Abstract
Objective
To compare the efficacy and safety of two fixed combination, preservative-free eye drops (bimatoprost 0.01% in combination with either timolol 0.1% or 0.5%) in a gel formulation, with bimatoprost 0.03%/timolol 0.5% in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).
Methods
Phase II, randomized, investigator-masked, multicenter, 3-arm parallel group (Eudract No. 2017-002823-46). Eighty-six patients aged ≥18 years with OAG or OHT, with intraocular pressure (IOP) initially controlled for at least 6 months by a combination therapy of a dual prostaglandin and timolol or insufficiently controlled by first-line monotherapy were included. Patients were randomized to receive T4030a (bimatoprost 0.01%/timolol 0.1%; N = 29), T4030c (bimatoprost 0.01%/timolol 0.5%; N = 29) or bimatoprost 0.03%/timolol 0.5% (N = 28), administered once daily in the evening for 12 weeks. Primary endpoint was defined as change in IOP from day 1 to week 12 measured at 08:00 (±1 h). Further efficacy, safety and pharmacokinetic endpoints were assessed as secondary outcomes.
Results
The mean change in IOP from baseline to week 12 was −9.8 ± 2.1 mmHg for T4030a, −10.1 ± 2.5 mmHg for T4030c and −10.0 ± 2.8 mmHg for bimatoprost 0.03%/timolol 0.5%. All treatments were well tolerated with no safety issues identified in any group. In patients treated with T4030a, the systemic concentration of timolol was significantly lower after 12 weeks than in patients treated with T4030c or bimatoprost 0.03%/timolol0.5%.
Conclusions
These study results suggest that the preservative-free ophthalmic formulation of T4030a (bimatoprost 0.01%/timolol 0.1%) can be regarded as a useful tool in the therapeutic management of OAG and OHT.
Introduction
Glaucoma is a chronic, progressive neurodegenerative ocular disease and a leading cause of irreversible blindness worldwideCitation1. Whilst the etiology of this disease is poorly understood, preventing the development or worsening of the disease is focused on lowering intraocular pressure (IOP), the most important and only treatable risk factorCitation2.
Despite advances in surgical interventions, lowering of IOP with topical drugs remains the initial treatment for most patients with open-angle glaucoma (OAG) or ocular hypertension (OHT) Citation3,Citation4. First choice pharmacotherapies include topical formulations containing prostaglandins/prostamides such as bimatoprost or non-specific β-blockers such as timolol as monotherapyCitation5,Citation6. However, this fails to achieve a satisfactory IOP reduction in 40 to 75% of glaucoma patients after more than 2 years of treatmentCitation7. Consequently, a dual therapy such as bimatoprost/timolol fixed combination (BTFC) can be prescribed. Clinical studies using a BTFC have demonstrated a stronger IOP lowering effect compared to either component administered aloneCitation8–11. A fixed combination may also offer a more convenient therapy scheme, leading to improvement in compliance and a reduction in costsCitation12.
GanfortFootnotei, a well-established BTFC (bimatoprost 0.03%/timolol 0.5%) has been shown to be well tolerated and effective in patients who are insufficiently responsive to monotherapyCitation13. However, conjunctival hyperemia is reported as the most common adverse reaction related to bimatoprost 0.03%Citation14. The ocular safety profile of timolol 0.5% is favorable. However, due to systemic exposureCitation15, there is a risk of cardiovascular and pulmonary systemic adverse events (AEs) Citation16. A timolol 0.1% eye gel (GeltimFootnoteii) has demonstrated similar IOP-lowering effects compared with timolol 0.5%Citation17,Citation18, with reduced systemic absorption and side-effectsCitation19. The change from an aqueous solution to a gel formulation also leads to better ocular bioavailability of timolol and the potential to reduce the daily regimen from twice to once daily, improving convenience and complianceCitation20.
This phase II study was conducted as part of the clinical development program for a preservative-free bimatoprost/timolol formulation to compare the efficacy, safety and pharmacokinetics of two gel formulations (bimatoprost 0.01%/timolol 0.1% [T4030a]; bimatoprost 0.01%/timolol 0.5% [T4030c]) versus preservative-free Ganfort UD in patients suffering with OAG or OHT after 12 weeks of treatment.
