mean (SD) for the observed factor IX activity levels for all participants (N = 55) at the analysis baseline (6 months post-infusion) was 38.8 (18.76) (). Stable and durable factor IX activity levels were observed up to 30 months post-analysis baseline (6 months post-infusion) ().
Figure 2. Distribution of observed factor IX activity levels over time. White point represents the mean value, the overlaid points on each boxplot show individual datapoints. Stable and durable factor IX activity levels are observed on average in the analysis population. Note: Most participants had not completed 24- and 30- month follow-up by database lock (participants are not lost to follow-up, just not observed yet).
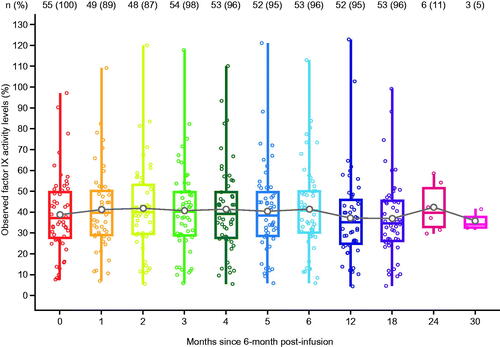
Table 1. Baseline characteristics of the analysis population.
Table 2. Number of participants and mean (SD) factor IX activity level at 6 months post-infusion by pre-infusion AAV5 NAb status and post-treatment ALT elevation.
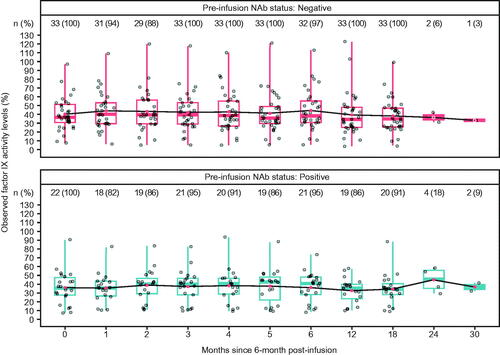
The number and mean factor IX activity levels according to pre-infusion AAV5 NAb status and ALT elevations are shown in . The observed mean (SD) factor IX activity levels for the AAV5 NAb-negative group (N = 33) and the AAV5 NAb-positive group (N = 22) were similar at the analysis baseline (6 months post-infusion): 40.6 (18.64) and 36.2 (19.05), respectively (). Stable and durable factor IX activity levels were also observed up to 30 months post-analysis baseline (6 months post-infusion) for both AAV5 NAb subgroups ().
Figure 3. Distribution of observed factor IX activity levels by AAV5 NAb status over time. Red point represents the mean value, the overlaid points on each boxplot show individual datapoints. Stable and durable factor IX activity levels are observed on average in both NAb subgroups in the analysis population. Note: Most participants had not completed 24- and 30- month follow-up by database lock (participants are not lost to follow-up, just not observed yet). Abbreviations. AAV5, adeno-associated virus 5; NAb, neutralizing antibody.
Bayesian model
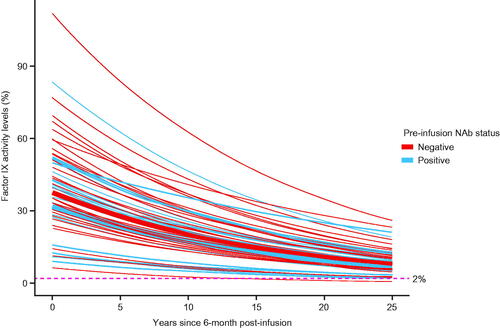
The model estimates (median) and 95% prediction intervals for factor IX activity levels extrapolated to 25 years post-analysis baseline (6 months post-infusion) are displayed in .
Figure 4. Estimated (median) factor IX activity levels extrapolated to 25 years post-analysis baseline with 95% prediction intervals: Bayesian approach with pre-infusion AAV5 NAb status as covariate (N = 55). Abbreviations. AAV5, adeno-associated virus 5; NAb, neutralizing antibody.
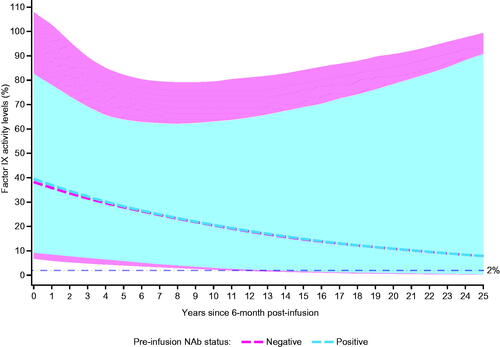
The model-based extrapolation of observed participants (N = 55) up to 25.5 years post-infusion shows long-term durability of factor IX activity levels. Overall, 6/55 (10.91%) participants were predicted to have factor IX activity levels <2% over 25.5 years, of which 3/33 (9.09%) participants belonged to AAV5 NAb-negative subgroup, and 3/22 (13.64%) participants belonged to the AAV5 NAb-positive subgroup (Figure S1).
