Abstract
Objective
Providing evidence on donepezil and memantine administration as extemporaneous combination (DM-EXT) to treat Alzheimer Disease (AD) in Italy, and describing demographic and clinical features of AD patients prescribed DM-EXT.
Methods
Retrospective observational study using IQVIA Italian LifeLink Treatment Dynamics (LRx) and Longitudinal Patient Database (LPD). Prevalent users of DM-EXT were identified on the databases (cohorts DMpLRx and DMpLPD) including patients with donepezil and memantine overlapping prescriptions during the selection period (DMpLRx: “July 2018–June 2021”; DMpLPD: “July 2012–June 2021”). Demographic and clinical profiles of patients were provided. Starting from cohort DMpLPD, new users of DM-EXT were selected to calculate treatment adherence. Three additional cohorts of prevalent users of DM-EXT were identified on IQVIA LRx over subsequent 12-month periods, from July 2018 to June 2021, to get national-level yearly estimates accounting for database representativeness.
Results
Cohorts DMpLRx and DMpLPD included 9862 and 708 patients, respectively. For both cohorts, two-third of patients were female, and more than half were aged 80+. Concomitant conditions and co-treatments prevalence was very high; most frequent comorbidities included psychiatric and cardiovascular diseases. An intermediate-to-high adherence was observed in 57% of DM-EXT new users. National-level yearly estimates showed an increasing trend (+4%) in DM-EXT prescription, which led to estimate about 10,000 patients being treated during the period “July 2020–June 2021”.
Conclusions
Prescription of DM-EXT is a common practice in Italy. Because the administration of fixed-dose (FDCs) instead of extemporaneous combinations improves treatment adherence, the introduction of an FDC containing donepezil and memantine might enhance AD patients’ management and reduce caregiver burden.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, accounting for 60–70% of all dementia casesCitation1. AD affects about 5% of adults over 60 years of ageCitation2, with this translating into an estimate of around 850,000 people currently suffering from this disease in ItalyCitation3. As of now, the only drugs approved for the treatment of AD are acetylcholinesterase inhibitors (AChEIs), namely donepezil, galantamine, and rivastigmine, and the N-methyl-d-aspartate antagonist, memantineCitation4. AChEIs have modest and temporary effects on improving cognitive and behavior functionCitation5, and are indicated for the treatment of mild to moderately severe ADCitation6; memantine helps improving memory, awareness, daily activitiesCitation7 attenuating aggressive behaviourCitation8, and has the indication for the treatment of moderate-to-severe ADCitation6. The joint use of memantine and AChEIs might favorably address both pathological aspects and it is commonly administered in patients who are in moderate to severe stages of the diseaseCitation9. In 2012, the European Medicine Agency (EMA) expressed a negative opinion to the marketing authorization of two fixed-dose combinations (FDCs) of memantine and donepezil, whose expected indication was the treatment of dementia in patients with moderate-to-severe AD who were already taking the two molecules at the same dosagesCitation10,Citation11.
Since 2012, many new studies on the combination therapy have been published, new decisions have been taken by Health AuthoritiesCitation12, and new Guidelines have been released by important Clinical Societies suggesting considering the addition of memantine in AD moderate-to-severe patients already assuming AchEIsCitation13–16. In particular, in 2014, the United States Food and Drug Administration (FDA) approved a FDC of memantine extended release and donepezil for patients with moderate-to-severe ADCitation12; in 2021 the commercialization of a FDC of memantine and donepezil was authorized also by the Spanish Medicine AgencyCitation17.
Many epidemiological studies have been published in Europe demonstrating a clinical utilization of the combination therapy in patients with moderate-severe ADCitation1,Citation18–28. Furthermore, a retrospective study conducted on seven Ambulatory Centers for Dementia in Italy and designed to assess effectiveness and safety of combined memantine and AChEIs found that the mean Mini-Mental State Examination (MMSE) total score increased from baseline up to the end of treatment in patients taking combined donepezil and memantine. Also, the proportion of patients with adverse events was in line with the expected range for an elderly and high-comorbid population receiving complex multidrug regimensCitation18. Nonetheless, a recent study by Gareri and colleagues endorsed the use of AChEIs plus memantine plus citicoline (a cholinergic precursor with neuroprotective properties), versus AChEIs plus memantine aloneCitation29.
In Italy, since the introduction in 2009 of the modification to Nota 85 by the Italian Medicine Agency (AIFA), prescriptions of AChEIs are reimbursed by the National Health System (SSN) only for patients with mild or moderate AD, while prescriptions of memantine are reimbursed only for patients with moderate AD. In addition, the diagnosis of AD must be performed by a specialist working in a CDCD (Centro Disturbi Cognitivi e Demenze), who also is in charge for filling a therapeutic plan for the patientCitation30. Currently, an FDC of memantine and AChEIs is not available in Italy.
The main objective of the present study was to produce real-world data on the use of donepezil and memantine as an extemporaneous combination to treat AD in Italy, whilst secondary objective was to provide a description in terms of demographic and clinical characteristics of AD patients prescribed with the extemporaneous combination of donepezil and memantine.
Methods
Data sources
This was a retrospective observational cohort study using data from the Italian IQVIA LifeLink Treatment Dynamics (LRx), which contains both GPs and specialists’ prescriptions information on a patient level collected from retail pharmacy computers, and IQVIA Longitudinal Patient Database (LPD) database, which collects patients’ information from GPs. Indeed, because AD is mostly managed in a specialist care setting and prescriptions of AChEIs and memantine is still regulated by the long-dated AIFA Note 85Citation30 the joint use of the above data sources allowed a much more overarching perspective on the real-world prescription habits related to AD.
