Abstract
Objective
Time To Discontinuation (TTD) is defined as the time from the start of treatment to the end of treatment, usually occurring due to loss of efficacy or occurrence of adverse events. It has become an important surrogate efficacy endpoint especially in real-world studies due to its correlation with endpoints such as Progression Free Survival (PFS). The aim of the study is to conduct a literature review of all studies reporting TTD in first-line therapy of Non-Small Cell Lung Cancer (NSCLC).
Methods
All articles that reported TTD for any first-line treatment of NSCLC as of 30 June 2022 were extracted from the PubMed search engine. From these articles, the drugs, study type, and TTD values were extracted. A descriptive analysis of the studies was made, dividing the TTD by subgroup according to the type of treatment (traditional chemotherapy, target therapy, immunotherapy) and study design (clinical trials, real world studies).
Results
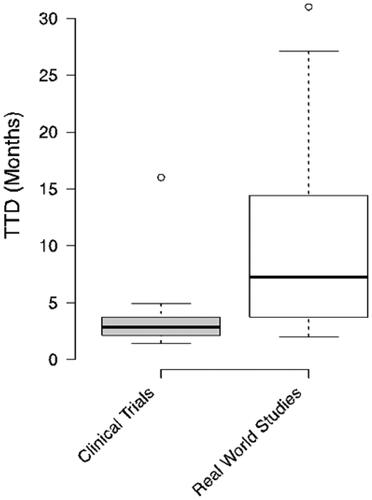
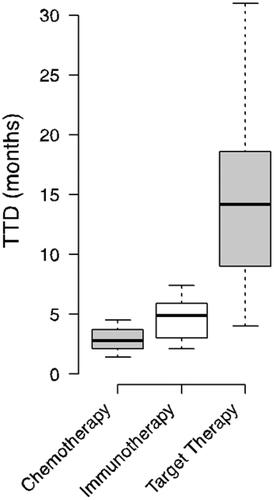
Fifty-five studies were considered for the analysis, of which 12 were published in 2021; 28 were clinical trials and 27 were real-world studies. Thirty of the studies considered involved conventional chemotherapy and expressed TTD values from 1.4 to 4.5 months, 5 of the studies considered involved immunotherapy with TTD values from 2.1 to 7.4 months and 18 of the studies considered target therapy, with TTD values from 4 to 31 months. The clinical trials reported TTD values from 1.4 to 16 months and the real-world studies from 2 to 31 months.
Conclusion
Studies reporting TTD are increasing, most notably real-world studies. Given the increasing importance of TTD as an efficacy endpoint, it becomes critical to measure and monitor it in various therapeutic settings such as NSCLC. This is the first study to review all TTD values of drugs used in first-line NSCLC.
Introduction
Non-Small Cell Lung Cancer (NSCLC) is a type of cancer affecting the epithelial tissues lining the bronchi and lung parenchyma, caused by pilot oncogenic mutations that have been identified mainly in adenocarcinomas.Citation1 In recent years, the range of therapies available for the treatment of NSCLC has expanded, with molecular-targeted therapies (target therapy) and immunotherapeutic drugs being added to traditional chemotherapy.Citation2–4 In order to be marketed for NSCLC, these drugs had to prove their efficacy in pre-marketing randomized clinical trials in terms of survival time, as measured by robust primary outcomes such as overall survival (OS) and progression free survival (PFS).Citation5
As is now well known, during the post-marketing experience these drugs are used in conditions that are not identical to those of clinical trials and on patients who would sometimes have been considered in trials as not eligible for treatment.Citation6 Thus, in recent years post-marketing studies, or real-world studies (RWS), have become increasingly important in providing additional efficacy and safety data that can support and complement the evidence gathered in clinical trials in order to improve the benefit-risk profile of marketed drugs.Citation7
Time To Treatment Discontinuation (TTD) is a surrogate efficacy endpoint used mainly in RWS, which is faster and more convenient than other endpoints and has been found to correlate with PFS in both RCTs and RWSs.Citation8
TTD is defined as the time elapsed between the first administration of the drug and its discontinuation, which may occur due to disease progression or because of the occurrence of serious adverse events attributable to the therapy and may also be identified by other synonyms such as persistence, treatment duration, time on treatment.Citation9
It is therefore important to investigate this new efficacy endpoint to understand its prevalence and to obtain a current status report. This study is an overview aimed at analysing the treatment duration data reported in the literature for first-line treatment of NSCLC.cos
Materials and methods
The literature search was conducted on 30 April 2022 using the PubMed search engine, the search string used was (first-line OR naive OR untreated) AND ((NSCLC) OR (non-small-cell-long-cancer)) AND ((time-to-discontinuation) OR (TTD) OR (time-to-treatment-discontinuation) OR (persistence) OR (time-on-treatment) OR (drug-survival) OR (treatment-duration)).
