Abstract
Objectives
Patients with type 2 diabetes nowadays have a wide range of new antidiabetic medications with better efficacy and safety. Physicians’ attitude toward selecting antidiabetic medications to reach targeted glycemic control and better quality of life (QOL) has not been studied prospectively. The global DISCOVER study aims to comprehensively provide a real-world assessment of the treatment pattern changes for patients with type 2 diabetes, in addition to QOL assessment. The Kingdom of Saudi Arabia was one of the countries participating in the DISCOVER study program.
Methods
This study is a part of the prospective, longitudinal multinational DISCOVER study conducted in 38 countries including Saudi Arabia, a country facing an epidemic of type 2 diabetes, recruited 519 adult patients with type 2 diabetes with a mean age of 52.4 ± 11 years, where, they were followed up for three years period, where 477 patients completed the follow-up period. The clinical, biochemical, and patient lifestyle data were assessed periodically during the study period. DISCOVER study is registered with ClinicalTrials.gov identifiers: NCT02322762.
Results
The most frequently used antidiabetic medications (ADMs) initially and during the follow-up were biguanides (metformin) and sulfonylureas (gliclazide, glibenclamide, glimepiride, glipizide, and glyclopyramide). Insulin (premix Insulin, basal insulin, and basal/bolus insulin) and dipeptidyl peptidase-4 (DPP-4) inhibitors (sitagliptin, vildagliptin, saxagliptin, and linagliptin) were the most frequent second and alternative of therapy. Other medications namely thiazolidinediones (TZds) (pioglitazone and rosiglitazone), incretins (exenatide and liraglutide), and Sodium-glucose co-transporter-2 (SGLT-2) inhibitors (canagliflozin) were used at a lesser rate. Drug availability, efficacy, and safety were the main determinants for choosing antidiabetic medications. The physical component score of the QOL had shown a significant decrease, while the mental component score has demonstrated an increase in QOL using SF36v2 Survey.
Conclusions
There is an increasing trend of using of newly available ADMs, mainly DPP-4 inhibitors. The major limitation of ADMs use is related to efficacy, availability, and safety. This warrant taking all the measures to overcome these limitations through adopting a multidisciplinary team approach for close monitoring of the patients and any unfavorable side effects. Additionally, global insurance coverage for all patients with type 2 diabetes could be a solution for the drug availability factor.
Introduction
Nowadays, there are several classes of anti-diabetic medications (ADMs) that are administered either orally or subcutaneously which differ in their mechanisms of action and both glycemic and extra-glycemic effectsCitation1. Although the management guidelines for type 2 diabetes are updated on regular bases according to evidence-based medicine, the trend of real-world prescription could be affected by several factors, such as medication efficacy, costs, and side effect profile, in addition to physicians’ choice and patient preferencesCitation2. With such broad choices of ADMs classes, the paradigm of therapy options for patients with highly heterogeneous glycemic and cardiovascular risk factors has changed significantlyCitation2. However, how this has occurred in a real-world setting, especially in the switching from old to new classes of ADMs as initial and intensification therapy options, has not been studied comprehensively.
The new classes of ADMs are associated with a significantly lower risk of hypoglycemia compared with sulfonylureas (SU) and insulinCitation3. The neutral effect on weight or benefits of weight reductions has also been well-established for some of the new classes including the incretinsCitation4. Given the benefits of the new agents, a fall in the use of SU or insulin as intensification therapies could be expected. However, studies assessing the possible benefits of using newer ADMs in terms of delaying the need for insulin are lackingCitation5,Citation6. Therefore, understanding the changing patterns of therapy initiation and intensifications with second and third-line therapies, in combination with the heterogeneous patients’ characteristics, is a vital requirement.
The pattern of prescribing anti-diabetic medications was previously assessed in some countriesCitation7,Citation8. In Taiwan for instance, the prescribing patterns of oral antidiabetic drugs over 7 years (1997–2003) were shifted from old classes, such as SU to newer classes with the prescribing patterns moving toward combination therapy, especially triple oral therapyCitation7. On the other hand, as per the data of the Adelphi Real World Diabetes Disease Specific Programmes, metformin monotherapy was most commonly prescribed by primary care providers, increasing to a peak of 44% in 2012 before dropping to 36% in 2015Citation8.