Italian IQVIA LRx database contains prescriptions’ information on a patient level collected from retail pharmacies computers with a 70% and a 50% coverage for retail and on behalf of Local Health Authorities (DPC) distribution channel, respectively. Indeed, thanks to a historical partnership with FEDERFARMA and ASSOFARM, IQVIA gets information, with monthly frequency, of SSN reimbursed products, which pharmacies have to communicate to Ministry of Health in order to get reimbursement. Italian IQVIA LRx collected information include encrypted date of birth, gender, age class (5 years bound), pharmacy anonymous code per micro-area, product\pack dispensed, and date of pharmacy dispensation. The concatenation between information of date of birth and sex (present in the prescription) in the micro-area of the dispensing pharmacy served as a proxy for the identification of the anonymous patient and allows tracking prescriptions by patient over time. Appropriate eligibility rules eliminate pseudo-patient codes that, with high probability, are spurious patients or patients with interrupted stories, providing solidity to the database. Prescription databases allow covering several specialty physicians and pharmacies and represent an important asset in terms of information. For this reason, IQVIA longitudinal prescription databases have been previoulsy employed in the conduction of a large number of drug utilization studies in multiple countriesCitation31–35 and in ItalyCitation36,Citation37.
The IQVIA Italian LPD provides routine care information from physician consultations, including diagnoses according to the International Classification of Diseases 9th revision (ICD-9), drug prescriptions according to the Anatomical Therapeutic and Chemical (ATC) classification system, as well as medical and demographic data obtained from the computer systems of a representative sample of general practitioners (GPs) throughout Italy. GPs voluntarily agreed to contribute to the general practice database and to attend specific training courses for data entry. Currently, about 900 GPs contribute to the database, providing data from routinely collected records of about 1.2 million patients. The IQVIA Italian LPD, which is representative of the Italian general population in the care of the GP in terms of age and genderCitation38, has been shown to be a reliable source of information in numerous previous studies for several disease areasCitation39–45.
Study populations and study design
Prevalent users of the extemporaneous combination of donepezil and memantine (i.e. both patients newly prescribed with the combination as well as patients already treated with the combination before start of observation) were separately identified using the two data sources, thus two different cohorts were built. Specifically, cohort DMpLRx represented prevalent users of the extemporaneous combination identified through IQVIA LRx, while DMpLPD represented the cohort of prevalent users of the extemporaneous combination built on IQVIA LPD. For both cohorts, in order to be included in the study, patients received at least one prescription of memantine whose duration overlapped with that of donepezil during the selection period, whichever occurred first. The selection period considered for cohort DMpLRx was the 3-year period “1st July 2018–30th June 2021”, while the selection period for the cohort DMpLPD was the 9-year period “1st July 2012–30th June 2021”. The date when donepezil and memantine first overlapped for each patient was termed Index Date. In addition, patients belonging to cohort DMpLPD were observed for a 12-month period before the Index Date (baseline) as well as for the six-month period starting on the Index Date (follow-up). For donepezil, only prescriptions with a strength of 10 mg were included in the analysis. Moreover, for both molecules, only SSN-reimbursed prescriptions were considered. Indeed, according to reimbursability criteria defined by AIFACitation30, the exclusion of non-reimbursed prescriptions prevented authors from including in the study patients who had not been correctly diagnosed with AD by a specialist working in a CDCD centre. Cohort DMpLRx was intended to provide a quantification of patients prescribed with the extemporaneous combination, while both cohorts served to delineate patients profile in terms of demographic (DMpLRx and DMpLPD) and clinical (DMpLPD) characteristics. Furthermore, with the aim of calculating treatment adherence during the six-month follow-up, starting from DMpLPD cohort, we excluded patients who had already received overlapping prescriptions of donepezil and memantine during the six-month period preceding the Index Date, and we identified incident users of the extemporaneous combination (cohort DMiLPD) (i.e. patients starting treatment during the selection period).
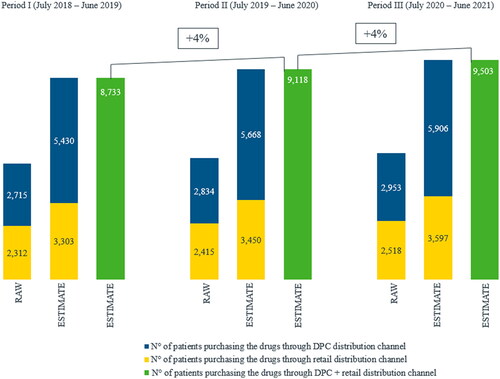
Three additional cohorts were generated on IQVIA LRx adopting the same approach used to define DMpLRx cohort over subsequent 12-month periods (namely, period I: “1st July 2018–30th June 2019”; period II: “1st July 2019–30th June 2020”; period III: “1st July 2020–30th June 2021”). Number of patients in the three cohorts were expanded according to the distribution channel through which patients purchased their Index Date’s prescriptions and to the representativeness of the distribution channels on IQVIA LRx (70% and 50% for retail and DPC channel, respectively). As such, we obtained national-level yearly estimates of the number of patients prescribed with the extemporaneous combination for the three periods so to understand whether there was a trend in term of prescriptions habits over time.