All studies reporting the duration of treatment with first-line anticancer drugs in NSCLC were included, regardless of how duration was defined, and irrespective of the design and the drug used.
On the other hand, studies that did not report TTD for first-line treatment with NSCLC indication, dose-finding studies, reviews, expert opinions, unpublished English-language papers, non-antitumour drugs, treatments with a maximum number of cycles regardless of clinical response, and evaluations of treatment sequences were excluded.
For each study, the following data were extracted: sample size of patients treated, study design, type of NSCLC, drug administered, inclusion and exclusion criteria, method of calculating TTD, duration and whether this was a study endpoint. For studies with infusion therapies in which the duration of treatment was not reported but the median number of cycles performed, the duration of treatment was calculated by multiplying the median number of cycles by the number of days in each cycle.
The results were presented in a descriptive manner reporting the characteristics of the studies, the sample of patients and treatment under study, as well as the method of calculating TTD. In addition, the TTD endpoint was reported by subgroup according to the type of treatment (dividing between traditional chemotherapy, target therapy and immunotherapy) and study design. The boxplots were created using BoxPlotR (http://shiny.chemgrid.org/boxplotr/).
Results
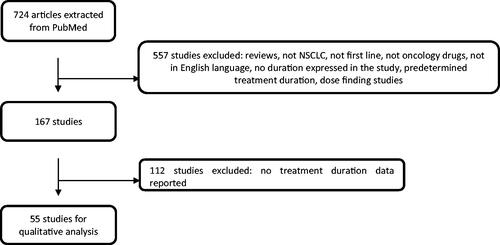
A total of 724 articles were extracted from the literature search, of which 55 studies were considered by applying the exclusion criteria, as shown in the flowchart in .
The results are summarized in .
Table 1. study characteristics and endpoint.
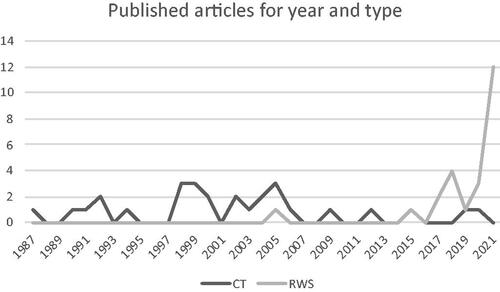
Over the years, there has been an increase in studies reporting TTD, as shown in . In the past it was mainly evaluated in CTs, whereas in recent years RWSs report it more frequently.
Figure 2. Number of articles reporting treatment duration, by year of publication and divided between RWSs and CTs.
The duration of treatment was defined differently in the various studies: Whilst in CT it is almost never reported as a primary or secondary endpoint but rather among the information on the use of the drug or sample characteristics, in pharmacy utilization and effectiveness studies in Real World studies it very often represents a primary or secondary efficacy endpoint.
In most cases, “treatment duration” was defined in 43 out of 55 studies either as the time between the first and last drug intake or discontinuationCitation10–19 without giving further details in the calculation methodology, otherwise as the time between the first and last drug administration + 1 day,Citation20 7 days,Citation21 21 days.Citation22
There were 28 Clinical trials (CTs) and 27 RWSs so they were equally represented.
The boxplot in shows the distribution of TTD in CTs and RWSs, which were 1.4 to 16 months for CTs and 2 to 31 months for RWSs.
Looking at the individual drugs, it was noted that the highest mTTD (median TTD) were related to target therapies, specifically Afatinib and Osimertinib, which were the most widely reported molecules, and which were almost always related to RWSs.
In terms of the type of therapy considered, 30 studies considered traditional chemotherapies, with Gemcitabine being the most cited; 18 studies considered Target Therapies, with Afatinib being the most widely cited molecule; immunotherapies were considered in 5 studies, of which 3 with Pembrolizumab and the remaining 2 studies considered all the treatments used in the first-line treatment of NSCLC.
The boxplot in , on the other hand, shows the distribution by drug class, with traditional chemotherapies showing TTD values from 1.4 to 16 months, immunotherapies showing TTD values from 2.1 to 7.4 months and target therapies showing TTD values from 4 to 31 months.
Dividing the studies into subgroups, the drugs considered within CTs differ from those considered within RWSs. In fact, traditional chemotherapeutics were more widely reported in CTs (24 out of 28 studies), whilst target therapies were more widely reported in RWSs (14 out of 27). Afatinib, Alectinib and Osimertinib were only to be found in RWSs and not in CTs.
