Abstract
Background
Sedation is common practice in endoscopic procedures to suppress a patient’s level of consciousness while maintaining the cardio-respiratory function. Midazolam and propofol are the sedatives most frequently used for procedural sedation at hospitals in Scandinavia. Remimazolam is a new ultra-short-acting benzodiazepine sedative and the present analysis aimed at estimating the economic benefits of introducing remimazolam for procedural sedation in colonoscopies and bronchoscopies in hospitals in Scandinavia.
Method
We developed a cost model applying a micro-costing approach that comprised the cost components that are affected by differences in the efficacy of remimazolam, midazolam, and propofol, and the model estimated the cost per successful colonoscopy and bronchoscopy when using remimazolam, midazolam or propofol as sedation. A micro-costing approach was applied, and the model consisted of six stages representing the journey for patients undergoing endoscopies and was informed primarily by data from clinical studies on remimazolam.
Results
We found a total cost of DKK 1200 per successful colonoscopy procedure when using remimazolam, a total cost of DKK 1320 when using midazolam, and a total cost of DKK 1255 when using propofol. Hence, the incremental saving per successful colonoscopy procedure of using remimazolam was estimated to be DKK 120 compared to midazolam and DKK 55 compared to propofol. The total cost per successful bronchoscopy procedure when using remimazolam was DKK 1353 and DKK 1724 for midazolam, resulting in an incremental saving per bronchoscopy of DKK 372 when using remimazolam. Performed sensitivity analyses identified the time in recovery as the largest contributor to uncertainty in the analyses of remimazolam compared to midazolam in colonoscopies and bronchoscopies. In the comparison of remimazolam and propofol in colonoscopies, procedure time was the largest contributor to uncertainty.
Conclusion
We found that procedural sedation with remimazolam was associated with economically meaningful savings compared to procedural sedation with midazolam and propofol in colonoscopies and to midazolam in bronchoscopies.
Introduction
Sedation is common practice in medical procedures and involves administrating a sedative to suppress a patient’s level of consciousness while maintaining the cardio-respiratory function. Patients who undergo a diagnostic or therapeutic procedure often experience anxiety before the procedure, due to anticipated pain or discomfort, or fear of the outcomes of the procedureCitation1,Citation2. Procedural sedation plays an important role in increasing patient comfort and tolerance by reducing the awareness, anxiety, and pain associated with some proceduresCitation1.
Most procedural sedation occurs in the endoscopy unit (e.g. for colonoscopies and bronchoscopies), and technical advancements in diagnostic and therapeutic procedures have resulted in an exponential growth of procedures that require procedural sedation in the last few decades, particularly in gastroenterologyCitation3.
Midazolam and propofol are the sedatives most frequently used for procedural sedation at hospitals in Scandinavia. Midazolam is a benzodiazepine and a short-acting agent that binds to alpha subunits of the gamma-aminobutyric acid (GABAa) receptors linked to the chloride channel complex. The time to onset of action of midazolam is ∼2 min after administration, with a maximum effect obtained in ∼5–10 minCitation4. Recovery from sedation with midazolam varies among patients but typically occurs within 30–40 minCitation5. Propofol is an alkyl phenol and an ultra-short-acting agent that increases the GABA-mediated inhibitory tone in the central nervous system (CNS). The time to onset of action of propofol is between 30 and 40 s, depending on the rate of injection. Recovery from sedation with propofol occurs within 10–20 min after the last doseCitation6,Citation7. According to the summary of product characteristics (SPC) of propofolCitation8, propofol should only be administered by doctors that have been trained in anaesthesiology or intensive care. A substantial economic burden is associated with complications of procedural duration, particularly when procedures are canceled, patients are hospitalized or the length of hospitalization is increasedCitation5. With a growing demand for diagnostic and therapeutic procedures, many centers are under pressure to conduct them as quickly as possible to avoid long waiting timesCitation9. It is therefore essential that procedural sedation is effective and safe, and that recovery times are fast to allow as many patients as possible to undergo procedures.