Study definitions
Adherence to therapy is the extent to which patients follow their medication schedules as prescribed by their doctorsCitation46. Treatment adherence was evaluated in terms of proportion of days covered (PDC), which corresponds to the total days of supply of medication dispensed over the length of the corresponding follow-up (i.e. 180 days). The PDC metric has been advocated by the Pharmacy Quality Alliance as the preferred indicator for estimating adherence to therapies for chronic diseasesCitation47 and it is known to provide a more conservative estimate of medication adherence compared to other measures in cases of concomitant multiple medications usageCitation48. The number of days supplied by each prescription was calculated by dividing the total amount of active drug in each prescription by the corresponding daily maintenance dose (DMD). The maximum daily dosage for memantine is 20 mg. Patients shall assume 5 mg, 10 mg, and 15 mg of memantine once daily during the first, second, and third week of treatment respectively; starting from the fourth week of treatment, patients shall assume 20 mg of memantine once dailyCitation49; a dosage of 20 mg was considered as the DMD for memantine. The maximum daily dosage for donepezil is 10 mg. Patients shall assume 5 mg of donepezil once daily for at least the first month of treatment, then the doctor can decide to increase the daily dosage to 10 mgCitation50; a dosage of 10 mg was considered as the DMD for donepezil. Days of supply contributed to the numerator only when memantine and donepezil overlapped.
Information extracted from the database
Information extracted from the databases to characterize prevalent users of the extemporaneous combination included age class and sex for IQVIA Italian LRx (cohort DMpLRx) and age, sex, body mass index, and comorbidities (defined through ICD-9 codes; both 10 most frequently recorded comorbidities and a pre-defined set of comorbidities of interest were collected), treatments (defined through ATC codes; both 10 most frequently recorded treatments and a pre-defined set of treatments of interest were collected), and neurologist and geriatrist visit referrals during baseline for IQVIA Italian LPD (cohort DMpLPD) . Moreover, the analysis on adherence to the treatment with the extemporaneous combination of donepezil and memantine performed on the incident users of the combination used information on all 10 mg donepezil prescriptions and on memantine prescriptions recorded during the 6-month follow-up period for each patient.
Statistical analysis
Descriptive statistics were used to provide an overview on demographic and clinical characteristics, and on treatment adherence for the extemporaneous combination users: qualitative variables were described in terms of frequencies and percentages, while quantitative variables were described in terms of mean value, standard deviation (SD), median, and first and third quartiles (Q1 and Q3). According to already published studiesCitation46,Citation51–53, treatment adherence was classified as low when PDC was lower than 40%, intermediate when PDC was in the range 40–79%, and high when PDC was at least 80%. Finally, because IQVIA LRx database includes reimbursed prescriptions dispensed through either retail (70% coverage) or DPC (50% coverage) distribution channel, we estimated the national-level number of patients prescribed with the extemporaneous combination during period I, II, and III and considering distribution channels representativeness on IQVIA LRx. In particular, patients were classified as retail or DPC depending on the distribution channel related to their Index Date prescription. Then, the estimate of the national-level number of patients for each period was calculated as the sum of the number of DPC patients multiplied by 100 and divided by 50, plus the number of retail patients multiplied by 100 and divided by 70. The yearly variation between period I and II was calculated as the difference in the estimate of the national-level number of patients of period II and that of period I, divided by the estimate of the national-level number of patients of period I and multiplied by 100. The same approach was applied to calculate the yearly variation between period II and III.
Finally, two sensitivity analyses were performed: (1) prevalent users of the extemporaneous combination of donepezil and memantine were identified and quantified using the approach described for the main analysis, but also 5 mg donepezil prescriptions contributed to patients inclusion in the cohorts for both IQVIA LRx and LPD cohorts; (2) we calculated the proportion of patients who had at least two donepezil and memantine overlaps and/or a single overlap lasting more than 28 days for both DMpLRx and DMpLPD cohorts.
All the analyses presented here were performed using SAS software (Version 9.4) and did not involve any new study of human or animal subjects performed by any of the authors: IQVIA LRx includes pseudo-patients’ data; IQVIA LPD is based on the information collected from approximately 900 GPs who, in compliance with Italian law, according to their usual clinical practice and using an ambulatory management software, record information related to their patients’ visits for reimbursement purposes. As such, IQVIA LPD data is not ad-hoc collected for studies purposes and there is no sponsor and, for this reason, all the analyses conducted on this data do not require an Ethical Committee approval; IQVIA LPD database contains anonymous data which are not originated by any clinical trial. Indeed, data is anonymized through a non-identifiable encryption process directly on GPs’ computer before being sent to IQVIA: patients’ names are not collected in the database. IQVIA LPD complies with the European Regulation 679/2016 and the ex-Legislative Decree 196/03 and following modificationsCitation43.
Results
Prevalent users of donepezil and memantine extemporaneous combinations
Patients on IQVIA LRx database who had at least one overlap of memantine and donepezil prescriptions during the 3-year selection period were 9862 (Cohort DMpLRx), while those identified on IQVIA LPD database during the 9-year selection period were 708 (Cohort DMpLPD). For both cohorts of prevalent users of the combination, about two-third of the patients were females and more than half of the patients were older than 79 years (). The joint distributions by sex and age class showed a higher proportion of women in all age classes for both cohorts, and the highest discrepancy between males and females was found among patients older than 79 years (data not shown). Mean age of patients in Cohort DMpLPD was 79 years, while 80 years was the median age ().
Figure 1. Prevalent users of donepezil and memantine extemporaneous combination (DMpLRx and DMpLPD cohort) stratified by sex and age class.
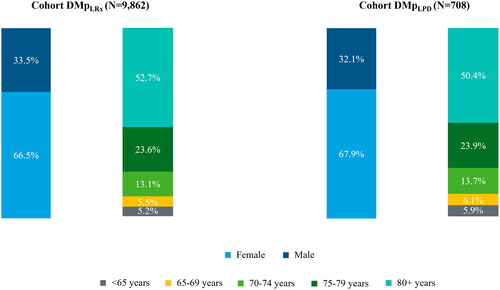
Table 1. Demographic characteristics of prevalent users of donepezil and memantine extemporaneous combination (DMpLPD cohort).