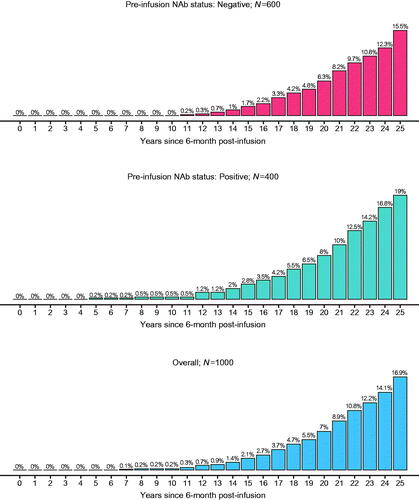
Model-based predictions of 1000 future participants resulted in <20% in each AAV5 NAb subgroup reaching factor IX activity levels below the bleeding threshold of 2% over the 25.5 years post-infusion ().
Figure 5. Predicted cumulative proportion of participants with factor IX activity levels <2% for 1000 future participants: Bayesian model-based predictions with pre-infusion AAV5 NAb status as a covariate. Percentages are based on N’s in each subgroup. Abbreviations. AAV5, adeno-associated virus 5; NAb, neutralizing antibody.
Frequentist approach
The estimated (mean) factor IX activity level trajectories for the 55 participants from the analysis baseline to 25.5 years post-infusion is shown in . The AAV5 NAb-positive subgroup had a slightly lower mean factor IX activity level, but the difference was not statistically significant. The model predicted that three participants (5.45%) in total, one (3.03%) in the AAV5 NAb-negative subgroup and two (9.09%) in the AAV5 NAb-positive subgroup would have factor IX activity levels <2% at 25.5 years after the analysis baseline (Table S3).
Discussion
Gene therapy provides a possible cure for people who suffer from diseases associated with faulty or missing genes. Ideally, those gene therapies target the root causes of the disease with a single (or limited) administration of treatment dose. For chronic diseases, gene therapy may be able to replace a lifetime of expensive maintenance therapies, which may lead to decreased treatment burden and to cost savings in the longer term. Yet, the uncertainty around the long-term durability of gene therapy is of concern to patients, treating physicians, regulators and payers. To address these concerns specifically related to etranacogene dezaparvovec, we analyzed 55 participants from the Phase 2 b and Phase 3 studies who responded to treatment, to predict the long-term durability of the selected outcome – etranacogene dezaparvovec produced factor IX activity levels as measured by central laboratory one-stage aPTT assay. More specifically, Bayesian and Frequentist linear mixed models were deployed to predict the factor IX activity level up to 25.5 years post-etranacogene dezaparvovec infusion at both the individual and population level.
All 55 participants showed durable factor IX activity levels up to 2 years after receiving etranacogene dezaparvovec. Durable factor IX activity levels were also observed for some participants (N = 3; 5.5%) who have had a longer follow-up observational period (up to 3 years after etranacogene dezaparvovec infusion). These findings are consistent with other gene therapy studies in hemophilia B where durable factor IX activity levels have been reported over 5Citation8 and 8 yearsCitation9. In addition, none of the 55 participants required prophylactic factor IX replacement products after receiving a single infusion of etranacogene dezaparvovecCitation4,Citation6,Citation7.
Linear mixed modeling approaches were implemented in this analysis to predict long-term factor IX activity. Linear mixed models can account for within- and between-participant variability in factor IX activity levels. Since not all of the included participants had factor IX activity levels recorded at each visit, the mixed modeling approach provides a simple alternative to handle missing data under the missing at random assumption without imputation. These features make it a viable and well-accepted approach for analysis of longitudinal dataCitation23,Citation24. Two modeling approaches are considered in this analysis: Bayesian and Frequentist. A Bayesian modeling approach is probabilistic in nature and views the model parameters as random variables while the analysis data are fixed. The Frequentist approach views the parameters in the model as fixed values and data as a random sample of the population. One advantage of this approach is the ability to estimate parameters without setting the prior distribution. An advantage of the Bayesian approach is the ability to predict outcomes for future data conditional on observed data. This model-based prediction accounts for uncertainty in future observations while considering inherent within- and between-participant variability.