Remimazolam is a new ultra-short-acting IV benzodiazepine sedative. It is an ester-based drug that is rapidly hydrolyzed in the body by esterases to an inactive carboxylic acid metabolite (CNS 7054)Citation10. Remimazolam delivers both a rapid onset of action (1–3 min) and rapid offset of sedation, with a predictable and short duration of action (∼15 minCitation11). In addition, remimazolam is associated with rapid recovery from sedation of ∼10 minCitation6,Citation7,Citation11. In clinical studies, remimazolam has shown a faster recovery profile (shorter time to fully alert and time to ready for discharge) than midazolamCitation12–15 and a similar time to fully alert to propofolCitation16,Citation17. Based on the fast recovery profile, the introduction of remimazolam in settings where midazolam is typically used for procedural sedation may result in shorter procedure times, thereby reducing the resource use and costs associated with procedures. Furthermore, shorter procedure times may make it possible to conduct a higher annual number of procedures. In settings where propofol is more commonly used for procedural sedation, the ability to use remimazolam without an anesthetist being present during the procedure may help to offset the higher treatment cost associated with remimazolam compared to propofolCitation18.
The present analysis aimed at estimating the economic benefits of introducing remimazolam for procedural sedation in colonoscopies and bronchoscopies in hospitals in Scandinavia. We developed a health economic model that comprised the cost components that are affected by differences in the efficacy of remimazolam, midazolam, and propofol, and the model estimated the cost per successful procedure using remimazolam, midazolam, or propofol as sedation.
Methods
We conducted a cost analysis applying a micro-costing approach to estimate the cost per successful procedure for patients undergoing a colonoscopy or bronchoscopy receiving procedural sedation with either remimazolam, midazolam, or propofol as accurately as possible.
With the micro-costing approach, we identified all resources associated with the procedures and valued the resources used based on Danish unit costs. The cost components of the model include drug acquisition costs, cost of time-use for relevant healthcare personnel, cost of utensils, and patient-incurred costs (patient time-use and transportation costs).
Model inputs for colonoscopies were primarily based on endpoints reported in three separate clinical studies evaluating the efficacy and safety of remimazolam relative to midazolam in patients undergoing colonoscopy (NCT01145222, NCT02290873, and RF-5Citation13,Citation14,Citation16). Safety endpoints from these clinical trials related to the drugs were not included in the cost model as it was assumed that “unsuccessful procedures” was the relevant safety endpoint to be modeled. In the Supplementary Appendices, we present the inputs and results from the similar analysis comparing the cost per successful procedure for patients undergoing bronchoscopies. Model inputs for this analysis were informed by one clinical study (NCT02296892Citation12).
Model structure
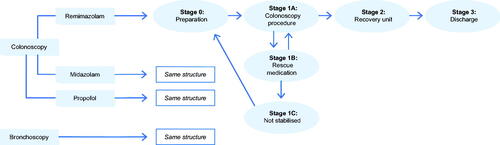
We divided the model into six stages representing the journey of patients undergoing endoscopies. illustrates the model structure. The different stages in the model are similar for remimazolam, midazolam, and propofol but differ between the three sedatives in terms of which costs are assigned.
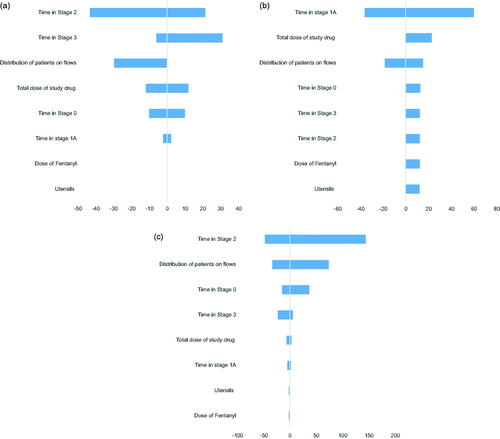
Figure 2. Tornado diagrams from DSA, change in incremental cost of remimazolam compared to midazolam and propofol in colonoscopies and midazolam in bronchoscopies.