Additionally, Type 2 diabetes as a chronic metabolic disease has a major impact on QoL in terms of various domains, such as social, physical, and mental well-being. A declining QoL score and depression can also strongly influence a patient’s commitment to controlling his disease and medication adherenceCitation9.
The progressive changes in the proportional distributions across all classes of ADMs, and determinants of switching to the second-and third-line ADMs, have not been explored enough. Additionally, the longitudinal assessment of different aspects of the QOL among patients with type 2 diabetes is also lacking.
One of the aims of the DISCOVER study is to comprehensively provide a real-world assessment of the treatment pattern changes for patients with type 2 diabetes, in addition to QOL assessment among patients with type 2 diabetes in the Kingdom of Saudi Arabia.
Methods
Study design and population
The current analysis is a part of the global DISCOVER study program that included two similar, 3-year, non-interventional, prospective studies that were conducted simultaneously in 38 countries: DISCOVER (NCT02322762) in 37 countries and J-DISCOVER (NCT02226822) in JapanCitation10,Citation11. The studies included a total of 15,992 adult participants and were conducted in six regions as per the World Health Organization categories including; Africa (812 participants); Americas (2002 participants); Southeast Asia (3360 participants); Europe (3479 participants); Eastern Mediterranean (2182 participants); and Western Pacific (4157 participants). The Kingdom of Saudi Arabia was one of the countries in the Eastern Mediterranean region in which the study was conducted in nine hospitals, from four out of the five provinces in the Kingdom. The study included a total of 519 adult patients with type 2 diabetes who were non-insulin users and were initiating a second-line glucose-lowering therapy (either as an add-on or switching from first-line oral monotherapy to dual therapy or triple therapy). Patients using an injectable agent, namely insulin or GLP-1-receptor agonist, were excluded. The exclusion criteria also included type 1 diabetes, pregnant women, patients using herbal remedies or natural medicines alone, and patients undergoing dialysis or with a history of renal transplantation. The patients were recruited between 31 December 2014 and 30 June 2016 and were followed up for 36 months after their initial enrollment.
Data source
The Data for all eligible patients who signed informed consent were collected by trained participating physicians using an electronic case report form (eCRF). All the clinical data including laboratory investigations were collected at baseline and during the follow-up periods. All eCRFs were audited for completeness and were saved in a central database. Data were reported according to routine clinical practice at each site. The participating physicians were mainly endocrinologists/diabetologists (77.8%), and other specialties included general practitioners (GP)/Family medicine (11.1%) and internal medicine (11.1%).
Baseline characteristics
At baseline, the demographic, socioeconomic, and risk factors data were collected along with vital signs, mainly blood pressure and pulse rate, anthropometric characteristics including weight, height, and body mass index, and laboratory tests including Aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (γ-GTP), alkaline phosphatase (ALP), serum creatinine, serum albumin, fasting serum insulin (IRI), hematocrit, white blood cell, hemoglobin, platelet, serum C-peptide, high-sensitivity C-reactive protein (CRP), serum amylase, a serum ketone body, serum uric acid, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, urinary test data (if routine urinary test data are available). Urinary test data include urinary glucose (qualitative), urine protein (qualitative), urine protein (quantitative), urine albumin (quantitative), urinary ketone (qualitative), urinary albumin to creatinine ratio, and urine protein to creatinine ratio. Diabetes past medical and treatment history along with concomitant medication and co-morbidities were also collected at baseline, see Supplementary Appendix 1.