Around 40% of patients belonging to DMpLPD cohort had a recorded body mass index (BMI) value; the majority of them (around 80%) were normo- to over-weight, very few were under-weight, and obese class accounted for approximately 15% (). Mean value of BMI was 26 ± 5 kg/m2 ().
The most frequently recorded comorbidity for DMpLPD cohort was essential hypertension, which affected half of the patients, followed by other cerebral degenerations (including AD), specific nonpsychotic mental disorders due to brain damage, and disorder of lipid metabolism. Dementia, anxiety, dissociative and somatoform disorders, diabetes mellitus, other disorders of bone and cartilage, osteoarthrosis and allied disorders, and diseases of esophagus also scored among the ten most frequently detected comorbidities, but they were all recorded in less than 20% of the population (). Among comorbidities of interest, psychiatric disorders affected more than two-thirds of DMpLPD cohort, including depression which was detected for almost one quarter of the population, whereas cardiovascular diseases were recorded for more than 60% of the patients (). Focusing on baseline treatment, most frequently recorded drugs fall into 5-digit ATC class B01AC (i.e. platelet aggregation inhibitors excl. heparin), which were detected for half of DMpLPD cohort, followed by drugs falling into 5-digit ATC class A02BC (i.e. proton pump inhibitors), drugs falling into 5-digit ATC class C10AA (i.e. HMG CoA reductase inhibitors), and drugs falling into 5-digit ATC class N06AB (i.e. selective serotonin reuptake inhibitors) ().
Figure 2. Top 10 baseline comorbidities and treatments of prevalent users of donepezil and memantine extemporaneous combination (DMpLPD Cohort). 1Proportion of patients with at least one registration of a disease falling under the specified 3-digit ICD-9 code. 2Proportion of patients with at least one registration of a drug prescription falling under the specified 5-digit ATC code.
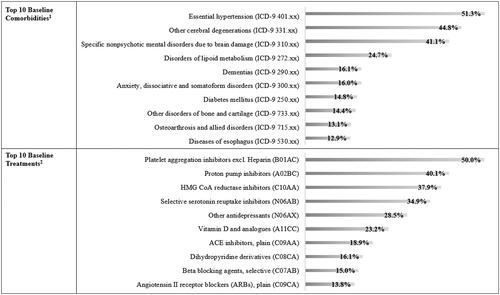
Table 2. Clinical characteristics of prevalent users of the combination (IQVIA DMpLPD cohort).
Looking at baseline treatment of interest, both antithrombotics and antidepressants were prescribed to half of the patients belonging to DMpLPD cohort, while antipsychotics were administered to one-fifth of the population ().
The analysis on specialist visit referrals during baseline for cohort DMpLPD showed that the proportion of patients with neurologist visit referrals is higher than that of patients with geriatrist visit referrals among prevalent users of the extemporaneous combination of donepezil and memantine. In particular, patients with at least one neurologist visit referral accounted for 44% of DMpLPD cohort compared to 32% of patients with at least one geriatric visit referral ().
National level yearly estimates of the number of patients prescribed with the extemporaneous combination of donepezil and memantine based on IQVIA LRx data accounting for database representativeness of retail and DPC distribution channels showed a 4% yearly increase. In particular, the number of patients prescribed with the extemporaneous combination ranged from 8733 for period I (i.e. July 2018–June 2019) up to 9503 for period III (i.e. July 2020–June 2021) ().
Treatment adherence
Adherence to treatment with the extemporaneous combination of donepezil and memantine was calculated on the cohort of incident users identified through IQVIA LPD database, thus the subgroup of patients belonging to cohort DMpLPD who were new for the treatment, who were 627 (cohort DMiLPD). Median value of PDC was 47.2%, with this meaning that half of the patients took both memantine and donepezil for at least 85 days out of 180 (i.e. follow-up duration) (). The stratification by PDC classes showed that more than half of the patients had an intermediate-to-high adherence ().
Table 3. Treatment adherence of incident users of memantine and donepezil extemporaneous combination (cohort MDiLPD).
Sensitivity analyses
Results from the sensitivity analyses found that (1) inclusion of 5 mg donepezil prescriptions when defining prevalent users of the extemporaneous combinations led to 14,499 patients against 9862 for DMpLRx cohort, and 1017 patients against 708 for DMpLPD cohort; (2) patients who had at least two molecules overlaps within the follow-up and/or a single overlap lasting more than 28 days represented 83% and 79% of DMpLRx and DMpLPD cohort, respectively.
Discussion
Main objective of this study was to produce real-world data on the use of memantine and donepezil as an extemporaneous combination to treat AD in Italy, and to provide a description in terms of demographic and clinical characteristics of AD patients prescribed with the extemporaneous combination.
Findings from the present analysis show that co-prescription of memantine and donepezil is a current practice in Italy, with this also being reinforced by the analysis on overlaps, which showed that around 80% of the patients identified on both data sources had more than one memantine and donepezil overlap or an overlap lasting more than 28 days during follow-up. Also, the very fact that patients prescribed with the extemporaneous combination have been detected on IQVIA LPD database, which receives data from a representative sample of GPs, is indicative that doctors indeed prescribe this kind of therapy. Moreover, national level yearly estimates found that AD patients receiving the extemporaneous combination of donepezil and memantine during the period July 2020–June 2021 were around 9500, and a constant increase in this prescription habit was observed. It is worth mentioning that this trend did emerge despite COVID-19 pandemic, which is known to have caused delays and interruptions of medical services particularly for old, chronically ill peopleCitation54, and the very complex management of AD treatment. Indeed, as a consequence of the long-dated AIFA Note 85, the prescription of AChEIs and memantine requires physicians to fill in two different therapeutic plansCitation4, with this likely discouraging such practice.