Stage 0: Preparation
Stage 0 in the model includes all preparations of the patient for the procedure. This stage consists of the “time to start” of the procedure defined as when patients achieved Modified Observer’s Alertness/Sedation scale (MOAA/S) ≤3, where the patient receives the induction and top-up dose of the procedural sedation, among other preparations. Fentanyl was included as a pain-relieving agent.
Stage 1A: Procedure
Stage 1A consists of the “procedure duration”.
Stage 1B: Rescue sedative medication
A share of the patients will experience complications (e.g. due to non-optimal response to the procedural sedation). In response to the complications, rescue sedative medication will be used to get the patient back on track with the procedure.
In most cases, the rescue sedative medication works as intended, and the patient will continue the procedure in the model. In other cases, the rescue sedative medication does not have the desired effect (achieving MOAA/S ≤ 3) and the patient will move to Stage 1C.
Stage 1C: Not stabilized and sent home
If patients are not stabilized with the rescue sedative medication in Stage 1B, they are sent for recovery, discharged, and sent home. Patients were defined as not stabilized if rescue sedative medication (defined as any medication given, over and above that of the assigned treatment arm) had failed to either induce or maintain a suitable sedation level to start or continue with the procedure. Patients will typically be re-scheduled for another procedure and will return to Stage 0 (preparation) as illustrated in .
Stage 2: Recovery unit
The share of patients who successfully undergo the treatment procedure will recover at the recovery unit after the procedure. This stage includes the “time to fully alert”. Time to fully alert was defined as the time to the first of three consecutive MOAA/S scores of 5 after the last dose of the trial medication.
Stage 3: Discharge
Upon being fully alert, patients are transferred to the waiting room, where they spend the remainder of the time until discharge. Patients are discharged when they can walk unassisted.
Model inputs
In the following, model inputs for colonoscopies are presented. Information on bronchoscopies can be found in the Supplementary Appendices.
Time-use
For remimazolam and midazolam in colonoscopies, time estimates primarily came from NCT01145222Citation14 and NCT02290873Citation13. Time in Stage 0 was based on “time to start of the procedure from administration of the first dose of study drug (mg)” from NCT01145222 and time in Stage 1 A (procedure duration) was based on clinical expert input. The estimated time spent in Stage 2 was based on the endpoint “time to fully alert after end of colonoscopy” from NCT02290873, and the time estimate in Stage 3 was based on “time to ready for discharge after end of colonoscopy” from NCT02290873.
Due to the lack of appropriate data that reflects clinical practice for propofol in Scandinavia in colonoscopies, we assumed that the time estimates for propofol were equal to the time estimates for remimazolam. We found this assumption acceptable, as the time estimates from study RF-5 were found to be clinically implausible in a Scandinavian setting because the propofol dose applied in study RF-5 was higher (an initial dose of 1.5 mg per kg was applied) than the dose applied in clinical practice in Scandinavia (an initial dose of 0.5–1.0 mg per kg). The higher propofol dose can cause patients to reach the desired level of sedation faster, which the results from study RF-5 implied.
We assumed no additional costs associated with healthcare personnel time-use in Stage 1B, and in Stage 1C, the time estimates were assumed to be the same as in Stage 2 plus Stage 3. In Stage 1C, a time-use associated with a porter transporting the patient to the waiting room was assumed.
Presence of healthcare personnel
All estimates of the presence of healthcare personnel were based on in-depth interviews with clinical experts with many years of experience with procedural sedation in Scandinavia.
When applying remimazolam or midazolam as procedural sedation, we assumed the presence of two nurses and one surgeon/endoscopist during preparation and the procedure (Stage 0 and Stage 1A), two nurses in the recovery unit and one nurse for the remainder of the stay until discharge. If the procedure was stopped due to healthcare personnel being unable to stabilise the patient after administrating rescue sedative medication, the cost associated with a porter transporting the patient to the waiting room was added in Stage 1C.
A slightly different setup is required when propofol is used in procedural sedation. In Stage 0, a nurse, a nurse anesthetist, and an anaesthesiologist are present until the patient is sedated. The anesthesiologist is also present for some time of the procedure when propofol is used for sedation, whereas the surgeon/endoscopist is responsible for the sedation when using remimazolam or midazolam.