The follow-up data were obtained from patient records at 6, 12, 24, and 36-month time points with a ± 2-month buffer period. This included vital signs (weight, height, BMI, waist circumference, pulse rate, systolic and diastolic blood pressure), new comorbidities, and concomitant medications of interventions. The HbA1c fasting, random, and 2-h post-prandial blood glucose levels at the time of the visit. were measured and recorded by routine clinical practice in each health care facility. Diabetes medication changes, other non-diabetic medication changes, from baseline, incidence, and progression of microvascular and macrovascular complications since the last visit were all collected during each follow-up visit. Minor hypoglycemic events in the last month and major hypoglycemic events in the last 12 months were collected at baseline and during each follow-up visit. Reasons for changing first-line therapy and reasons for choosing second-line therapy were entered into the data capture system by the participating physicians, who selected one or several reasons from predefined lists.
Quality of life assessment
The QoL was assessed at baseline and through the follow-up period using the Arabic version of the 36-item Short Form Health Survey version 2 (SF-36v2) which is a generic measure covering eight dimensions: physical functioning, role limitations caused by physical health problems, bodily pain, general health perception, vitality, social functioning, role limitations caused by emotional health problems, and mental healthCitation12. Scores on all the subscales are transformed linearly to a possible range of 0–100; higher scores indicated more favorable physical functioning/psychological well-being. These eight domains were further aggregated into two summary measures: the physical component summary and the mental component summary measuresCitation13. The questionnaire was administered according to the RAND Corporation terms and conditions available at (https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/terms.html).
Statistical methods
The mean values were presented with standard deviations (±SD) and the medians with interquartile ranges (IQR). The categorical data are presented as numbers and percentages. Multiple imputations were used to account for unreported data and missing values. Imputation was carried out using IVEware (University of Michigan). All statistical analyses were performed using the SAS statistical software system (SAS Institute, Inc., Cary, NC, USA). QOL results were summarized descriptively and stratified as per different follow-up points. The bubble chart presentation is used to statistically calculate the different dynamics of the used drugs during the study period.
All patients consented to be enrolled in this study. This study was reviewed and approved by the Institutional Review Board, College of Medicine, King Saud University for the university hospitals (E-14-1299), the Institutional Review Board of King Fahad Medical City for the Ministry of Health hospitals (FWA0001877), and the International Medical Center Ethics Committee for the International Medical center (2016-02-044 A1), and the Armed Forces Hospital, southern region (H-06-KM-001). The Saudi German Hospital Local Ethics Committee reviewed and approved the study, for the Saudi German Hospital (IRBC/865/15). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The study is registered in Clinical Trial.gov with ClinicalTrials.gov identifiers: NCT02322762.
Results
Baseline characteristics
A total of 519 patients were recruited with a mean age of 52.4 ± 11 years and median (IQR) of 53 (44.9–58.3) years with 54.7% males and 45.3% females with a mean body mass index (BMI) of 31.9 ± 6.6 kg/m2 and mean HbA1c of 8.8 ± 1.7%. Hypertension affected 37.8% of the patients, while hyperlipidemia was reported among 43.5% as shown in Supplementary Appendix 1. A total of 477 patients (91.9%) completed the 36 months of follow-up visits and had clinical and biochemical assessments, while 42 (0.9%) patients were lost to follow-up.
Rate of ADMs initiation and discontinuation
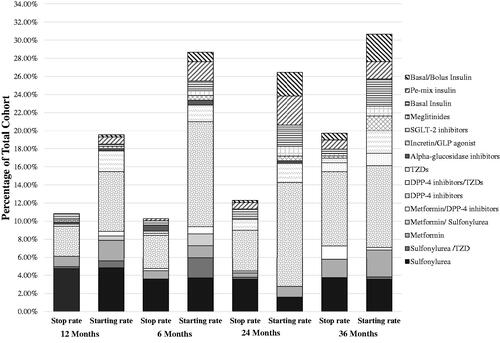
The study has shown an increasing trend for changing ADMs during the three years study duration, especially in the first year. The most frequently initiated medication is insulin regardless of its type, while DPP-4 inhibitors are the most frequently chosen as a second-choice oral medication. The ADMs stopping rate ranged from 10.84% during the 6 months follow-up to 19.71% during 36 months follow-up, where the sulfonylureas and DPP-4 inhibitors were the most frequently stopped. The rate of stopping or initiating metformin was stable during the follow-up period. During the three years follow-up, 30.65% of the patients needed to initiate new ADMs, where DPP-4 inhibitors alone or with TZDS were the most frequently used as shown in , and Supplementary Appendix 3.