Nonetheless, confirmation about the established use of the extemporaneous combination of memantine and AChEIs also comes from literature. Castagna and colleagues, in the CITIMEA study, used data from Italian CDCD centers and retrospectively identified almost 200 patients receiving memantine in combination with an AChEICitation27. Another study designed to assess the costs and resource use associated with AD patients and caregivers provided a picture of AD treatments pattern by severity predominantly involving memory clinics and CDCD centers in Italy. Patients assuming memantine in combination with an AChEI accounted for 10% and 22% of moderate and severe patients, respectivelyCitation26. A retrospective observational study conducted by Scuteri and colleagues using data from the provincial prescription database of the health district of Cosenza and involving around 80,000 patients who were 60 years older found that, over a 2-year period (2017–2018), 153 patients concomitantly assumed memantine and an AchEIsCitation28. Of note, among AChEIs, donepezil was the first to get market authorization in Italy and it is the most widely used in patients with dementiaCitation55,Citation56.
The combined used of AChEIs and memantine is further supported by their mechanisms of action. Indeed, differently from that of donepezil, memantine’s mechanism of action does not involve inhibition of acetylcholinesterase. For this reason, memantine is commonly administered in combination with AChEIs in patients who are in moderate to severe stages of the diseaseCitation57. Indeed, memantine normalizes dysfunction of the glutamatergic signalling system, which causes impairment of cognition and neuronal loss, while AChEIs help to mitigate the effect of progressive loss of cholinergic function in AD by raising acetylcholine (ACh) concentrations. Since the combined dysfunction of the glutamatergic and cholinergic neuronal systems is crucial for the onset of AD symptoms, the joint use of memantine and AChEIs might beneficially address both pathological aspectsCitation9.
Demographic characteristics of patients receiving the extemporaneous combination of donepezil and memantine were very similar when comparing the two cohorts obtained from IQVIA LRx and LPD: a higher proportion of women was observed, with discrepancies between sexes increasing with increasing age, and about half of the patients being aged 80 years or older. This perfectly fits with the overview in terms of demographic characteristics provided by the scientific literatureCitation26,Citation27,Citation58.
The characterization of clinical profile performed based on IQVIA LPD data showed that among patients treated with the extemporaneous combination of donepezil and memantine the prevalence of comorbid conditions and of co-treatments was very high. In particular, the analysis on top 10 comorbidities delineates a clinical profile that is in line with the demographic one, with most frequently reported diseases being those that are typical of an old population, such as cardiovascular disease, essential hypertension, hypercholesterolemia, diabetes, osteoporosis, and osteoarthrosis. In addition, also a very high proportion of patients affected by psychiatric conditions was observed. On the other hand, the proportion of patients with dementias, which was 16%, might seem low. However, it should be considered that the ICD-9 code defining dementias in is 290 and it is not specific for Alzheimer disease-related dementia. Nonetheless, it is possible that prevalence of dementias in this cohort was underestimated as the research of ICD-9 codes that defined comorbidities was limited to each patient’s 12-month baseline period. A confirmation about the very high prevalence of comorbid conditions in Italian AD patients once again comes from the scientific literature. The study by Bruno and colleagues found the presence of comorbidities in more than 80% of AD patientsCitation26, and evidence on possible genetic factors relating AD and psychiatric conditions has been reported previouslyCitation59. Moreover, co-treatment profile of AD patients in the present study agrees with comorbid conditions observed, with very high proportions of patients receiving drugs to treat cardiovascular disease, hypercholesterolemia, osteoporosis, and psychiatric conditions. With particular reference to drug used to treat psychiatric conditions, the study by Bruno and colleagues reported almost half of AD patients receiving any psychiatric/hypnotic treatmentCitation26. The study by Scuteri found that antipsychotics and antidepressants were used by around 21% and 50% respectively of dementia patients treated with AChEIs and/or memantine, with such data being a perfect match with results from the present studyCitation28.
On average, treatment adherence of patients starting the extemporaneous combination of memantine and donepezil is low. However, the proportion of patients with a high level of adherence, as well as mean adherence, is higher than that found by two previous studies using the same data source and measuring the same outcome in patients assuming an extemporaneous combination of two antihypertensive drugsCitation45,Citation46. Considering that the present study investigated old patients affected by dementia and with comorbid conditions that require drug treatment, it is worth mentioning that even if generally low, treatment adherence is higher than expected. Consistently, a study by Sinforiani comparing AD management between two different periods found an improvement in treatment persistency of patients assuming an AChEI and/or memantine, with a discontinuation rate at six months of 8% in the latter periodCitation60. The study by Scuteri reported that the proportion of patients assuming both an AChEI and memantine and who had a high level of treatment adherence was greater than 70%, thus even much higher than the one we found. However, no explanations have been provided by the authors on the methodology used to calculate neither treatment combination nor treatment adherenceCitation28.