Patients and methods
Study design and patients
This was a phase II, randomized, investigator-masked, 12-week treatment study conducted in 24 centers in 4 countries (Austria, Belgium, Hungary and Poland) (Eudract No. 2017-002823-46). The study was conducted in accordance with Good Clinical Practice ICH (E6 (R2)) applicable and the Declaration of Helsinki. Independent ethics committee approval for the study was obtained from Austria (Medical University of Vienna [1690/2018]), Belgium (University of Leuven [S62741]), Hungary (ETT FEB [38147-0/2019-EKL] and Poland (Medical University of Silesia [KNW/022/KB1/74/VIII/18]) before the start of the study. Prior to enrolment, written informed consent was obtained from each patient. The study took place between September 2018 and February 2020.
Patients eligible for inclusion were aged ≥18 years, with OAG (primary and secondary) or OHT initially treated and with an IOP controlled for at least 6 months by combination therapy of prostaglandin and timolol (fixed combination or not) and a history of IOP insufficiently controlled with first-line monotherapy OR initially treated with first-line monotherapy for at least 6 months, insufficiently controlled and requiring a dual therapy, and 500 µm ≤ central corneal thickness ≤600 µm in both eyes. At randomization visit, IOP had to be between 22–36 mmHg in both eyes, with asymmetry between eyes ≤3 mmHg. The main exclusion criteria were: advanced stage of glaucoma (defined by an absolute defect in the ten degrees central point of the visual field, severe visual field loss [MD < −18dB], or risk of visual field worsening due to trial participation according to the investigator’s judgement); history of non-response to bimatoprost and/or timolol; far best-corrected visual acuity (BCVA) (≤20/100 Snellen); presence of severe conjunctival hyperemia, superficial punctate keratitis, blepharitis, dry eye; ongoing or known history of ocular allergy, uveitis and/or viral infection; clinically significant or progressive retinal disease; corneal ulceration; history of trauma, infection or clinically significant inflammation within the previous 3 months; any contraindications to timolol.
Each patient attended the study center at the screening visit (day −42 ± 3 days), randomization visit (day 1), week 6 (day 42 ± 3 days) and week 12 (day 84 ± 7 days). Patients were given brinzolamide (AzoptFootnoteiii) eye drops b.i.d for 5 weeks, followed by a 7-day washout period before the randomization visit. At baseline (day 1), eligible patients were randomly assigned to receive either T4030a (preservative-free bimatoprost 0.01%/timolol 0.1%), T4030c (preservative-free bimatoprost 0.01%/timolol 0.5%; both from Laboratoires Théa, Clermont-Ferrand, France), or Ganfort UD (reference group, preservative-free bimatoprost 0.03%/timolol 0.5%; Allergan) at 20:00, once daily for 12 weeks in both eyes. Patients were questioned at each post-baseline visit to check compliance with the treatment regimen and the time of instillation. The number of used and unused single dose units were counted at the end of the study.
Efficacy assessments
Tonometry IOP assessment
At day 1 and week 12, IOP in each eye was measured using a calibrated Goldmann applanation tonometer at 08:00, 10:00 and 16:00 (±30 mins). At week 6, measurement was taken at 08:00. Two measurements were taken; if these differed by >2 mmHg, a third reading was taken.
Global efficacy assessment by investigator
The investigator evaluated the efficacy of the treatment based on a 4-point scale as very satisfactory, satisfactory, not very satisfactory, or unsatisfactory at week 6 and week 12. The investigator based this assessment on the reduction of the patients IOP and his/her knowledge of the patients previous antiglaucoma treatments and its effectiveness.
Safety assessments
Adverse events
Adverse events (AEs) were collected throughout the study. All AEs observed by the investigators or reported by patients were recorded, along with their severity and potential relationship to the study treatment.
Ocular symptoms and Global tolerance
Ocular symptoms (irritation/burning, stinging, itching, tearing, eye dryness feeling, foreign body sensation) that occurred throughout the day (recorded at each visit) and upon instillation (recorded at week 6 and week 12) were assessed by the patient based on a 4-point scale (none, present not disturbing, disturbing, very disturbing). A total severity score (sum of the 6 ocular symptoms) was calculated. Due to the exploratory nature of the study, this qualitative evaluation was considered sufficient to have a general understanding of symptomology. Global tolerance was assessed by the investigator and by the patient at week 6 and week 12 as very satisfactory, satisfactory, not very satisfactory, or unsatisfactory.
