Abstract
Schizophrenia is a chronic, heterogeneous, severe psychiatric disorder characterized by a spectrum of symptomology and is associated with substantial morbidity and mortality. For the last 70 years, available treatments have shared blockade of dopamine D2 receptors as their primary mechanism of action (MOA), the efficacy of which has been limited by incomplete resolution of all symptoms as well as treatment non-response in a select subset of patients. In addition, antipsychotics are associated with class-related side effects attributed to this primary MOA, including extrapyramidal symptoms (EPS). The need for non-D2 treatment options for patients which offer a novel risk/benefit profile is therefore apparent. There has been substantial investment in the research and development of non-D2 drug candidates. However, none of these programs have received successful regulatory approval by the FDA (as of Oct 2022). In this article, the scale of industry-sponsored clinical trials for D2-based investigational pharmacological treatments in schizophrenia was quantified and compared with investigational compounds with non-D2 MOAs. In a dataset of 545 clinical trials identified in ClinicalTrials.gov from January 2002 to July 2022, total enrollments in trials of non-D2-based compounds for the treatment of schizophrenia summed to approximately 34,000 patients, compared with 27,144 patients for D2-based compounds. These data indicate that there remains substantial and ongoing investment in the development of novel non-D2 options for schizophrenia, with a success rate measured by regulatory approval that is well-below recent benchmarks for the broader category of CNS drugs. Improved trial design, conduct, endpoints, and analyses/methods may influence signal detection and reliability to support development and registration of non-D2 compounds.
Introduction
Since the introduction of chlorpromazine in the mid-1950s, more than 30 different antipsychotics have been developed and launched globally. Structurally, these compounds share a common pharmacophore that interacts and blocks dopamine D2 receptors as a requirement for clinical efficacy. Though incremental improvements have been made in these antipsychotics by varying receptor pharmacology, treatment response is limited to what is possible with this mechanism of action (MOA). Positive symptoms of schizophrenia can often be successfully treated via D2 receptor antagonism, consistent with what is known about the neurobiology of schizophrenia, including its genetic diathesis, and underlying abnormalities in the dopamine systemCitation1,Citation2. This mechanism, however, has limited effectiveness in the treatment of other key symptom domains, most notably negative symptoms (anhedonia, avolition, and social withdrawal) and cognitive symptoms (deficits in working memory and cognitive flexibility). Except for clozapine, lack of efficacy in treating positive symptoms occurs in about 30% of patients, which accounts for the conclusion by Leucht et al.Citation3 that few patients (23%) achieved a “good” response during short-term treatment.
In addition to limitations in the efficacy of D2 agents, antagonist activity at this receptor is associated with poor tolerability, and high rates of nonadherence and discontinuationCitation4,Citation5. For example, extrapyramidal side effects (EPSs) and hyperprolactinemia are significantly correlated with dopamine D2 receptor occupancyCitation6,Citation7. The prevalence of D2 receptor-related EPS is 25 − 30% for akathisia, 30–40% for Parkinsonism, and ∼25% for tardive dyskinesiaCitation8–10. Similarly, elevations in prolactin are another direct consequence of antagonist activity at the D2 receptor in the lactotrophCitation11. Antipsychotics (especially second-generation compounds) are also associated with clinically important adverse effects, most notably weight gain and metabolic disturbances that stem from actions related to their binding profiles, including binding to a range of non-D2 receptors. Due to the chronicity of schizophrenia and the need for long-term therapy, careful assessment of the benefit-risk profile of each antipsychotic agent is crucial for choosing the right medication for a given patient. The choice is frequently dictated by the adverse event profile of a drug, since differences in the efficacy of currently available antipsychotic drugs are relatively small, with exception of clozapine, compared with differences in their tolerability and safetyCitation12.
The low rates of “good” therapeutic outcomes in schizophrenia treated with currently available D2 compounds, coupled with the poor tolerability, and high rates of nonadherence and discontinuation during long-term treatment with these compounds, has spurred development programs that target drugs with non-D2 binding MOAs. These development programs are supported by a growing body of research that has broadened the understanding of the underlying pathophysiology of schizophrenia to include dysfunction in several different neurotransmitter circuits, including glutamatergic, muscarinic, GABAergic, and trace amine-associated receptor (TAAR1) receptor systemsCitation12–14.