Bayesian model-based predictions conditional on observed data further demonstrate the long-term durable factor IX expression for those who received etranacogene dezaparvovec, as over 80% are predicted to be free from prophylactic factor IX replacement products 25.5 years postinfusion. This finding is consistent with the model-based extrapolation of the 55 observed participants. Of all participants, 10.9% were predicted to have factor IX activity levels <2% 25.5 years after receiving the etranacogene dezaparvovec infusion, possibly requiring further prophylactic factor IX replacement products and, therefore, not achieving long-term durability with the gene therapy. Both the Bayesian and Frequentist linear mixed models demonstrate that the predicted factor IX activity levels are not significantly influenced by a participants’ pre-infusion AAV5 NAb status (). The impact of ALT elevation post-infusion was explored in a similar modeling approach and may impact on factor IX activity levels; however, these predictions need to be viewed with caution as the sample size in each subgroup is extremely small as seen in ; there are only three participants in the observed data with a positive pre-infusion AAV5 NAb status and a post-infusion ALT elevation (Figures S2–S5; Table S4).
Thus, based on the data observed, we predict that a single, full dose of etranacogene dezaparvovec will likely lead to durable factor IX expression for up to 25.5 years and prevent most people with hemophilia B from returning to prophylactic factor IX replacement products.
There are some limitations to this analysis. Firstly, since the predictions are based on 2–3 years of currently available data, long-term predictions need to be viewed with caution. Secondly, the Bayesian and Frequentist linear mixed models assume a linear relationship of log-transformed factor IX activity level over time, which is equivalent to an exponential growth/decay model in the original scale (i.e. the change of factor IX activity level would be more dramatic at the beginning but less so later on). While this assumption is biologically plausible, it is not completely verifiable due to limited available data. In fact, the UCL/St. Jude study demonstrated a durable factor IX activity level up to 8 years post-gene therapy infusion with no trend for declineCitation9. The model does not account for any sudden changes that can make an individual’s path deviate from the observed trajectory. For example, if a participant contracted hepatitis C; the relationship between the factor IX activity level will change over time (i.e. is not linear on the log-scale anymore). In the future clinical trial data, the model and predictions might need to be updated to account for this change. However, these sudden changes are difficult to predict as the frequency of such events is very low and no sudden changes in factor IX activity levels have been observed in the currently available clinical dataCitation4,Citation6,Citation7. In addition, over time hepatocyte cell turnover may reduce transgene expression, but this rate may be higher depending on a recipient’s liver health and individual characteristics. How AAV persists effectively in an epichromosomal state is unknown. They would not be expected to persist beyond the expected turnover of hepatocytesCitation25. However, clinical data clearly indicates that episomes can persist for yearsCitation26. Episomal persistence is critical to durability, the episome may be retained or replicated during hepatocyte cell turnover. The inverted terminal repeat of the gene therapy vector is likely to be involved in mediating plasmid persistence and prevention of epigenetic silencingCitation27–29. Further research is needed to enhance the knowledge and understanding of promoter silencing in gene therapy with tissue specific promoters before being able to draw definitive conclusions. Thirdly, a lot of variability was observed, especially at higher factor IX activity levels, which may cause bias in predicting factor IX activity levels in the long run through modeling, because of the inaccuracy of the model input data. Additionally, our model does not consider the development of factor IX inhibitors, however, to date no clinical trial assessing gene therapy for hemophilia has observed the development of factor inhibitors (for factor IX or factor VIII)Citation4,Citation7–9,Citation30–39. Preclinical evidence suggests that gene therapy for hemophilia A reduces the risk of inhibitor developmentCitation40. Lastly, two participants were not included in this analysis due to a lack of response and requirement of prophylactic factor IX replacement products. While one non-response was due to receiving only a partial dose of etranacogene dezaparvovec, for the other it is hypothesized that the non-response was due to an exceptionally high pre-infusion AAV5 NAb titer value. To understand the exact impact of these types of events, further data are required.
Conclusion
In summary, data analyzed from the 55 participants in the etranacogene dezaparvovec clinical trials demonstrated durable factor IX activity levels during the available follow-up period, after receiving one single dose of etranacogene dezaparvovec. As presented here, further predictive analysis based on Bayesian and Frequentist linear mixed modeling showed that those receiving etranacogene dezaparvovec would likely achieve durable factor IX activity levels and remain free from prophylactic factor IX product replacement therapies for up to 25.5 years following a single, full-dose infusion. These analyses suggest that etranacogene dezaparvovec can generate long-term, sustained, and durable factor IX activity levels. Having etranacogene dezaparvovec as a novel, alternative treatment option would ultimately benefit people with hemophilia B. The long-term factor IX durability predictions are based on statistical methods and results in vivo may differ.