The estimates for healthcare personnel time-use are presented in .
Table 1. Time-use (in minutes) and presence of HCPs during colonoscopies and bronchoscopies by procedural sedation.
Dosing and drug acquisition costs
Drug acquisition costs applied in the analysis of procedural sedation in colonoscopies were based on available list prices and the observed total dosing of remimazolam, midazolam, propofol, and fentanyl from referencesCitation14,Citation16. The mean total dose of remimazolam and midazolam used in referenceCitation14 was 11.0 and 4.9 mg, respectively. Similarly, the mean total dose of propofol in referenceCitation16 was 125.1 mg.
All patients received fentanyl 100 µg IV immediately before the administration of remimazolam and midazolam.
Use of rescue sedative medication and canceled procedure
In response to complications caused by non-optimal response to the procedural sedation, patients received rescue sedative medication. The number of patients assumed to require any rescue sedative medication during colonoscopies, as well as the mean dose of required rescue sedative medication, originated from referencesCitation13,Citation14,Citation16 for the comparison of remimazolam to midazolam and propofol.
If the use of rescue sedative medication did not stabilize the patient, it was assumed that the procedure would be canceled and rescheduled. For patients whose procedure was canceled, we assumed similar time-use and presence of healthcare personnel during the recovery phase and for the time ready to discharge as for patients who completed a successful procedure, although an additional cost of time-use associated with a porter transporting the patient to the waiting room was added.
presents the share of patients with successful and unsuccessful procedures as well as the requirement for rescue sedative medication.
Table 2. Procedure outcomes and use of rescue sedative medication.
Patient-incurred costs
Patient-incurred costs associated with the value of patient time-use and transportation costs are included in the model base case. Patient time-use was set equal to the total time spent at the hospital from preparation to discharge. Transportation costs to and from the hospital were incurred once for patients who successfully completed the procedure and twice for patients whose procedure were canceled, assuming a successful outcome of the second procedure.
Unit costs
presents the unit costs applied in the model. Costs associated with the presence of healthcare personnel follow the Danish Medicines Council’s micro-costing methodology guideCitation22. According to the clinical experts, one nurse can monitor three patients in recovery, thus, the hourly cost for nurses during recovery was assumed to be one-third of the hourly wage. The costs associated with the use of physical facilities were calculated based on Sørensen et al.Citation20.
Table 3. Unit costs.
Utensils included in the analysis were informed by clinical experts (see ). The utensil costs are based on estimates from the report “Differences in costs of administering biological drugs”, KORA 2015Citation23.
Results
In this section, we present the results from the cost analysis of procedural sedation in colonoscopies. The analysis in bronchoscopies showed similar results and can be found in the Supplementary Appendices.
presents the results from the comparison of remimazolam, midazolam, and propofol in colonoscopies. We found that the total cost of using remimazolam was DKK 1200 per colonoscopy procedure. In comparison, the total cost per colonoscopy procedure of using midazolam and propofol was DKK 1320 and DKK 1255, respectively. The incremental saving per colonoscopy procedure of using remimazolam was estimated to be DKK 120 compared to midazolam and DKK 55 compared to propofol.
Table 4. Results: procedural sedation in colonoscopies.
We found the difference in time spent in the preparation phase (time to start of the procedure) and the time spent in the recovery phase (time to fully alert after the end of the procedure) to be the main contributors to the difference in total cost in the comparison of remimazolam and midazolam. The two phases lead to an incremental time-use of 9.2 min, amounting to a monetary saving of DKK 219.
In the comparison of remimazolam to propofol, we identified the requirement of the presence of a nurse anesthetist and an anaesthesiologist during the preparation and the procedure to be the main driver for the difference in the total cost. Even though the time spent in each phase is expected to be lower for patients treated with propofol, the presence of an anaesthesiologist increased the cost by DKK 154 during the procedure and DKK 66 during the recovery phase.