The pharmaceutical industry has invested more into the treatment of schizophrenia than for any other mental disease disorder including Major Depression Disorder and Bipolar Disorder. Assuming typical average costs globally for a completer in an acute schizophrenia trial, in the experience of the authors, the cost is in the range of $100–150,000 per patient over the last 10 years, with costs steadily increasing. If considered separately, current costs for clinical trials for schizophrenia in the United States are considerably higher and on the order of close to $200,000 per patient. The overall research and development cost, based on total enrolled patients, is in the range of $9–12-billion over the past 20 years. However, despite this substantial research investment, the yield in approved drugs is surprisingly low, and does not appear to be improving as the focus of research has increasingly turned to drugs with a non-D2 binding MOAs. To characterize the current state of clinical drug development in schizophrenia, we have used the trial registry ClinicalTrials.gov to investigate clinical drug development in schizophrenia over the last 20 years, with specific focus spent on the magnitude of research devoted to D2 vs. non-D2 compounds.
Methods
A selection of 545 clinical trials was identified in ClinicalTrials.gov since 2002 using the following search terms: Funder = “Industry,” Condition = “Schizophrenia”, and Phase = “Phase 2 or Phase 3.” The search was last updated on 8 July 2022. Trials including bipolar patients or patients where psychosis was a comorbid diagnosis were excluded. Trials were excluded if the primary outcomes were safety or pharmacokinetic endpoints, or if the trial involved a new formulation of a previously FDA-approved D2 compound. Investigational compounds were classified in terms of their non-proprietary name and dominant pharmacological activity and were classified as having D2-based pharmacological activity (“D2”), or not (“non-D2”). Trials were classified according to primary endpoints: 1) trials targeting reduction in a scale of total schizophrenia symptoms, 2) trials targeting improvement in negative symptoms, 3) trials targeting cognition in schizophrenia, or 4) trials targeting relapse criteria. Enrollment numbers (n) were summed across different trials of the same compound, across different trials of the same endpoints, and across class of compound pharmacology. Total enrollments were reported rounded to the nearest two significant figures.
Results
According to ClinicalTrials.gov, there were 203 industry-sponsored clinical trials conducted since January 2002 designed to test the efficacy of a novel treatment in schizophrenia patients. Of these, 138 (∼68%) were conducted with compounds having non-D2 mechanisms compared to 65 (32%) with D2-based mechanisms. The combined total enrollment in efficacy studies of non-D2 compounds was ∼34,000 patients compared with ∼27,000 patients enrolled in studies of D2-based compounds. More non-D2 compounds (n = 82) were tested in efficacy studies relative to D2 compounds (n = 23).
For D2 compounds, there were nine drugs evaluated in 2 or more Phase 2/3 efficacy trials (, left panel): asenapine (8 trials), lurasidone and paliperidone (6 trials each), cariprazine and brexpiprazole (5 trials each), lumateperone (3 trials), and iloperidone (1 trial) and 3 additional drugs that were not approved for schizophrenia: bifeprunox (3 trials), sertindole (3 trials), and armodafinil (2 trials).
Figure 1. Summary of 203 industry-sponsored Phase II/III clinical trials conducted since January 2002 designed to test the efficacy of novel dopamine D2 and non-D2 treatments for schizophrenia. The pie charts summarize the proportion of subjects randomized in Phase 2/3 clinical trials. In the line graph (for both D2 and non-D2 mechanisms), the top black line represents the cumulative number of subjects enrolled into Phase 2/3 efficacy studies over time. The green line (total symptoms) represents the cumulative number of subjects enrolled in efficacy studies where the primary measure was “total symptoms” (i.e. PANSS total score); the blue line represents the cumulative number of subjects where a cognitive assessment was the primary measure; and the lower black line represents the cumulative number of subjects enrolled in studies investigating compounds that are not listed in the linear time-plots at the bottom of each panel. In the linear time-plots of D2 drugs, the hatched lines represent start dates of Phase 2/3 studies that were completed prior to FDA approval of the listed compound. In the linear time-plots of non-D2 drugs, the hatched lines represent the start dates of the Phase 2/3 clinical studies.