The sample size was extremely variable, with a median of 73 patients and a range of 4 to 11112 patients; the sample sizes for CTs and RWSs were different, with a median value of 42 for CTs and 216 for RWSs.
Discussion
Target therapy and immunotherapy show higher TTD than conventional chemotherapy, confirming the efficacy data evaluated with more robust efficacy endpoints.
Target therapies have higher mTTD values than both immunotherapies and conventional chemotherapy. It must be specified that the target therapies that showed the highest TTD (Osimertinib, Afatinib and Alectinib) were evaluated exclusively in RWSs and never in CTs. Immunotherapies showed higher TTD than conventional chemotherapy and lower TTD than target therapies, albeit in fewer studies than the other therapies (5 studies), all of which were carried out under Real World conditions.
CTs and RWSs are different kinds of studies and have different objectives. Nevertheless, we decided to consider them both since the aim of this review is to provide an overview of the literature reporting on treatment duration in first-line NSCLC, irrespective of study type and treatment.
CTs are the gold standard for evaluating the efficacy of a treatment, whilst RWSs can have different objectives and consider several treatments at once, thus providing an insight of how drugs are actually used in clinical practice.
shows how the number of RWSs reporting TTD has exploded in recent years, in line with the role of real-world evidence and an increasing number of publications in this area. TTD has in fact taken on the important value of a surrogate efficacy endpoint in RWSs in recent years, and for this reason has often been evaluated as a primary outcome in RWSs on drug utilization.
Prior to this “revolution”, the duration of therapy was simply part of the description of how the drug was used in clinical trials or seen as part of the characteristics of the patient sample, where it was almost always not even considered among the endpoints.
There are 2 critical issues in the definition and calculation of treatment duration:
(1) The definition of treatment discontinuation: after how many days since the last dispensing/administration of medication can a treatment be deemed “discontinued”, and not simply suspended for a period? The value is at the discretion of the authors and varies from 6021,Citation23,Citation24 to 120 daysCitation20,.Citation25 Therefore, depending on the choice of interval, the number of patients considered to be persistent on treatment may vary significantly. For this reason, to assess the statistical robustness of the results, some studies such as Ganti et al.Citation23 also perform a sensitivity analysis using a gap <30 days in the definition of persistence.
The choice of the maximum interval is an important aspect in the calculation of persistence and should be made according to the drug analysed and according to the dosage and the quantity dispensed.
Compliance with these variables would help standardize the method of analysis, making the results comparable. Certainly, the details of the methodology are necessary to better interpret the results presented and to lay the foundations for comparisons with other similar studies.
(2) The method by which the duration of treatment is calculated is also not unequivocal. For example, in the case of intravenous drugs, there are studies that add up the duration of an entire cycle to the time between the first and last administration,Citation22 studies that add up to only 1 dayCitation20 and studies that do not describe the calculation method at all.
In the case of oral drugs, no study specifically describes the method of calculating the duration of treatment, so it is not clear whether the date of last administration was determined by observation, questionnaire or by adding the number of days of dispensed dosage units to the last date of dispensing of the drug, or whether this corresponds to the last date of dispensing of the drug.
As far as the sample size of patients is concerned, it differs significantly between CTs and RWSs. In fact, the sample size in CTs is predetermined on the basis of statistical methods and expected results,Citation26 whereas in RWSs the sample size cannot be established a priori but reflects the availability of patients taking the treatment in clinical practice.
Conclusion
Given the great interest and large production of RWSs in recent years, the availability of data on treatment duration will continue to increase. In the first-line treatment of NSCLC it would be interesting to compare the values in RWSs with those in CTs, to assess whether in clinical practice the number and timing of discontinuations are comparable to those in CTs.
Transparency
Declaration of funding
No funding was received to assist with the preparation of this manuscript.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
RL contributed to the conception and design of the study, contributed to the acquisition, analysis, interpretation of data, and gave the final approval and agrees to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AZ, VI, AR, FM and FS contributed to acquisition, analysis, interpretation of data, and gave the final approval.
Acknowledgements
None.
Data availability statement
All data generated or analysed during this study are included in this published article.
References
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454.
- Rosell R, Tonato M, Sandler A. The activity of gemcitabine plus cisplatin in randomized trials in untreated patients with advanced non-small cell lung cancer. Semin Oncol. 1998;25(4 Suppl 9):27–34.
- Tartarone A, Lapadula V, Di Micco C, et al. Beyond conventional: the new horizon of targeted therapy for the treatment of advanced non-small cell lung cancer. Front Oncol. 2021;11:632256.
- Dong J, Li B, Lin D, et al. Advances in targeted therapy and immunotherapy for non-small cell lung cancer based on accurate molecular typing. Front Pharmacol. 2019;10:230.