Figure 1. The percentages of stopped and started anti-diabetic agents according to the classes during point time follow-up.
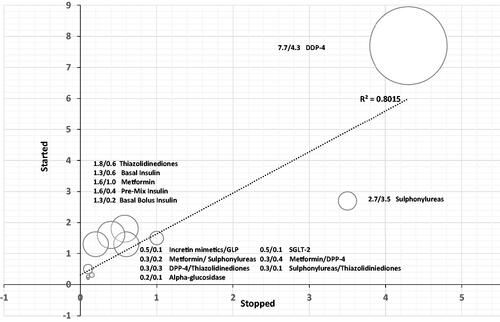
The trend of ADMs use is demonstrated by the percentage of starting any medication presented on the y-axis or stopping any medication on the x-axis as shown in the bubble graph (, and Supplementary Appendix 4). The bubble size is representing the actual number of patients subjected to the medication change. The DPP-4 inhibitors had a higher starting rate compared to the discontinuation rate and a large number of patients presented by larger bubble size, while the sulfonylureas (SUs) had a higher rate of discontinuation compared with initiation and a smaller number of patients with smaller bubble size. Metformin, TZDs, and insulin had higher rates of starting than discontinuation but with small differences ranging from 0.6 to 1.2% compared to what has been observed with DPP-4 inhibitors at 3.4%. Other ADMs showed either no or very small differences between starting and discontinuation rates.
Figure 2. Bubble chart presenting the relationship between the start and stop of different anti-diabetic drugs when chosen as the second choice.
The reasons for changing previous therapies and choosing the new therapy during the study follow-up are shown in , where efficacy was the most common reason for switching and/or intensifying the regimen in 53.4% of cases. The accessibility was the second common reason behind either stopping or starting ADMs during the first year of follow-up. Changing the ADMs based on the physician preference was found in up to 11.4% of cases, while side effects were behind changing the therapy in only 5.7% of the patients. The patient request or convenience played a minor role as a reason for changing the regimen. The beneficial effect on weight and the lower risk of hypoglycemia were considered as reasons for selecting the new ADMs rather than reasons for changing the old therapy. The cost of the ADMs had minimal effect on the physicians’ or patients’ decision in changing or selecting the ADMs.
Table 1. Reason for changing previous therapies and choosing the new therapy during the study follow-up.
Through the study time points, the rate of using concomitant medications, mainly antihypertensive and lipid-lowering agents was increasing. One-third of the studied cohort (35.6%) was receiving antihypertensive medications at baseline reaching 54.9% by the end of 36-month follow-up period. The Angiotensin-converting enzyme inhibitors (ACIs) were the most commonly used class to control blood pressure at a rate ranging from 30.1 to 46.3% during the entire follow-up period. This was followed by calcium channel antagonists, beta blockers then diuretics with rates reaching 15.7, 14.7, and 11.7%, respectively at the end of the three years of follow-up. At baseline, 53.9% of patients were receiving lipid-lowering agents increasing to 86.2% by the end of the follow-up period. Statins, either in low or high dose, was the most commonly used lipid-lowering agent. During the first six months of follow-up, one-third of the cohort used antiplatelet therapy and this rate reached up to 60.4% by the end of the follow-up period, where Aspirin is the most commonly used antiplatelet agent. A total of 11.3% of the studied cohort received treatment for thyroid disease mainly hypothyroidism as shown in .
Table 2. Non-diabetic medications were used by the study participants over the follow-up period.
Quality of life assessment
demonstrates the dimensions of the QOL of the study participants throughout the study period. Out of the eight dimensions of the SF-36v2 QOL survey, only the physical function score had shown a significant drop, while the other seven dimensions have demonstrated a significant increase. When looking at the total physical and mental component score, there was a clear and significant decrease in the physical component score with a significant increase in the mental component score. The physical component score decreased by 1.2 points, while the mental component score increased by 5.1 points.
Table 3. The patient’s quality of life score was reported during each follow-up visit using a quality of life questionnaire (SF-36v2 Health Survey).