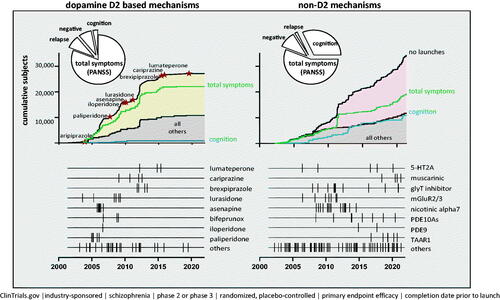
For non-D2 compounds, the number of Phase 2/3 efficacy trials conducted, by MOA, were as follows: glycine-1 transporter inhibitors (n = 17 trials; n = 8 trials for bitopertin), nicotinic alpha7 agonists or positive allosteric modulators (n = 16 trials; n = 4 trials for EVP-6124), phosphodiesterase (PDE)-10 inhibitors (n = 8 trials), TAAR1 agonists (ulotaront [4 trials], ralmitaront [2 trials]; muscarinic agonists (xanomeline + trospium chloride [N = 4 trials] (, right panel).
The total enrollment in Phase 2/3 studies evaluating the efficacy of non-D2 compounds (∼34,000 patients in 138 trials) represents a substantial fraction of the overall investment in clinical development programs for the indication schizophrenia. Not surprisingly, given the crowded competitive landscape, the interest in the development of novel D2 compounds has waned somewhat over the last 20 years. Over this period of time, 140 clinical trials (∼57,000 patients) have been recorded in ClinicalTrials.gov on D2 compounds with efficacy endpoints (total symptoms, cognition, negative symptoms, and relapse). However, 75 of these clinical trials (>30,000 patients) investigated new formulations (extended release) or alternative routes of administration (IM depot) for D2 compounds that had previously been approved by the FDA. Consequently, only 65 clinical studies (27,000 patients) have investigated novel D2 compounds.
In terms of primary efficacy endpoints, trials targeting reductions of total schizophrenia symptoms (i.e. Positive and Negative Syndrome Scale [PANSS] total score) were the most represented, based on the regulatory precedence of demonstrating clinical benefit in patients with schizophrenia. Alternative efficacy endpoints targeting reductions in negative or cognitive symptoms have been incorporated most frequently in study designs with non-D2 compounds, likely in an attempt to demonstrate efficacy in key schizophrenia symptom domains that are minimally responsive to treatment with D2 compounds. For example, in D2 clinical trials, approximately 81% of registered Phase 2/3 trials reported a measure of total symptoms (PANSS, Brief Psychiatric Rating Scale [BPRS]) as the primary endpoint, while only ∼3% reported a cognitive measure of efficacy (as either a primary or secondary endpoint). By contrast, 32% of Phase 2/3 trials of non-D2 compounds reported a cognitive measure of efficacy (either as a priori primary or secondary endpoint): 10,856 patients enrolled in 53 trials of 38 different compounds utilized a cognitive measure. Furthermore, 3500 patients in 16 trials of 13 different non-D2 compounds utilized a measure of negative symptoms as an efficacy endpoint.
Discussion
The results of this clinical trial registration database review highlight the substantial continued interest in the development of non-D2 medications for schizophrenia. Patient enrollment in non-D2-based schizophrenia trials (∼34,000 patients) in the last 20 years has surpassed that of D2 compounds (∼27,000 patients). This investment appears to be motivated by two main factors. First, the limited efficacy of both first- and second-generation D2 antipsychotics, especially in the treatment of negative and cognitive symptom domainsCitation15,Citation16. This has led to a notable increase in the inclusion, for example, of cognitive measures of efficacy (∼3% of studies of D2 vs. 32% of non-D2 antipsychotics). Nonetheless, it should be noted that total number of trials designed to evaluate the efficacy of non-D2 compounds on the cognitive symptom domain in schizophrenia remains relatively small.