Slit lamp examination
A slit lamp examination was performed at each visit to assess conjunctival hyperemia (McMonnies 0–5 scale) and corneal fluorescein staining (Oxford 0–5 grading scale). Other ocular signs recorded at each visit (blepharitis, eyelid oedema, abnormal eyelashes aspect, folliculo-papillary conjunctivitis, and iris pigmentation) were graded using a 4-point severity score (none, mild, moderate, severe). Change from baseline was also assessed for each ocular sign based on 3 classes (improvement, no change/stable or worsening).
Other Safety parameters
Fundoscopy, central corneal thickness and visual field examinations were performed in each eye (if not done in preceding 6 months) at Screening and week 12 if judged necessary by the investigator. BCVA was measured at screening, day 1 and week 12. Cardiovascular parameters (blood pressure [mmHg] and heart rate [bpm]) were measured at day 1 and week 12.
Pharmacokinetic Assessments
Blood samples were taken pre-instillation and 30 min, 1 h 30 min, 4 h, 8 h and 12 h after the first instillation at day 1 and week 12. Blood was immediately centrifuged, and plasma frozen at −80 °C. At the end of the study, the frozen samples were shipped to SGS (France) for the measurement of plasma concentrations of bimatoprost and timolol by enzyme immunoassay. The lower limit of quantification (LOQ) of the assay was 0.100 ng/mL.
Statistical analyses
As this was a pilot study, the analyses were considered as exploratory, and no formal sample size was calculated. The primary efficacy endpoint (change in IOP from baseline at week 12 at 08:00 in the worse eye) was analyzed using a Mixed Model for Repeated Measures (MMRM) including as fixed factors: treatment, scheduled visit time point (week 6 and week 12), baseline IOP and previous treatment type (monotherapy or dual therapy) as covariates; and treatment by visit interaction, baseline IOP by visit interaction; and patient as random factor. The difference between treatments with the 95% confidence intervals (CI) were estimated at week 12 in this model. Sensitivity analyses (analysis of covariance [ANCOVA]) considering all observed data or using the last observation carried forward (LOCF) method were performed. Supporting analyses to investigate possible treatment by covariate (baseline IOP, previous treatment type) interaction were performed using separated MMRM.
Treatment comparisons for the secondary efficacy endpoints and safety endpoints were performed using a MMRM (ANCOVA for sensitivity analysis) or Cochran-Mantel-Haenzel (CMH) test with modified ridit scores.
For data recorded in both eyes, the analysis was performed separately for each eye. The worse eye was defined as the eligible eye with the highest average IOP at baseline (if IOP was the same in both eyes, the right eye was considered the worse eye).
The pharmacokinetic (PK) parameters (AUC0–12h, Cmax, t½, and tmax) were calculated and quantitative analysis was performed if more than 50% of patients in a subgroup had a plasma concentration ≥ LLOQ.
The modified intent-to-treat (mITT) set was defined as all randomized patients who received at least one dose and with at least one post-baseline efficacy evaluation. The per protocol (PP) set comprised all mITT patients without a major protocol deviation and the ITT set, all randomized patients who received at least one dose. The Safety set was all enrolled patients who received at least one dose. Efficacy assessments were performed on the mITT set, confirmed by the PP and ITT sets. Safety analyses were performed on the Safety set. Pharmacokinetic analyses were performed on the PK set (patients having received at least one dose, with at least one blood sampling performed).
Results
Patients studied
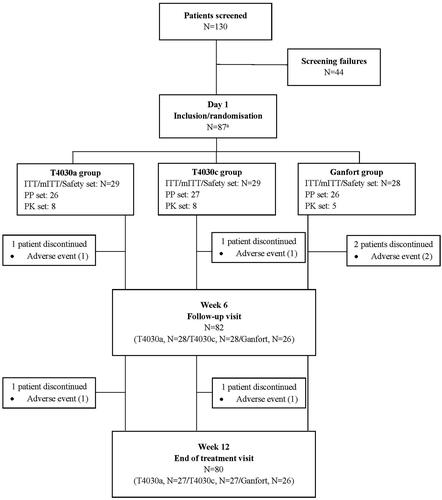
Of 130 patients screened, 86 were randomized on day 1 and received T4030a (n = 29), T4030c (n = 29) or Ganfort (n = 28) (). The most common reason for screen failure (40% of screen failed patients) was for patients excluded from entering the study due to the contraindications associated with timolol, reactive airway disease and cardiac disease. All but two patients from each treatment group (discontinuation due to AE) completed the study. The mean treatment compliance was >98% in all treatment groups.