Discussion
In a country facing an epidemic of type 2 diabetes, proper selection for antidiabetic medications would minimize chronic complications that will reflect both the direct and indirect costs of this disease. Both physicians’ and patients’ behaviors toward drug selection are mainly affected by the health system, drug availability, drug safety, and cost. The international multicenter global DISCOVER study aimed at assessing the characteristics, treatment, and outcomes of patients with type 2 diabetes mellitus failing first-line management had demonstrated the management discrepancies in the 37 studied countriesCitation10.
In the studied cohort, the mean HbA1c had dropped significantly from the baseline level during the 36 months of follow-up (8.8–8.2%) indicating a beneficial outcome related to the second-line management used in the nine recruited centers, although it did not reach the optimal levelCitation14. This finding should be interpreted with caution since other factors that could have negatively affected diabetes control, such as poor medication adherence and unhealthy lifestyleCitation15.
The most common first-line drug used in the studied cohort was metformin either as a monotherapy or in combination with other oral agents followed by sulfonylureas that match what has been observed in the global dataCitation14. This was expected due to the low cost and long history of use for such medications which are the first drugs of choice as per the guidelinesCitation16.
The picture was different when looking at the choice for second-line medications, where DPP4-inhibitors and insulin were the most commonly prescribed medications in this cohort which was different from the global dataCitation14 since metformin and sulfonylureas continued leading the second-line therapy in Africa, South-East Asia, and the West Pacific region. Such variation shed the light on the fact that the selection of second-line therapy is likely to be driven by the low cost of the drugs rather than clinical evidence, particularly in low- to middle-income countries, where the use of metformin as an add-on to sulfonylurea is the predominant pattern of prescriptionCitation17. This difference could be also due to the free healthcare system provided to the patients with type 2 diabetes in this study which limits the effect of the cost as a barrier to initiating the new ADMs.
The high rate of prescribing insulin in the current study despite the clinical inertiaCitation18 could be due to several factors related to the study cohort including poor glycemic control (HbA1c of 8.8%) and the old age of the study population (mean age of 52.4 years), especially when 75% of the study population aged ≥45 years old, as reported by previous DISCOVER publication from Saudi ArabiaCitation19. This observation also reflects the attempt of the treating physicians to adhere to the American Diabetes Association (ADA) guidelines, where prescribing insulin as second-line therapy is recommended if the HbA1c exceeds 8.5%Citation16.
The most frequently stopped medication was sulfonylurea which was replaced by insulin therapy being also one of the most frequently selected therapies as second-line for 8.7% in the total cohort which was not different from what has been observed in the global data reflecting the effect of guidelines adoption by most of the recruited centers globallyCitation14.
The current study data revealed that both old and new drug classes for managing diabetes are currently used for optimal treatment. Other ADMs namely alpha-glucosidase inhibitors (AGIs), TZDs, meglitinides, or new medications namely: GLP-1 agonists, and SGLT-2 inhibitors had almost equal chances of being selected as second-line management. Acarbose, a type of AGI, which lowers post-prandial blood glucose, is still used as add-on therapy for type 2 diabetes in this cohort which is consistent with the observation in another ethnic group (Taiwan)Citation20. This is probably because of its relatively cheap price and lesser possibility of hypoglycemia, particularly in elderly patients.
Despite the risks associated with its useCitation21,Citation22, pioglitazone, being the predominantly used TZDs drug, was started at almost the same rate over the three years of follow up which is indicating that pioglitazone is still having a role in the treatment of type 2 diabetes mellitus. Such a finding reflects the debate and contradictory opinions concerning the benefits and risks of TZD therapy, especially when a recent meta-analysis demonstrated that patients treated with TZDs had a ∼30% lower risk of developing atrial fibrillationCitation23. Therefore, a more balanced view weighing the potential risks and benefits of TZDs is warranted to individualize the use of this medication with patients who will get the maximum benefits with lower risks, such as younger patients with prediabetes or early T2DMCitation24.