Transparency
Declaration of funding
Funding for this analysis was provided by CSL Behring. The two clinical trials from which the data was collected for this analysis were sponsored by uniQure and CSL Behring.
Declaration of financial/other relationships
JS, KS, PEM and MF are employees of, and have stock ownership in, CSL Behring. HK is employed by Techdata service, funded by CSL Behring. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors have been involved in the conception and design of the analysis and involved in the assessment and interpretation of the data. All authors drafted and critically reviewed the paper for intellectual content. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work.
Ethical approval
All of the participants involved in the two clinical studies assessed in the manuscript have given informed consent to participate.
Reviewer_response_Suppl_Mat_Durability_assessment_of_Etranacogene_Dezaparvovec_FINAL_04102022.docx
Download MS Word (1.6 MB)Acknowledgements
The authors thank the study participants and acknowledge Nicholas Galante, Silpa Nuthalapati and Xiang Zhang of CSL Behring for their support and contribution to the analysis and manuscript development. Medical writing support was provided by Danielle Walsh and Danielle Lindley of Chrysalis Medical Communications, part of Nucleus Global Ltd, funded by CSL Behring.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, Jinesh Shah. The data are not publicly available as they contain information that could compromise the privacy of study participants.
References
- Srivastava A, Santagostino E, Dougall A, the WFH Guidelines for the Management of Hemophilia panelists and co-authors, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(S6):1–158.
- Buckner TW, Witkop M, Guelcher C, et al. Impact of hemophilia B on quality of life in affected men, women, and caregivers – assessment of patient-reported outcomes in the B-HERO-S study. Eur J Haematol. 2018;100(6):592–602.
- Batty P, Lillicrap D. Hemophilia gene therapy: Approaching the first licensed product. Hemasphere. 2021;5(3):e540.
- Von Drygalski A, Giermasz A, Castaman G, et al. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 2019;3(21):3241–3247.
- Miesbach W, Meijer K, Coppens M, et al. Gene therapy with adeno-associated virus vector 5–human factor IX in adults with hemophilia B. Blood. 2018;131(9):1022–1031.
- Gomez E, Giermasz A, Castaman G, et al. Etranacogene dezaparvovec (AAV5-Padua hFIX variant, AMT-061), an enhanced vector for gene transfer in adults with severe or moderate-severe hemophilia B: 2.5 year data from a phase 2b trial. Res Pract Thromb Haemost. 2021;5(Suppl 2):LPB0020.
- Miesbach W, Leebeek F, Recht M, et al. Final analysis from the pivotal phase 3 HOPE-B gene therapy trial: Stable steady-state efficacy and safety of etranacogene dezaparvovec in adults with severe or moderately severe hemophilia B. Haemophilia. 2022;28:25–126 (PO143).
- Samelson-Jones BJ, Sullivan SK, Rasko JEJ, et al. Follow-up of more than 5 years in a cohort of patients with hemophilia B treated with fidanacogene elaparvovec adeno-associated virus gene therapy. Blood. 2021;138(Suppl 1):3975.
- Nathwani AC, Reiss U, Tuddenham E, et al. Adeno-associated mediated gene transfer for hemophilia B: 8 year follow up and impact of removing "empty viral particles" on safety and efficacy of gene transfer. Blood. 2018;132(Suppl 1):491.
- Jones HE, Ohlssen DI, Neuenschwander B, et al. Bayesian models for subgroup analysis in clinical trials. Clin Trials. 2011;8(2):129–143.
- Majowicz A, Ncete N, van Waes F, et al. Seroprevalence of pre-existing nabs against AAV1, 2, 5, 6 and 8 in South african hemophilia a patient population. Blood. 2019;134(Suppl 1):3353.
- Colella P, Ronzitti G, Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev. 2018;8:87–104.
- Gross DA, Tedesco N, Leborgne C, et al. Overcoming the challenges imposed by humoral immunity to AAV vectors to achieve safe and efficient gene transfer in seropositive patients. Front Immunol. 2022;13:857276.
- Falese L, Sandza K, Yates B, et al. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24(12):768–778.
- Manno C, Pierce G, Arruda V, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–347.
- Nathwani AC, Reiss UM, Tuddenham EGD, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994–2004.