Figure 1. Flow chart of patient disposition. Abbreviations. ITT, intent-to-treat; mITT, modified intent-to-treat; PP, perprotocol; PK, pharmacokinetics. A one patient performed screening visit but withdrew from the study before the randomisation visit due to abnormal ECG. The patient was considered a screen failure but was randomised incorrectly in the Ganfort group. As only the screening visit was performed, the patient was excluded from the study. Consequently, the total number of patients considered in the study was 86.
Patient demographics were comparable at screening, except for the median time from diagnosis of OAG/OH in the T4030c group (). Most patients were diagnosed with primary OAG (81.4% of patients) and had previously been treated with a dual therapy (62.8%). Five (4.7%) patients in the study had secondary OAG: 1 (3.4%) patient in the T4030a, 2 (6.9%) patients in the T4030c and 2 (7.2%) patients in the Ganfort groups.
Table 1. Demographic and other characteristics at Screening (mITT set).
Efficacy
Primary efficacy variable
The mean change in IOP (measured at 08:00) from baseline to week 12 in the mITT set was similar between treatment groups (). Using a MMRM, the adjusted mean difference between T4030a minus Ganfort was 0.16 ± 0.60 mmHg (95% CI: −1.04; 1.36), between T4030c minus Ganfort, 0.18 ± 0.60 mmHg (95% CI: −1.03; 1.39) and between T4030a minus T4030c, −0.02 ± 0.60 mmHg (95% CI: −1.21; 1.17). These results were confirmed in the PP and ITT set, and in the sensitivity analyses (ANCOVA).
Table 2. IOP (mmHg) in the worse eye at baseline (day 1; 08:00), week 6 and week 12 (primary efficacy analysis) and change from baseline (mITT Set).
Supporting analyses demonstrated no statistically significant treatment-by-baseline IOP (p = 0.788) or treatment-by-previous treatment type (p = 0.129) interaction.
Secondary endpoints
Results were similar between treatment groups at week 6 (08:00; ) and week 12 (10:00 and 16:00) in the worse eye, and at all timepoints measured in the contralateral eye, supporting the primary analysis.
The proportion of patients with an IOP <18 mmHg at week 12 at 08:00 was higher in the T4032a group (92.6% [95% CI: 76.6; 97.9]) compared to the T4030c (78.6% [95% CI: 60.5;89.8]) and Ganfort (76.9% [57.9; 89.0]) groups, but was similar between treatment groups at 16:00 (85.2% [67.5; 94.1], 82.1% [64.4; 92.1] and 84.6% [66.5; 93.8] for the T4030a, T4030c and Ganfort groups, respectively). Based on the 95% CIs, these differences in IOP <18 mmHg at 08:00 was not considered as statistically significant. IOP variation (in classes; ≤6 mmHg and >6 mmHg) throughout the day was consistent between treatment groups at week 12, with the majority of patients having a diurnal IOP variation ≤6 mmHg (100% in the T4030a and Ganfort groups, and 96.4% in the T4030c group). Results were also similar between treatment groups for the mean change in diurnal IOP from baseline at week 12: −9.0 ± 2.0 mmHg in the T4030a group, −9.3 ± 2.8 mmHg in the T4030c group and −9.5 ± 2.8 mmHg in the Ganfort group.
Investigators assessed the global efficacy as satisfactory or very satisfactory for most patients at week 6 and week 12 (>92% in all treatment groups).
Safety and tolerability
Adverse events
Treatment-related ocular AEs were reported in 4 (13.8%) patients (5 AEs) in the T4030a group, 5 (17.2%) patients (6 AEs) in the T4030c group and 6 (21.4%) patients (6 AEs) in the Ganfort group (). All ocular AEs were mild or moderate in severity. Six patients prematurely withdrew from the study due to an AE; 2 patients in the T4030a group (1 with erythema of eyelid and eye irritation and 1 with blepharitis and conjunctivitis), 2 patients in the T4030c group (1 eye allergy and 1 eye irritation) and 2 patients in the Ganfort group (1 eye symptom and 1 documented hypersensitivity to administered product). One treatment-related systemic AE was reported in the Ganfort group (headache). One patient in the T4030a group had a serious systemic AE (osteoarthritis), which was not related to the study treatment.