This study presents some limitations, which are those typical of real-world evidence studies. First, only data on prescriptions dispensed (Italian IQVIA LRx) or on written prescriptions (Italian IQVIA LPD) were available, therefore we assumed that any written or dispensed prescription was actually consumed, so it is possible that the true adherence to the therapeutic regimens was overestimated. Second, Italian IQVIA LRx includes information on all SSN reimbursed drugs, but information on patients’ diagnoses is not available, thus it was not possible to filter patients based on the presence of AD diagnosis. However, memantine and donepezil prescriptions are reimbursable by the SSN only if the patient has been diagnosed with AD in a CDCD center. Therefore, it is intended that all patients included in the study do have an AD diagnosis performed in a CDCD. Third, patients with AD can be difficult to identify in electronic medical records such as IQVIA LPD due to a lack of precision in clinicians’ recording of the diagnosis. In addition, AD can be difficult to discriminate from other causes of dementiaCitation1. However, in order to overcome these issues, the analysis on IQVIA LPD only used memantine and donepezil prescriptions when administered in an SSN reimbursable regimen. Fourth, including only SSN-reimbursed prescriptions might have caused an under-estimation in the number of patients actually prescribed with the extemporaneous combination. Indeed, while donepezil is reimbursed by SSN for mild and moderate AD, memantine is reimbursed only in case of moderate ADCitation4. Being so, we were able to detect only patients who have extemporaneous prescriptions of memantine and donepezil due to moderate AD, and we were not able to track neither mild nor severe AD patients eventually prescribed with out-of-the-pocket memantine and donepezil combination. However, it should be noted that memantine is not indicated for mild ADCitation49, thus we do not expect patients with mild AD to be prescribed with a combination including this drug. Differently, we should be aware that not having detected severe AD patients because not responding to SSN reimbursability criteria might have caused an underestimation in the number of patients actually assuming the extemporaneous combination of memantine and donepezil. Finally, the exclusion of 5 mg donepezil prescriptions in our study might have resulted in an underestimation of patients treated with the extemporaneous combination, and this is the reason why we performed the sensitivity analyses accounting also for 5 mg donepezil prescriptions. In conclusion, estimates from the present study might be somewhat conservative, but it is authors’ opinion that this consideration contributes to support findings suggesting a consolidated practice of donepezil and memantine co-prescription.
On the other hand, to authors’ knowledge, this is the first study specifically focused on the co-prescription of donepezil and memantine in a real-world setting in Italy. Importantly, the concomitant use of two very large databases offered two different perspectives on AD management, but basically told the same story and provided a perfectly comparable demographic profile of patients prescribed with the extemporaneous combination of donepezil and memantine. This, together with very similar results in terms of demographic and clinical profile compared to scientific literature, give robustness to our findings.
Finally, it is the authors’ opinion that further studies investigating the concomitant use of memantine and AchEIs other than donepezil should be encouraged to provide an even more overarching overview of the management of patients affected by AD.
Conclusions
In summary, the results of this real-world analysis suggest that in Italy, the prescription of the extemporaneous combination of donepezil and memantine is a common clinical practice. EMA guidelines for the development of FDCs list several rationales supporting the use of combination therapies: potential enhancement in patients with inadequate response to monotherapy, greater and/or more rapid effect, improvement in safety due to one active substance counteracting the adverse drug reactions of another, or by combining doses that are sub-therapeutic when used in monotherapyCitation61. Under this perspective, and because the administration of FDCs instead of extemporaneous ones has demonstrated to improve adherence to treatment, and thus its outcomes, in different therapeutic areasCitation62,Citation63, the introduction of an FDC of memantine and donepezil in Italy might further improve adherence to medication and help reducing caregiver burden also in AD patients.
Transparency
Acknowledgements
All named authors take responsibility for the integrity of the work as a whole and have given final approval for the version to be submitted.
Declaration of financial/other relationships
A.P. declares no competing interests. S.F. and V.P. have disclosed that they are employees of IQVIA.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- James G, Collin E, Lawrance M, et al. Treatment pathway analysis of newly diagnosed dementia patients in four electronic health record databases in Europe. Soc Psychiatry Psychiatr Epidemiol. 2021;56(3):409–416.
- Alzheimer’s Disease. Statistics from Epicentro – Epidemiology for public health. Istituto Superiore di Sanità. Available from https://www.epicentro.iss.it/en/alzheimer/.
- Italian National Statistics Institute – ISTAT. Italian resident population stratified by age class. Available from http://dati.istat.it/Index.aspx?DataSetCode=DCIS_POPRES1.
- Italian Medicines Agency. Nota 85. Available from https://www.aifa.gov.it/nota-85.
- Kaushik V, Smith ST, Mikobi E, et al. Acetylcholinesterase inhibitors: beneficial effects on comorbidities in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2018;33(2):73–85.
- Molino I, Colucci L, Fasanaro AM, et al. Efficacy of memantine, donepezil, or their association in moderate-severe Alzheimer’s disease: a review of clinical trials. ScientificWorldJournal. 2013;2013:925702.
- Conway ME. Alzheimer’s disease: targeting the glutamatergic system. Biogerontology. 2020;21(3):257–274.
- Reisberg B, Doody R, Stöffler A, et al. Memantine study group. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–1341.
- Parsons CG, Danysz W, Dekundy A, et al. Memantine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res. 2013;24(3):358–369.
- European Medicine Agency. Refusal of the marketing authorisation for Acrescent (memantine hydrochloride/donepezil hydrochloride). EMA/CHMP/816079/2012. Available from www.ema.europa.eu/en/documents/smop-initial/questions-answers-refusal-marketing-authorisation-acrescent_en.pdf.
- European Medicine Agency. Refusal of the marketing authorisation for Balaxur (memantine hydrochloride/donepezil hydrochloride). EMA/CHMP/816057/2012. Available from www.ema.europa.eu/en/documents/smop-initial/questions-answers-refusal-marketing-authorisation-balaxur_en.pdf.
- Deardorff WJ, Grossberg GT. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe alzheimer’s disease. Drug Des Devel Ther. 2016;10:3267–3279.
- Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889–898.