- George LA, Sullivan SK, Giermasz A, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med. 2017;377(23):2215–2227.
- Konkle BA, Walsh CE, Escobar MA, et al. BAX 335 hemophilia B gene therapy clinical trial results: Potential impact of CpG sequences on gene expression. Blood. 2021;137(6):763–774.
- Pipe S, Leebeek FWG, Ferreira V, et al. Clinical considerations for capsid choice in the development of liver-targeted AAV-based gene transfer. Mol Ther Methods Clin Dev. 2019;15:170–178.
- Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7(5):316–324.
- Chowdary P, Shapiro S, Makris M, et al. Phase 1–2 trial of AAVS3 gene therapy in patients with hemophilia B. N Engl J Med. 2022;387(3):237–247.
- Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian approaches to clinical trials and health-care evaluation: Statistics in practice. 1st ed. Hoboken (NJ): Wiley; 2004.
- O'Kelly M, Ratitch B. Clinical trials with missing data: a guide for practitioners (statistics in practice). 1st ed. Hoboken (NJ): Wiley; 2014.
- Verbeke G, Molenberghs G. Springer series in statistics: Linear mixed models for longitudinal data. 1st ed. New York (NY): Springer; 2000.
- Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137(2):466–481.
- Kattenhorn LM, Tipper CH, Stoica L, et al. Adeno-associated virus gene therapy for liver disease. Hum Gene Ther. 2016;27(12):947–961.
- Berns KI, Muzyczka N. AAV: an overview of unanswered questions. Hum Gene Ther. 2017;28(4):308–313.
- Ehrhardt A, Xu H, Kay MA. Episomal persistence of recombinant adenoviral vector genomes during the cell cycle in vivo. J Virol. 2003;77(13):7689–7695.
- Nichols T, Rakhe S, Arruda V, et al. Effect of growth on AAV-mediated expression of FIX-R338L in juvenile hemophilia B dogs. Res Pract Thromb Haemost. 2022;6(Suppl 1):OC 21.4.
- Xi M, Makris M, Marcucci M, et al. Inhibitor development in previously treated hemophilia a patients: a systematic review, meta-analysis, and meta-regression. J Thromb Haemost. 2013;11(9):1655–1662.
- Santoro C, Quintavalle G, Castaman G, et al. Inhibitors in hemophilia B. Semin Thromb Hemost. 2018;44(06):578–589.
- Miesbach W, Meijer K, Coppens M, et al. Five year data confirms stable FIX expression and sustained reductions in bleeding and factor IX use following AMT-060 gene therapy in adults with severe or moderate-severe hemophilia B. Res Pract Thromb Haemost. 2021;5(Suppl 2):OC 26.3.
- Chowdary P, Shapiro S, Makris M, et al. Factor IX expression within the normal range prevents spontaneous bleeds requiring treatment following FLT180a gene therapy in patients with severe hemophilia B: Long-Term follow-up study of the B-Amaze program. Blood. 2021;138(Suppl 1):3967.
- Pasi KJ, Laffan M, Rangarajan S, et al. Persistence of haemostatic response following gene therapy with valoctocogene roxaparvovec in severe haemophilia A. Haemophilia. 2021;27(6):947–956.
- Pasi KJ, Rangarajan S, Robinson TM, et al. Hemostatic response is maintained for up to 5 years following treatment with valoctocogene roxaparvovec, an AAV5-hFVIII-SQ gene therapy for severe hemophilia A. Res Pract Thromb Haemost. 2021;5(Suppl 2):OC 67.1.
- Ozelo MC, Mahlangu J, Pasi KJ, et al. Valoctocogene roxaparvovec gene therapy for hemophilia A. N Engl J Med. 2022;386(11):1013–1025.
- Visweshwar N, Harrington TJ, Leavitt AD, et al. Updated results of the alta study, a phase 1/2 study of giroctocogene fitelparvovec (PF-07055480/SB-525) gene therapy in adults with severe hemophilia a. Blood. 2021;138(Suppl 1):564.
- George LA, Monahan PE, Eyster ME, et al. Multiyear factor VIII expression after AAV gene transfer for hemophilia A. N Engl J Med. 2021;385(21):1961–1973.
- Monahan PE, Négrier C, Tarantino M, et al. Emerging immunogenicity and genotoxicity considerations of Adeno-Associated virus vector gene therapy for hemophilia. JCM. 2021;10(11):2471.
- Arruda AR, Samelson-Jones BJ. Gene therapy for immune tolerance induction in hemophilia with inhibitors. J Thromb Haemost. 2016;14(6):1121–1134.