A second factor motivating the investment in non-D2 compounds is the adverse safety profile of D2 antipsychotics that includes EPSs, prolactinemia, weight gain, metabolic syndrome, and increased risk of cardiovascular morbidity and mortality.
With a 30-year interval between the advent of the first- and second-generation antipsychotics, another 30 years has now passed without the introduction of a new class of drugs with a novel, non-D2 MOA. The lack of marketed non-D2 options is not due to lack of investment in clinical development programs, but rather to the lack of successful trials leading to regulatory approval.
Before reviewing possible reasons for this state of affairs, it is worth stepping back and examining the comparative success rate for regulatory approval in the US of the broader category of CNS drugs compared to other therapeutic categories. Several analyses are available that have examined trends in clinical development success rates from 2000 to 2017Citation17,Citation18. Both analyses found CNS drugs to have one of the lowest regulatory approval success rates, with one study estimating that a CNS drug entering Phase I had a 19.3% probability of successfully being launched during the time-period of January 2000 to October 2015Citation17. The more recent analysisCitation18, examining drugs in development from 2010 to 2017, found a 3% probability of successful launch for a CNS drug entering Phase I, and an 8% probability for a CNS drug that had entered Phase II. Both CNS drug success rates were the lowest launch success probabilities of any drug category. For CNS drugs that have entered Phase III, the launch probability was 49%, perhaps somewhat encouraging, but still one of the lowest success rates of all therapeutic categoriesCitation18.
The clinical development success rate identified in the current review for antipsychotic drugs is even lower than the rates reported for the broader category of CNS drugsCitation19. The magnitude of the failure rate would be expected to be higher for drugs with a novel and unproven MOA (such as non-D2 antipsychotics), in part because of the obvious possibility that a novel MOA simply might not be effective for a given non-D2 CNS target.
The “non-D2” category included non-D2 compounds which do not occupy, directly interact with, or block the dopamine D2 receptor; however, many of them may still indirectly affect dopamine neurotransmission. For example, serotonergic compounds (5-HT2A antagonists, 5-HT1A partial agonists) increase prefrontal dopamine levels via mesocortical neuronsCitation20,Citation21. Conversely, phosphodiesterase (PDE10a) inhibitors alter dopamine receptor signaling leading to inhibition of dopamine neurotransmission, particularly in the basal ganglia where PDE10a is expressedCitation22. Agonists and positive allosteric modulators at mGluR2 and mGluR5 receptors attenuate DA release in the nucleus accumbensCitation23. Lastly, drugs with TAAR1/5-HT1a agonist activity, such as ulotaront, are thought to modulate dopamine, serotonin, and glutamate neurotransmission, as well as affecting dopamine synthesisCitation14,Citation24.
Candidate non-D2 compounds failing in schizophrenia trials may, indeed, be “true negatives” in that the targeted MOA is actually ineffective. However, uncertainties around clinical development failures persist, along multiple other factors, several of which are especially relevant for CNS drug development, including the lack of identification of proper drug targets due to the complexity of the disease and the inadequacy of animal models in terms of both their limited translational value and their lack of predictive validity in human populations. This is especially true for models of cognitive function and negative symptoms. In Phase 2/3 trials of non-D2 compounds over the past 10–15 years, the marked increase in utilization of negative symptom and cognitive efficacy measures has exposed the extent to which animal models of these schizophrenia symptom domains have minimal-to-no face validity, construct validity, or predictive validity for human trialsCitation19,Citation25,Citation26. Recently, artificial intelligence algorithms have been utilized in an attempt to identify novel MOAs and to improve the ability of pre-clinical models to predict the efficacy of candidate compounds in human populationsCitation27.