- National Institute for Health and Care Exellence (NICE) Guidelines. Dementia: assessment, management and support for people living with dementia and their carers. Available from www.nice.org.uk/guidance/ng97/resources/dementia-assessment-management-and-support-for-people-living-with-dementia-and-their-carers-pdf-1837760199109.
- Cartabellotta A, Eleopra R, Quintana S. Linee guida per la diagnosi, il trattamento e il supporto dei pazienti affetti da demenza. Evidence. 2018;1:e1000190.
- Caltagirone C, Bianchetti A, Di Luca M, et al. Guidelines for the treatment of Alzheimer’s disease from the Italian Association of Psychogeriatrics. Drugs Aging. 2005;22(Suppl 1):1–26.
- Agenzia Espanola de medicamentos y productos sanitarios. Ficha tecnica donepezilo/memantina tecnigen 10 mg/10 mg comprimidos recubiertos con pelicula. Available from https://cima.aemps.es/cima/pdfs/ft/85693/FT_85693.pdf.
- Gareri P, Putignano D, Castagna A, et al. Retrospective study on the benefits of combined Memantine and cholinEsterase inhibitor treatMent in AGEd patients affected with Alzheimer’s disease: the MEMAGE study. JAD. 2014;41(2):633–640.
- Tifratene K, Duff FL, Pradier C, et al. Use of drug treatments for Alzheimer’s disease in France: a study on a national level based on the national Alzheimer’s data bank (Banque Nationale Alzheimer). Pharmacoepidemiol Drug Saf. 2012;21(9):1005–1012.
- Brewer L, Bennett K, McGreevy C, et al. A population-based study of dosing and persistence with anti-dementia medications. Eur J Clin Pharmacol. 2013;69(7):1467–1475.
- Taipale H, Tanskanen A, Koponen M, et al. Antidementia drug use among community-dwelling individuals with Alzheimer’s disease in Finland: a nationwide register-based study. Int Clin Psychopharmacol. 2014;29(4):216–223.
- Kuronen M, Koponen H, Nykänen I, et al. Use of anti-dementia drugs in home care and residential care and associations with neuropsychiatric symptoms: a cross-sectional study. BMC Geriatr. 2015;15:100.
- Wübbeler M, Wucherer D, Hertel J, et al. Antidementia drug treatment in dementia networks in Germany: use rates and factors associated with treatment use. BMC Health Serv Res. 2015 May 22;15(1):205.
- Donegan K, Fox N, Black N, et al. Trends in diagnosis and treatment for people with dementia in the UK from 2005 to 2015: a longitudinal retrospective cohort study. Lancet Public Health. 2017;2(3):e149–e156.
- Calvó-Perxas L, Turró-Garriga O, Vilalta-Franch J, et al. Trends in the prescription and Long-Term utilization of antidementia drugs among patients with Alzheimer’s disease in Spain: a cohort study using the registry of dementias of girona. Drugs Aging. 2017;34(4):303–310.
- Bruno G, Mancini M, Bruti G, et al. Costs and resource use associated with Alzheimer’s disease in Italy: results from an observational study. J Prev Alzheimers Dis. 2018;5(1):55–64.
- Castagna A, Fabbo A, Manzo C, et al. A retrospective study on the benefits of combined citicoline, memantine, and acetylcholinesterase inhibitor treatments in older patients affected with Alzheimer’s disease. JAD. 2021;79(4):1509–1515.
- Scuteri D, Vulnera M, Piro B, et al. Pattern of treatment of behavioural and psychological symptoms of dementia and pain: evidence on pharmacoutilization from a large real-world sample and from a Centre for cognitive disturbances and dementia. Eur J Clin Pharmacol. 2021;77(2):241–249.
- Gareri P, Cotroneo AM, Orsitto , et al. The CITIDEMAGE study: stressing the cholinergic hypothesis for the best outcomes in dementia patients: human/human trials: cognitive enhancement. Alzheimers Dement. 2020;16:e038178.
- Italian Medicines Agency. Modifica nota 85. Gazzetta Ufficiale. AIFA. [cited 2009 Mar 19]. Available from www.gazzettaufficiale.it/do/atto/serie_generale/caricaPdf?cdimg=09A0268300100010110001&dgu=2009-03-19&art.dataPubblicazioneGazzetta=2009-03-19&art.codiceRedazionale=09A02683&art.num=1&art.tiposerie=SG.
- Richter H, Dombrowski S, Hamer H, et al. Use of a German longitudinal prescription database (LRx) in pharmacoepidemiology. Ger Med Sci. 2015;13:Doc14.
- Helwig U, Kostev K, Schmidt C. Comparative analysis of 3-year persistence with vedolizumab compared with antibodies against tumor necrosis factor-alpha in patients with inflammatory bowel disease in Germany: retrospective analysis of a large prescription database. J Clin Gastroenterol. 2021;55(1):e1–e7.
- Richette P, Allez M, Descamps V, et al. Impact de la COVID-19 sur les initiations et les renouvellements des biothérapies et traitements synthétiques ciblés en France. Rev Rhum Ed Fr. 2020;87:a14.
- Joumaa H, Sigogne R, Maravic M, et al. Artificial intelligence to differentiate asthma from COPD in medico-administrative databases. BMC Pulm Med. 2022;22(1):357.
- Vilcu AM, Blanchon T, Sabatte L, et al. Cross-validation of an algorithm detecting acute gastroenteritis episodes from prescribed drug dispensing data in France: comparison with clinical data reported in a primary care surveillance system, winter seasons 2014/15 to 2016/17. BMC Med Res Methodol. 2019 May 31;19(1):110.
- Putignano D, Bruzzese D, Orlando V, et al. Differences in drug use between men and women: an italian cross sectional study. BMC Womens Health. 2017;17(1):73.