On the other hand, although the rates of starting and using the most recent classes, such as SGLT-2 inhibitors and incretins are increasing, they were not widely used except during the last visit that occurred in 2017. This is an expected finding since these medications were recently approved, have higher prices than the old classes, and are in an injectable form (only incretins)Citation25. In addition, many countries participating in DISCOVER have limited formularies with SGLT-2 inhibitorsCitation26.
Reasons for changing the previous therapy and selecting the new therapy were investigated by the DISCOVER study. In this study, seeking for better drug efficacy, drug accessibility, and physician preference were the most common reasons for stopping or changing the previous medication throughout the three years of follow-up. This was the same observation of Grant et al.Citation27, where the drug efficacy and achieving the target HbA1c level were the main reasons for changing the old therapy. Although drug efficacy and accessibility were the main determinants for choosing the new therapy, other reasons were behind this decision including drug tolerance, weight gain, hypoglycemic events, and patients’ preference. This was in line with the findings from a systematic review showing that the most important attributes for preferences for type 2 diabetes medications included changes in fasting blood glucose and HbA1c level, hypoglycemia events, weight changes, side effects, and drug costCitation28. However, for the studied population, the cost was not a factor due to the free availability of the medications either through government hospitals or insurance coverage.
The study results have shown a pronounced and significant increasing trend in prescribing antihypertensive medications and lipid-lowering agents compared with baseline (p-value < .0001). This high rate of prescribing these medications could be due to adherence to the international guidelinesCitation29 since the achievement of the target low-density lipoprotein cholesterol (LDL-C) and blood pressure levels are established to be associated with a substantial decrease in the overall CVD riskCitation30. This finding also does not exclude the fact that the treating physician of patients participating in DISCOVER study is more likely to adhere to guidelines in terms of prescribing both lipid-lowering agents and antihypertensive medications during the 3 years follow-up period. One of the most striking observations of the current study was the low prescription rate of the antiplatelet for such a relatively old cohort. Despite that the prescription significantly increased from 34.9% at the baseline to 60.4%, this is still below what is expected according to the guidelinesCitation31.
Health-QOL of life is a central parameter for the assessment of the burden of diabetes as well as the clinical effectiveness of care and treatmentCitation32. The QOL score related to the studied cohort at the baseline reflects being near to normative values. In the current study, over the follow-up period, all the mean score for all domains was near to or exceeding the normative value, except for the physical function score which significantly decreased. Although the mean physical component score significantly decreased, the difference in the mean score between the baseline and follow-up did not exceed the minimal clinically important difference for the SF-36v2 score which is more than three pointsCitation33,Citation34. On the other hand, the mental component means score increased significantly with a difference in mean scores approaching double the minimal clinically important difference. This observation could be explained by the fact that physical QOL could be hindered by the negative impact on patients’ perception that is associated with increased regimen complexity and the consequent increased risk of hypoglycemia. On the other hand, an increased sense of empowerment associated with improved glycemic control positively impacted the mental component of QOLCitation35,Citation36. Despite good health care and drug accessibility, patients’ QOL did not demonstrate major change or improvement.
This study has the strength of being part of an international multicenter study and representing different provinces and health sectors in addition to the low dropout rate of 8.1%. The study limitations are related to using the medical file review as a source of data which resulted in having some missing variables. The second limitation of this study is that it did not assess the patient’s adherence to their anti-diabetic medication. Another limitation of the study is that it did not assess the confounding factors that could be behind the improved scores on the quality of life assessment.
Conclusion
This study demonstrated that, even though patients had significantly reduced HbA1c, but they did not reach the target regardless of the medication used as a second choice. This warrant better patients’ and physicians’ education on the importance of good glycemic control that would reduce morbidity, mortality, and disease economic impact. Additionally, poor medication adherence could be a major confounding factor for achieving the targeted outcomes of diabetes management, therefore the multidisciplinary team approach should be considered and the role of pharmacists and diabetes educators in terms of following up on the patient’s level of adherence should be maximized. It was clear that drug selection is affected by efficacy, availability, and safety. This could be overcome by improving drug availability and close monitoring of the ADMs side effects. The new classes of ADMs had a good chance of being whenever they are available and affordable as observed with DPP-4 inhibitors and incretin use. Additionally, global insurance coverage for all patients with type 2 diabetes could be a solution for the drug availability factor. The improvement in the mean mental component score of QOL reflects the effect of improved diabetes control and close patients’ follow-up. Further longitudinal studies are needed to determine other factors associated with improved patients’ quality of life rather than good diabetes control.