In addition to the reduced utility of pre-clinical models, clinical trial designs (in Phases 1/2/3) have not always been optimal due various factors (sometimes attributable to financial constraints), including lack of power to detect moderate effect sizes (or, conversely, early phase studies that are so underpowered that the risk of a false positive study is high…eventuating in clinical development failure in Phase 3). A major contributor to clinical development failure is lack of assay sensitivity in the clinical trial itself. Many factors can contribute to lack of assay sensitivity in clinical trials, including high placebo response, which has been growing over timeCitation28,Citation29 (the hypothesized reasons for which are beyond the scope of this article). The heterogeneity of the clinical presentation of schizophrenia and the typically chronic and complex history of individual patients (e.g. in terms of treatment history – especially if recently discontinued, and comorbidity) also contribute significant heterogeneity that is not well-controlled even by the most stringent entry criteria.
Finally, standard outcome measures, such as the PANSS, for assessing efficacy in schizophrenia RCTs may also have poor reliability and poor assay sensitivity, especially of for detecting drug effects in negative and cognitive symptom domains. The current authors have previously reportedCitation30 that the items that comprise the PANSS subscales have a relatively high degree of between-domain correlation. For this reason, improvement, for example, in the negative symptom domain may largely be attributable to improvement in the positive symptom domainCitation30.
In an effort to demonstrate efficacy in poorly responsive treatment domains (e.g. negative and cognitive symptoms), enrichment strategies have been utilized intermittently over the past few decades in schizophrenia RCTs. Enrichment strategies in treatment studies of negative symptoms in schizophreniaCitation31, have not been particularly successful. In an attempt to address some of the shortcomings of current enrichment strategies, we have recently tested a novel alternative data-analytic enrichment strategy that limits inclusion to patients with low (screen-to-baseline) measurement variability for the specific symptom domain of interestCitation32.
Study limitations
The main limitation of the current survey of clinical trials over the past decade is that the search was conducted using only one clinical trials registry (clinicaltrials.gov). There are 17 registries included in the WHO’s International Clinical Trials Registry Platform (ICTRP)Citation33. Notably, the clinicaltrials.gov registry does not share trial data with ICTRP; furthermore, the overlap between ICTRP and clinicaltrials.gov is not known.
Conclusions
The investment footprint of industry-sponsored clinical trials of novel, non-D2 compounds in schizophrenia is substantial and currently is comparable to the investment in the class of D2-based antipsychotics. However, over the last 20 years, efforts across broad arrays of pharmacological classes of 56 total non-D2 compounds have yielded no new approvals. Given the scale of these investments and their lack of success to-date, alternative designs are needed for more efficient Phase 2/3 signal-detection to support development and registration of non-D2 compounds in schizophrenia.
Transparency
Declaration of funding
Supported by funding from Sunovion Pharmaceuticals Inc., Marlborough, MA.
Declaration of financial/other relationships
Seth C. Hopkins, Robert Lew, Courtney Zeni, and Kenneth S. Koblan are employees of Sunovion Pharmaceuticals Inc. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception of this study, the interpretation of the clinicaltrials.gov data, the drafting and revising of the article, and the final approval of the version to be published; all authors agreed to be accountable for all aspects of the work.
Acknowledgements
Dr. Edward Schweizer of Paladin Consulting Group, funded by Sunovion Pharmaceuticals, Inc., provided editorial assistance in preparing revised drafts of the manuscript. He has provided permission to be acknowledged.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19(1):15–33.
- Kraguljac NV, McDonald WM, Widge AS, et al. Neuroimaging biomarkers in schizophrenia. Am J Psychiatry. 2021;178(6):509–521.
- Leucht S, Leucht C, Huhn M, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174(10):927–942.
- Higashi K, Medic G, Littlewood KJ, et al. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3(4):200–218.
- Taub S, Krivoy A, Whiskey E, et al. New approaches to antipsychotic medication adherence - safety, tolerability and acceptability. Expert Opin Drug Saf. 2022;21(4):517–524.
- Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514–520.
- Kapur S, Roy P, Daskalakis J, et al. Increased dopamine d(2) receptor occupancy and elevated prolactin level associated with addition of haloperidol to clozapine. Am J Psychiatry. 2001;158(2):311–314.
- Miller DD, Caroff SN, Davis SM, et al. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br J Psychiatry. 2008;193(4):279–288.