- Memmott RM, Wolfe AR, Carbone DP, et al. Predictors of response, progression-free survival, and overall survival in patients with lung cancer treated with immune checkpoint inhibitors. J Thorac Oncol. 2021;16(7):1086–1098.
- Lasala R, Santoleri F, Romagnoli A, et al. Randomized clinical trials and real-life studies: comparison of baseline characteristics of patients in oral target therapies for renal cell carcinoma. J Oncol Pharm Pract. 2022;28(4):870–883.
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence – What is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297.
- Walker B, Boyd M, Aguilar K, et al. Comparisons of real-world time-to-event end points in oncology research. J Clin Oncol Clin Cancer Inform. 2021;5:45–46.
- Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47.
- Agulnik JS, Kasymjanova G, Pepe C, et al. Real-world pattern of treatment and clinical outcomes of EGFR-mutant non-small cell lung cancer in a single academic centre in Quebec. Curr Oncol. 2021;28(6):5179–5191.
- Davis KL, Kaye JA, Masters ET, et al. Real-world outcomes in patients with ALK-positive non-small cell lung cancer treated with Crizotinib. Curr Oncol. 2018;25(1):e40–e49.
- Gan CL, Stukalin I, Meyers DE, et al. Outcomes of patients with solid tumour malignancies treated with first-line immuno-oncology agents who do not meet eligibility criteria for clinical trials. Eur J Cancer. 2021;151:115–125.
- Horvat P, Gray CM, Lambova A, et al. Comparing findings from a friends of cancer research exploratory analysis of real-world end points with the cancer analysis system in England. J Clin Oncol Clin Cancer Inform. 2021;5:1155–1168.
- John A, Yang B, Shah R. Clinical impact of adherence to NCCN guidelines for biomarker testing and first-line treatment in advanced non-small cell lung cancer (aNSCLC) using Real-World electronic health record data. Adv Ther. 2021;38(3):1552–1566.
- Lee J, Park S, Jung HA, et al. Evaluating entrectinib as a treatment option for non-small cell lung cancer. Expert Opin Pharmacother. 2020;21(16):1935–1942.
- Lester J, Escriu C, Khan S, et al. Retrospective analysis of real-world treatment patterns and clinical outcomes in patients with advanced non-small cell lung cancer starting first-line systemic therapy in the United Kingdom. BMC Cancer. 2021;21(1):515.
- Nadler E, Arondekar B, Aguilar KM, et al. Treatment patterns and clinical outcomes in patients with advanced non-small cell lung cancer initiating first-line treatment in the US community oncology setting: a real-world retrospective observational study. J Cancer Res Clin Oncol. 2021;147(3):671–690.
- Planchard D, Boyer MJ, Lee JS, et al. Post progression outcomes for osimertinib versus standard-of-care EGFR-TKI in patients with previously untreated EGFR-mutated advanced non-small cell lung cancer. Clin Cancer Res. 2019;25(7):2058–2063.
- Yang JC, Schuler M, Popat S, et al. Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: a database of 693 cases. J Thorac Oncol. 2020;15(5):803–815.
- Velcheti V, Hu X, Li Y, et al. Real-world time on treatment with first line pembrolizumab monotherapy for advanced NSCLC with PD-L1 expression ≥50%: 3-year follow-up data. Cancers. 2022;14(4):1041.
- Mudad R, Patel MB, Margunato-Debay S, et al. Comparative efficacy and safety of nab-paclitaxel plus carboplatin vs gemcitabine plus carboplatin in first-line treatment of advanced squamous cell non-small cell lung cancer in a US community oncology setting. Lung Cancer. 2017;8:179–190.
- Weiss J, Force RW, Pugmire BA, et al. Comparative effectiveness and resource usage in patients receiving first-line taxane-based chemotherapy for stage IV Non-Small-cell lung cancer in a US community oncology setting. Clin Lung Cancer. 2017;18(4):372–380 e371.
- Ganti AK, Lin CW, Yang E, et al. Real-world adherence, and persistence with anaplastic lymphoma kinase inhibitors in non-small cell lung cancer. J Manag Care Pharm. 2022;28(3):305–314.
- Lim J, Samuelsen C, Golembesky A, et al. Duration of treatment among patients prescribed afatinib or erlotinib as first-line therapy for EGFR mutation-positive non-small-cell lung cancer in the USA. Future Oncol. 2019;15(13):1493–1504.
- Jahanzeb M, Lin HM, Wu Y, et al. Real-world efficacy and tolerability of brigatinib in patients with non-small cell lung cancer with prior ALK-TKIs in the United States. The Oncologist. 2022;27(9):790–798.
- Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clinical Trials. 1981;2(2):93–113.