Sensitivity analyses
To assess the uncertainty associated with the base case analysis, we conducted various deterministic sensitivity analyses (DSAs) (see ). The performed DSAs were changes in the time spent in each stage, assuming 100% of patients have an uncomplicated procedure (scenario 1), +10% of patients required rescue sedative medication and experienced an unsuccessful procedure (scenario 2), a DSA on the total dose of study drug as the dose of midazolam, propofol, and remimazolam varies between patients and a DSA on the fentanyl dose in all arms as this dose also varies between patients. The DSAs were conducted on all treatment arms simultaneously, i.e. in the DSA with changes in the time spent in each stage, the changes in time were applied to both the remimazolam, midazolam, and the propofol arms. More information and results on performed DSAs are presented in the Supplementary Appendices.
Discussion and limitations
This cost analysis estimated the cost per successful procedure when using remimazolam compared to midazolam or propofol for procedural sedation in colonoscopies and bronchoscopies. We found that remimazolam is a cost-saving sedative compared to midazolam and propofol in endoscopies. The difference in time spent in the preparation phase (time to start of the procedure) and the time spent in the recovery phase (time to fully alert after the end of the procedure) were the two main contributors to the difference in total cost in the comparison of remimazolam and midazolam. In the comparison of remimazolam and propofol, the requirement of the presence of a nurse anesthetist and an anaesthesiologist during the preparation and the procedure were identified as the main driver of the difference in the total cost between remimazolam and propofol. The value of reducing resources in terms of healthcare personnel (HCP) is essential due to the technical advances in diagnostic and therapeutic procedures that have resulted in an exponential growth of procedures that require procedural sedation in the last few decades, particularly in gastroenterology and colonoscopiesCitation24. This growing demand for diagnostic and therapeutic procedures has resulted in many centers being under pressure to conduct the procedures as quickly as possible and conduct as many as possible to avoid long waiting times for patientsCitation25. The increasing demand for procedures requiring procedural sedation results in an unmet need for sedatives that aside from being effective and offer rapid recovery can also release time from HCPs to perform more procedures and reduce the waiting times.
Monitoring equipment was not specified in the clinical study reports on the included trials. Monitors, such as Bispectral Index (BIS) and Entropy could provide a more subjective starting point for the procedure. All three trials applied in the present study used the MOAA/S to assess the starting point for the procedure, and the initial doses of the sedatives were administered as IV injections. It is expected that the choice of monitoring equipment might impact the “Time to start of the procedure”. This parameter was included in the DSA and showed that applying the 95% CIs to this parameter had a minor impact on the result of the base case. Thus, it is expected that the usage of different monitoring equipment will be minor.
In clinical studies, remimazolam has been shown to increase the number of successful procedures, reduce the need for rescue sedative medication, reduce the time spent on preparing for the procedure, reduce the length of the procedure itself and reduce the time needed for recoveryCitation12–14. These are all components that influence the cost of performing the included procedures, as seen when using a micro-costing approach as applied in our analysis. In addition, these components are also relevant to the patient. Procedural sedation is a central component in many procedures and may encourage patients to attend diagnostic screenings, such as colonoscopies, which is the gold standard method for screening for colorectal cancer. Colorectal cancer is a common type of cancer in Scandinavia, and fear and anxiety associated with colonoscopies have been cited as barriers to colorectal cancer screening, causing the patient to refuse the procedure with possible negative implications for both diagnosis and treatmentCitation26.
The focus of our cost analysis was procedural sedation in endoscopies; however, procedural sedation is also used for a wide range of procedures in other settings, including cardiology procedures, diagnostic and emergency medical proceduresCitation27. Endoscopies were the focus of our analysis, as the majority of procedural sedation occurs in endoscopy unitsCitation28, and clinical studies of remimazolam have been conducted in colonoscopy and bronchoscopy procedures. No data is available on the use of remimazolam in the other above-mentioned procedures. We were unable to identify reliable data on relevant inputs for propofol in bronchoscopies, and for that reason, this analysis was not conducted.