- Caroff SN, Hurford I, Lybrand J, et al. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127–148, viii.
- Carbon M, Hsieh CH, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264–e278.
- Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28(5):421–453.
- Kaar SJ, Natesan S, McCutcheon R, et al. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704.
- Correll CU, Abi-Dargham A, Howes O. Emerging treatments in schizophrenia. J Clin Psychiatry. 2022;83(1):SU21204IP1.
- Dedic N, Dworak H, Zeni C, et al. Therapeutic potential of TAAR1 agonists in schizophrenia: evidence from preclinical models and clinical studies. Int J Mol Sci. 2021;22(24):13185.
- Spark DL, Fornito A, Langmead CJ, et al. Beyond antipsychotics: a twenty-first century update for preclinical development of schizophrenia therapeutics. Transl Psychiatry. 2022;12(1):147.
- Köster LS, Carbon M, Correll CU. Emerging drugs for schizophrenia: an update. Expert Opin Emerg Drugs. 2014;19(4):511–531.
- Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273–286.
- Dowden H, Munro J. Trends in clinical success rates and therapeutic focus. Nat Rev Drug Discov. 2019;18(7):495–496.
- Zhu T. Challenges of psychiatry drug development and the role of human pharmacology models in early development. Front Psychiatry. 2020;11:562660.
- Díaz-Mataix L, Scorza MC, Bortolozzi A, et al. Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J Neurosci. 2005;25(47):10831–10843.
- Pehek EA, Nocjar C, Roth BL, et al. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31(2):265–277.
- Zagórska A, Bucki A, Partyka A, et al. Design, synthesis, and behavioral evaluation of dual-acting compounds as phosphodiesterase type 10A (PDE10A) inhibitors and serotonin ligands targeting neuropsychiatric symptoms in dementia. Eur J Med Chem. 2022;233:114218.
- Gupta I, Young AMJ. Metabotropic glutamate receptor modulation of dopamine release in the nucleus accumbens shell is unaffected by phencyclidine pretreatment: in vitro assessment using fast-scan cyclic voltammetry rat brain slices. Brain Res. 2018;1687:155–161.
- Dedic N, Jones PG, Hopkins SC, et al. SEP-363856, a novel psychotropic agent with a unique, Non-D2 receptor mechanism of action. J Pharmacol Exp Ther. 2019;371(1):1–14.
- Gribkoff VK, Kaczmarek LK. The need for new approaches in CNS drug discovery: why drugs have failed, and what can be done to improve outcomes. Neuropharmacology. 2017;120:11–19.
- Scannell JW, Bosley J, Hickman JA, et al. Predictive validity in drug discovery: what it is, why it matters and how to improve it. Nat Rev Drug Discov. 2022. DOI:10.1038/s41573-022-00552-x
- Vincent F, Nueda A, Lee J, et al. Phenotypic drug discovery: recent successes, lessons learned and new directions. Nat Rev Drug Discov. 2022. DOI:10.1038/s41573-022-00472-w
- Kemp AS, Schooler NR, Kalali AH, et al. What is causing the reduced drug-placebo difference in recent schizophrenia clinical trials and what can be done about it? Schizophr Bull. 2010;36(3):504–509.
- Rutherford BR, Pott E, Tandler JM, et al. Placebo response in antipsychotic clinical trials: a meta-analysis. JAMA Psychiatry. 2014;71(12):1409–1421.
- Hopkins SC, Ogirala A, Loebel A, et al. Transformed PANSS factors intended to reduce pseudospecificity among symptom domains and enhance understanding of symptom change in antipsychotic-treated patients with schizophrenia. Schizophr Bull. 2018;44(3):593–602.
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–534.
- Hopkins SC, Tomioka S, Ogirala A, et al. Assessment of negative symptoms in clinical trials of acute schizophrenia: test of a novel enrichment strategy. Schizophr Bull Open. 2022;3:sgac027.
- Juneja A, Gupta J, Yadav N, et al. An overview of primary registries of WHO's international clinical trial registry platform. Ayu. 2019;40(3):141–146.