Table 3. Summary of AEs related to treatment (Safety set).
Ocular symptoms
The mean total score of ocular symptoms throughout the day decreased from baseline to week 12 in the T4030a (from 1.8 ± 3.1 to 1.4 ± 2.5) and Ganfort (0.9 ± 1.3 to 0.4 ± 0.6) groups, while it remained stable in the T4030c group (from 1.2 ± 1.8 to 1.1 ± 2.2), but no statistically significant between-group difference was observed. No statistically significant between-group difference was observed for any individual ocular symptom throughout the day for change from baseline to week 6 or week 12.
The mean total score of ocular symptoms upon instillation was similar at week 6 in the T4030a, T4030c and Ganfort groups (0.8 ± 1.2, 1.0 ± 2.2 and 1.3 ± 2.5, respectively) and reduced in all treatment groups at week 12 (0.5 ± 1.0, 0.6 ± 1.3 and 0.5 ± 1.1, respectively), with no statistically significant between-group difference observed. There was no statistically significant difference between groups for any individual ocular symptom upon instillation, except for itching at week 6 (in favor ofT4030c versus Ganfort; p = 0.047, CMH).
Ocular signs
At week 12, conjunctival hyperemia was present in 42.9% of patients in the T4030a group, 64.3% in the T4030c group and 42.3% in the Ganfort group for the worse eye. There was a higher percentage of patients experiencing a worsening of conjunctival hyperemia at week 12 in the T4030c group (39.3%), compared to T4030a (10.7%) and Ganfort (19.2%) groups. However, based on 3 classes (improvement, no change or worsening), there were no statistically significant between-group difference in the change from baseline to week 12 (T4030a vs Ganfort, p = 0.557; T4030c vs Ganfort, p = 0.585; T4030a vs T4030c, p = 0.298, CMH). The percentage of patients with worsening of corneal fluorescein staining from baseline at week 12 was similar in the T4030a (25.0%), T4030c (21.4%) and Ganfort (21.4%).
The percentage of patients with worsening of blepharitis, eyelid oedema, folliculo-papillary and iris pigmentation from baseline to week 12 for the worse eye was low in all treatment groups (<4% of patients). Worsening of abnormal eyelashes aspect from baseline to week 12 was noted in a higher percentage of patients in the T4030c (10.7%) and Ganfort (15.4%) groups compared to the T4030a group (3.6%).
Similar results were observed for the contralateral eye.
Other Safety parameters
There were no clinically important changes in corneal thickness, fundoscopy, visual field examinations, BCVA, or cardiovascular parameters in any group during the study. The majority of investigators and patients in all treatment groups regarded the tolerance as very satisfactory or satisfactory (>86%) at week 6 and week 12.
Pharmacokinetics
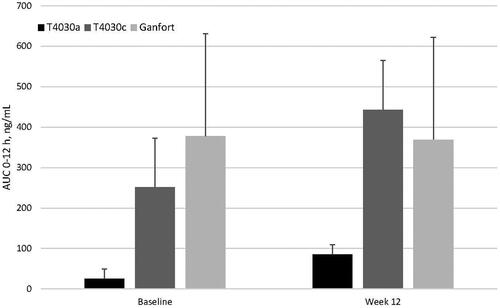
Systemic concentrations of bimatoprost were below the lower limit of detection of 0.100 ng/mL. For timolol, the mean (AUC 0–12 h) following the first instillation at day 1 was lower in the T4030a (25.5 ± 24.3 ng*min/mL) group compared to the T4030c (251.2 ± 121.9 ng*min/mL) and Ganfort (378.0 ± 253.0 ng*min/mL) groups. This was also observed at week 12 (85.5 ± 45.9, 442.9 ± 227.2 and 369.2 ± 149.3 ng*min/mL, respectively; ). Due to the small number of patients analyzed in the pharmacokinetic subgroup (8 patients in the T4030a group, 8 patients in the T4030c group and 5 patients in the Ganfort group), the observed difference between T4030a versus T4030c and Ganfort was not confirmed with a statistical test.