Key model parameters were based on available data from the clinical studies, designed in collaboration with the FDA with published data from high ranked peer-reviewed journals. By relying on high-quality objective data, we sought to enhance the external validity of the cost estimates. Estimates that were not available from the clinical trials were provided by clinical experts with vast experience in procedural sedation and the included procedures. We emphasize that the use of estimated data in the model is a limitation of our study, however, the purpose of the model was to estimate the economic benefits of remimazolam in a Scandinavian context and we believe that the information provided by the clinical experts on clinical practice in Scandinavia are appropriate to inform the model. The clinical experts provided inputs on the presence of HCPs, usage of utensils, procedure time, and the use of rescue medication. The same procedure time and utensils were assumed for all drugs and therefore, we do not expect this to have an impact on the result of our analysis. However, a limitation associated with using data from clinical studies that are performed in a controlled environment is the potential risk that our findings will not result in the same cost savings in a clinical setting. Clinical practice, as well as the preferred choice of sedative, varies greatly across the hospital departments performing the procedures in Scandinavia, e.g. the use of nurse-administered propofol sedation (NAPS) at some hospital departments can compromise the applicability of our results to local departments in Scandinavia. Therefore, it is challenging to establish the potential heterogeneity in the effect on costs that the implementation of remimazolam will have. We tried to overcome this by conducting several DSAs. The parameter with the largest impact on our base case of remimazolam compared to midazolam was the time in recovery. In the comparison of remimazolam and propofol, the procedure time was the parameter with the largest impact on the base case. However, we found remimazolam to consistently be the cost-saving treatment choice. In addition, propofol has a narrow therapeutic window and even minor dose adjustments may affect vital signsCitation5. The adverse events associated with propofol include injection site pain, hypotension, bradycardia, and respiratory depressionCitation6,Citation7. Due to its potentially serious side effects, the use of propofol might be more restricted than the use of remimazolam needs to be. It should be noted that other factors can influence the cost of endoscopies, such as the patient’s body type, and the metabolism time of the drugs, which vary from patient to patient. Also, the physical condition of patients can impact the cost of the endoscopy, and all these factors can have an impact on the overall cost.
Conclusion
Procedural sedation with remimazolam is associated with economically meaningful savings compared to procedural sedation with midazolam and propofol in colonoscopies and to midazolam in bronchoscopies.
The main drivers of the savings in the comparison with midazolam are a reduction in the time spent in the preparation phase and the time spent in the recovery phase. In the comparison with propofol, the main drivers were the requirement of the presence of a nurse anesthetist and an anaesthesiologist during the preparation and the procedure.
Transparency
Declaration of funding
Supported by PAION AG.
Declaration of financial/other relationships
Remimazolam is a product of PAION (funder). PAION provided support in the form of payments to EY Godkendt Revisionspartnerselskab. Authors L.K. and E.E. are employees at PAION. EY Godkendt Revisionspartnerselskab was a paid vendor to PAION and authors M.H.P., A.D., and E.M. are paid employees of EY Godkendt Revisionspartnerselskab. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
M.H.P., A.D., and E.M. contributed to the study design, development of the economic model, and interpretation of the results. E.M. and M.H.P. drafted the manuscript. L.K. and E.E. contributed to the interpretation of the results, revision of the manuscript, and provided clinical expert knowledge. All authors have approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (31.7 KB)Acknowledgements
The authors would like to thank Frank Pott, Department of Anesthesiology and Intensive Care at Bispebjerg Hospital, Denmark, for providing insight into the clinical practice for procedural sedation and review of and feedback on the paper to the authors.
References
- Gurbulak B, Üçüncü MZ, Yardımcı E, et al. Impact of anxiety on sedative medication dosage in patients undergoing esophagogastroduodenoscopy. Wideochir Inne Tech Maloinwazyjne. 2018;13:192–198.
- Felley C, Perneger TV, Goulet I, et al. Combined written and oral information prior to gastrointestinal endoscopy compared with oral information alone: a randomized trial. BMC Gastroenterol. 2008;8:22.
- Vaessen HHB, Knape JTA. Considerable variability of procedural sedation and analgesia practices for gastrointestinal endoscopic procedures in Europe. Clin Endosc. 2016;49:47–55.
- Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49(6):924–934.
- Saunders R, Davis JA, Kranke P, et al. Clinical and economic burden of procedural sedation-related adverse events and their outcomes: analysis from five countries. Ther Clin Risk Manag. 2018;14:393–401.