- Federici MO, McQuillan J, Biricolti G, et al. Utilization patterns of Glucagon-Like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Italy: a retrospective cohort study. Diabetes Ther. 2018;9(2):789–801.
- Research Institute of SIMG. XIV report Health Search. 2021. Available from https://report.healthsearch.it/2021/Report_XIV.pdf.
- Ravera M, Cannavò R, Noberasco G, et al. High performance of a risk calculator that includes renal function in predicting mortality of hypertensive patients in clinical application. J Hypertens. 2014;32(6):1245–1254.
- Katz P, Pegoraro V, Liedgens H. Characteristics, resource utilization and safety profile of patients prescribed with neuropathic pain treatments: a real-world evidence study on general practices in Europe – the role of the lidocaine 5% medicated plaster. Curr Med Res Opin. 2017;33(8):1481–1489.
- Di Marco F, Santus P, Terraneo S, et al. Characteristics of newly diagnosed COPD patients treated with triple inhaled therapy by general practitioners: a real world Italian study. NPJ Prim Care Respir Med. 2017;27(1):51.
- Agostoni E, Barbanti P, Frediani F, et al. Real-world insights on the management of migraine patients: an Italian nationwide study. Curr Med Res Opin. 2019;35(9):1545–1554.
- Colombo GL, Heiman F, Peduto I. Utilization of healthcare resources in osteoarthritis: a cost of illness analysis based on real-world data in Italy. Ther Clin Risk Manag. 2021;17:345–356.
- Pegoraro V, Heiman F, Levante A, et al. An Italian individual-level data study investigating on the association between air pollution exposure and covid-19 severity in primary-care setting. BMC Public Health. 2021 May 12;21(1):902.
- Volpe M, Pegoraro V, Peduto I, et al. Extemporaneous combination therapy with nebivolol/zofenopril in hypertensive patients: usage in Italy. Curr Med Res Opin. 2022;38(10):1673–1681.
- Levi M, Pasqua A, Cricelli I, et al. Patient adherence to olmesartan/amlodipine combinations: fixed versus extemporaneous combinations. J Manag Care Spec Pharm. 2016;22(3):255–262.
- Prieto-Merino D, Mulick A, Armstrong C, et al. Estimating proportion of days covered (PDC) using real-world online medicine suppliers’ datasets. J Pharm Policy Pract. 2021;14(1):113.
- Asamoah-Boaheng M, Osei Bonsu K, Farrell J, et al. Measuring medication adherence in a population-based asthma administrative pharmacy database: a systematic review and Meta-Analysis. CLEP. 2021;13:981–1010.
- Italian Medicines Agency. Ebixa. Riassunto delle caratteristiche del prodotto. Available from https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000911_035681_RCP.pdf&sys=m0b1l3.
- Italian Medicines Agency. Donepezil Teva. Riassunto delle caratteristiche del prodotto. Available from https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000813_041733_RCP.pdf&sys=m0b1l3.
- Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186.
- Nishimura S, Kumamaru H, Shoji S, et al. Adherence to antihypertensive medication and its predictors among non-elderly adults in Japan. Hypertens Res. 2020;43(7):705–714.
- Khan R, Kaul P, Islam S, et al. Drug adherence and long-term outcomes in non-revascularized patients following acute myocardial infarction. Am J Cardiol. 2021;152:49–56.
- Gualano MR, Corradi A, Voglino G, et al. Beyond COVID-19: a cross-sectional study in Italy exploring the covid collateral impacts on healthcare services. Health Policy. 2021;125(7):869–876.
- Franchi C, Lucca U, Tettamanti M, et al. Cholinesterase inhibitor use in Alzheimer’s disease: the EPIFARM-elderly project. Pharmacoepidemiol Drug Saf. 2011;20(5):497–505.
- Pariente A, Helmer C, Merliere Y, et al. Prevalence of cholinesterase inhibitors in subjects with dementia in Europe. Pharmacoepidemiol Drug Saf. 2008;17(7):655–660.
- Mattace-Raso F. Is memantine + acetylcholinesterase inhibitor treatment superior to either therapy alone in Alzheimer’s disease? JAD. 2014;41(2):641–642.
- Chiatti C, Rimland JM, Bonfranceschi F, et al. Up-TECH research group. The up-TECH project, an intervention to support caregivers of Alzheimer’s disease patients in Italy: preliminary findings on recruitment and caregiving burden in the baseline population. Aging Ment Health. 2015;19(6):517–525.
- Porcelli S, Calabrò M, Crisafulli C, et al. Alzheimer’s disease and neurotransmission gene variants: focus on their effects on psychiatric comorbidities and inflammatory parameters. Neuropsychobiology. 2019;78(2):79–85.
- Sinforiani E, Bernini S, Picascia M, et al. Treatment adherence in patients with Alzheimer’s disease referred to an Italian center for dementia. Aging Clin Exp Res. 2015;27(3):395–396.
- European Medicines Agency. EMA/CHMP/158268/2017. Guideline on clinical development of fixed combination medicinal products. Available from www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-development-fixed-combination-medicinal-products-revision-2_en.pdf.
- Degli Esposti L, Perrone V, Veronesi C, et al. Modifications in drug adherence after switch to fixed-dose combination of perindopril/amlodipine in clinical practice. Results of a large-scale Italian experience. The amlodipine-perindopril in real settings (AMPERES) study. Curr Med Res Opin. 2018;34(9):1571–1577.
- Cho SJ, Oh IS, Jeong HE, et al. Long-term clinical outcomes of oral antidiabetic drugs as fixed‐dose combinations: a nationwide retrospective cohort study. Diabetes Obes Metab. 2022;24(10):2051–2060.