Transparency
Declaration of funding
Funding was provided by AstraZeneca Company.
Declaration of financial/other relationships
All authors declared no competing interest. All views, scientific findings, conclusions, and recommendations mentioned in the study represent the sole opinion of the research team and do not in any way reflect the sponsoring company’s views. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
K.A. contributed to the conceptualization and design of the study. K.A., F.A., E.S., D.A., A.A., N.A., A.H., D.E., and A.Y. contributed to the study conduct. K.A. and A.Y. contributed to data analysis and interpretation of results. All authors contributed to revising the article critically for intellectual content. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Ethical approval
This study was reviewed and approved by the Institutional Review Board, College of Medicine, King Saud University, for the university hospitals (IRB# E-14-1229), by the Institutional Review Board of King Fahad Medical City for the MOH hospitals, and the International Medical Center Ethics Committee for the International Medical center, and the Armed Forces Hospital, southern region. The Saudi German Hospital Local Ethics Committee reviewed and approved the study, for the Saudi German Hospital. All patients were consented through a written informed consent form to be enrolled in this study.
Supplementary_file.docx
Download MS Word (18.3 KB)Acknowledgements
We acknowledge the support given by staff in different participating hospitals and the staff of the research and scientific center at Sultan Bin Abdulaziz Humanitarian City for the support given in writing up this manuscript.
Data availability statement
The data that support the findings of this study are available from the DISCOVER study database, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
References
- American Diabetes Association. Standards of medical care Diabetes–2017: summary of revisions. Diabetes Care. 2017;40(Suppl. 1): S4–S5.
- Montvida O, Shaw J, Atherton JJ, et al. Long-term trends in anti-diabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018;41(1):69–78.
- Paul SK, Maggs D, Klein K, et al. Dynamic risk factors associated with non-severe hypoglycemia in patients treated with insulin glargine or exenatide once weekly. J Diabetes. 2015;7(1):60–67.
- Waldrop G, Zhong J, Peters M, et al. Incretin-based therapy in type 2 diabetes: an evidence-based systematic review and meta-analysis. J Diabetes Complications. 2018;32(1):113–122.
- Mamza J, Mehta R, Donnelly R, et al. Important differences in the durability of glycaemic response among second-line treatment options when added to metformin in type 2 diabetes: a retrospective cohort study. Ann Med. 2016;48(4):224–234.
- Inzucchi SE, Tunceli K, Qiu Y, et al. Progression to insulin therapy among patients with type 2 diabetes treated with sitagliptin or sulphonylurea plus metformin dual therapy. Diabetes Obes Metab. 2015;17(10):956–964.
- Chiang CW, Chiu HF, Chen CY, et al. Trends in the use of oral antidiabetic drugs by outpatients in Taiwan: 1997–2003. J Clin Pharm Ther. 2006;31(1):73–82.
- Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380.
- Timar R, Velea I, Timar B, et al. Factors influencing the quality of life perception in patients with type 2 diabetes mellitus. Patient Prefer Adherence. 2016;10:2471–2477.
- Ji L, Bonnet F, Charbonnel B, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: rationale and methods of the DISCOVER observational study program. J Diabetes Complications. 2017;31(7):1188–1196.
- Katakami N, Mita T, Takahara M, et al. Rationale and design for the J-DISCOVER study: DISCOVERing the treatment reality of type 2 diabetes in a real-world setting in Japan – a protocol. Diabetes Ther. 2018;9(1):165–175.
- Ware J, Snow K, Kosinski M, et al. SF-36 health survey: manual and interpretation guide. Boston (MA): The Health Institute, New England Medical Center; 1993.