- Triantafillidis JK, Merikas E, Nikolakis D, et al. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol. 2013;19:463–481.
- Keam SJ. Remimazolam: first approval. Drugs. 2020;80:625–633.
- EMC. Propofol 20 mg/ml Emulsion for injection/infusion [Internet]. Available from: https://www.medicines.org.uk/emc/product/11294/smpc#gref
- Shenbagaraj L, Thomas-Gibson S, Stebbing J, et al. Endoscopy in 2017: a national survey of practice in the UK. Frontline Gastroenterol. 2019;10:7–15.
- Hinkelbein J, Lamperti M, Akeson J, et al. European society of anaesthesiology and european board of anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur J Anaesthesiol. 2018;35:6–24.
- Pambianco DJ, Brooks DC. New horizons for sedation: the ultrashort acting benzodiazepine remimazolam. Tech Gastrointest Endosc. 2016;18:22–28.
- Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation During bronchoscopy. Chest. 2019;155:137–146.
- Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88:427–437.e6.
- Pambianco DJ, Borkett KM, Riff DS, et al. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83:984–992.
- Rex DK, Bhandari R, Lorch DG, et al. Safety and efficacy of remimazolam in high risk colonoscopy: a randomized trial. Dig Liver Dis. 2021;53:94–101.
- Yichang Humanwell Pharmaceutical Co Ltd. RF-5 clinical trial report: a multicentre, randomised, single-blind, positive-controlled, parallel group, phase IIb clinical study of the efficacy and safety of remimazolam besylate for injection in patients undergoing colonoscopy for diagnostic or therapeutic reasons. Data on file; 2018.
- Yichang Humanwell Pharmaceutical Co Ltd. RF-6 clinical trial report: a multicentre, randomised, single-blind, positive-controlled, parallel group, phase III clinical study of the efficacy and safety of remimazolam besylate for injection in patients undergoing colonoscopy for diagnostic or therapeutic reasons. Data on file; 2018.
- European Medicines Agency. Summary of product characteristics: remimazolam (Byfavo) [Internet]. EMA; 2021. Available from: https://www.ema.europa.eu/en/documents/product-information/byfavo-epar-product-information_en.pdf
- The Danish Medicine Council. Valuation of unit costs. Copenhagen (Denmark): The Danish Medicines Council; 2020.
- Sørensen. Omkostninger ved administration af biologiske lægemidler; 2011.
- Jakobsen M, Rasmussen SR, Kjelberg J. Differences in costs of administration of biologics – a case study within gastroenterology [Forskelle i omkostninger ved administrering af biologiske lægemidler – Et casestudie inden for gastroenterologien]. Aarhus and Copenhagen: Vive; 2015.
- The Danish Medicines Council. Værdisætning af enhedsomkostninger; 2020.
- Jakobsen M, Rasmussen SR, Kjellberg J. Forskelle i omkostninger ved administrering af biologiske lægemidler: et casestudie inden for gastroenterologien [Internet]. KORA; 2015. Available from: https://pure.vive.dk/ws/files/2041662/10724_administrering_biologiske_laegemidler.pdf
- Eurostat. Surgical operations and procedures statistics [Internet]. [cited 2021 May 19]. Available from: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Surgical_operations_and_procedures_statistics#Increasing_and_decreasing_surgical_operations_and_procedures
- Region Nordjylland. Sedation and analgesia for endoscopies: about the drugs [sedation og analgesi ved endoskopi; vedr. medikamenterne] [Internet]. Available from: https://pri.rn.dk/Sider/19475.aspx#a_Toc277144764
- Trevisani L, Zelante A, Sartori S. Colonoscopy, pain and fears: is it an indissoluble trinomial. World J Gastrointest Endosc. 2014;6:227–233.
- Blayney MR. Procedural sedation for adult patients: an overview. Contin Educ Anaesth Crit Care Pain. 2012;12:176–180.
- South West Anaesthetic Research Matrix. Sedation practice in six acute hospitals – a snapshot survey. Anaesthesia. 2015;70:407–415.