- Ware JE, Kosinski M, Keller SD. SF-36 physical and mental health summary scales: a user’s manual. Boston (MA): The Health Institute, New England Medical Center; 1994.
- Nicolucci A, Charbonnel B, Gomes MB, et al. Treatment patterns and associated factors in 14 668 people with type 2 diabetes initiating a second‐line therapy: results from the global DISCOVER study programme. Diabetes Obes Metab. 2019;21(11):2474–2485.
- Feldman BS, Cohen-Stavi CJ, Leibowitz M, et al. Defining the role of medication adherence in poor glycemic control among a general adult population with diabetes. PLOS One. 2014;9(9):e108145.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2009;32(1):193–203.
- Bazargani YT, de Boer A, Leufkens HG, et al. Selection of essential medicines for diabetes in low and middle income countries: a survey of 32 national essential medicines lists. PLOS One. 2014;9(9):e106072.
- Reach G, Pechtner V, Gentilella R, et al. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017;43(6):501–511.
- Al-Rubeaan K, Bana FA, Alruwaily FG, et al. Physicians’ choices in the first-and second-line management of type 2 diabetes in the kingdom of Saudi Arabia. Saudi Pharm J. 2020;28(3):329–337.
- Chu WM, Ho HE, Huang KH, et al. The prescribing trend of oral antidiabetic agents for type 2 diabetes in Taiwan: an 8-year population-based study. Medicine. 2017;96(43):e8257.
- Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macroVascular events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289.
- Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–2135.
- Zhang Z, Zhang X, Korantzopoulos P, et al. Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc Disord. 2017;17(1):9.
- American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1):S14–S80.
- Health G. Expanding access to newer medicines for people with type 2 diabetes in low-income and middle-income countries: a cost-effectiveness and price target analysis. Lancet Diabetes Endocrinol. 2021;9:825–836.
- Tummalapalli SL, Montealegre JL, Warnock N, et al. Coverage, formulary restrictions, and affordability of sodium-glucose cotransporter 2 inhibitors by US insurance plan types. JAMA Health Forum. 2021;2(12):e214205.
- Grant RW, Wexler DJ, Watson AJ, et al. How doctors choose medications to treat type 2 diabetes: a national survey of specialists and academic generalists. Diabetes Care. 2007;30(6):1448–1453.
- Toroski M, Kebriaeezadeh A, Esteghamati A, et al. Patient and physician preferences for type 2 diabetes medications: a systematic review. J Diabetes Metab Disord. 2019;18(2):643–656.
- American Diabetes Association. 9: Cardiovascular disease and risk management. Diabetes Care. 2017;40(Suppl 1):S75–S87.
- Fung CSC, Wan EYF, Chan AKC, et al. Statin use reduces cardiovascular events and all-cause mortality amongst Chinese patients with type 2 diabetes mellitus: a 5-year cohort study. BMC Cardiovasc Disord. 2017;17(1):166.
- Liu M, Zhuang X, Chen X, et al. Antiplatelet strategy in primary and secondary prevention of cardiovascular disease in patients with type 2 diabetes mellitus: a perspective from the guideline appraisal. J Diabetes Investig. 2021;12(1):99–108.
- Schunk M, Reitmeir P, Rückert-Eheberg IM, et al. Longitudinal change in health-related quality of life in people with prevalent and incident type 2 diabetes compared to diabetes-free controls. PLOS One. 2017;12(5):e0176895.
- Frendl DM, Ware JE Jr. Patient-reported functional health and well-being outcomes with drug therapy: a systematic review of randomized trials using the SF-36 health survey. Med Care. 2014;52(5):439–445.
- Maruish ME. User’s manual for the SF-36v2 health survey. 3rd ed. Lincoln (RI): Quality Metric Incorporated; 2011.
- Nomura M, Fujimoto K, Higashino A, et al. Stress and coping behavior in patients with diabetes mellitus. Acta Diabetol. 2000;37(2):61–64.
- Lau CY, Qureshi AK, Scott SG. Association between glycaemic control and quality of life in diabetes mellitus. J Postgrad Med. 2004;50(3):189–193; discussion 194.