Discussion
In this phase II study, a 12-week treatment with a fixed combination of bimatoprost 0.01%/timolol 0.1% (T4030a) or bimatoprost 0.01%/timolol 0.5% (T4030c) gel formulation was shown to be comparable to bimatoprost 0.03%/timolol 0.5% aqueous solution (Ganfort) in terms of IOP-lowering effect in OAG or OHT patients. This study also provides new pharmacokinetic and safety data of a new BTFC formulation. Both formulations (T4030a and T4030b) were well tolerated with no safety concerns. A review of the 5 patients with secondary OAG showed that IOP was well controlled throughout the study, with no spikes in IOP reported. Treatment-related ocular AEs were experienced by more patients in the Ganfort group (6 [21.4%] patients) compared to the T4030a (4 [13.8%] patients) and T4030c (5 [17.2%] patients) groups, but due to the small sample size it is considered unlikely that a difference of 2 patients is clinically significant. Whilst these findings are mainly descriptive, it will serve as a basis for the phase III clinical development.
For all timepoints, a significant reduction in IOP from baseline was observed in each treatment group. Whilst this study had a small sample size, the results are in accordance with results obtained from larger phase III non-inferiority studies of BTFCCitation10,Citation21. A study by Hommer et al. demonstrated non-inferiority of BTFC versus BT non-fixed combination after 3-weeks, where the mean diurnal IOP change from baseline was −8.8 mmHg for BTFC and −9.6 mmHg for BT unfixed combinationCitation10. Similar results were observed in a large, multicenter study in 235 patients with OAG or OHT, where the between-group difference (BTFC minus unfixed combination) in the change from baseline of mean IOP was −0.566 mmHg (95% CI: −1.68; 0.57) Citation21.
As bimatoprost and timolol are dosed at the same or lower concentration in T4030 compared to Ganfort, the ocular safety of T4030 were expected to be comparableCitation22, and was demonstrated in this study. Previous studies have demonstrated that the most common adverse reaction with BTFC is conjunctival hyperemiaCitation23. In our study, the presence of conjunctival hyperemia at week 12 was in 39.3% of patients in the T4030a group, 46.4% in the T4030c group and 42.3% in the Ganfort group. As conjunctival hyperemia is associated with the concentration of bimatoprost, the incidence of this sign was expected to be less frequent with T4030 compared to Ganfort. However, it has been suggested that conjunctival hyperemia takes several months to decrease, with one study reporting conjunctival hyperemia in 45.2% of patients at 6 months versus 9.5% at 12 monthsCitation24.
Plasma concentrations of bimatoprost in all treatment groups was below the lower limit of detection, with no evidence of accumulation at week 12, which is in agreement with the literatureCitation25. In contrast, timolol was detected at a significantly higher concentration in T4030c and Ganfort (timolol 0.5%) compared to T4030a (timolol 0.1%) at baseline and week 12, with AUC values ranging between 4-fold and 15-fold higher. This is to be expected as timolol has a high systemic bioavailability following topical administration with up to 80% being systemically absorbedCitation26.
The main limitation of this study was the small sample size, however the IOP and pharmacokinetic data are in accordance with previously reported dataCitation8,Citation27,Citation28. Another potential limitation is the relatively short treatment period, which may make assessments on visual functionCitation29, and assessing the evolution of ocular signs in patients previously treated with a preserved medication difficultCitation30.
At this point, it is important to discuss the main clinical implication of our findings. The results suggest a similar IOP-lowering effect of a lower concentration of both bimatoprost and timolol in regard to T4030a. A major phase III clinical study comparing this formulation with Ganfort to confirm these results is planned.
Conclusion
Overall, these study results suggest that the preservative-free ophthalmic formulations T4030a and T4030c are safe with good efficacy and therefore could be considered as potential new options in the therapeutic management of glaucoma and OHT.
Transparency
Declaration of funding
Editorial support for this study was funded by Laboratoires Théa, Clermont-Ferrand, France. No author received payment for their contribution to this article.
Declaration of financial/other relationships
Ewa Mrukwa-Kominek; Marta Misiuk-Hojlo; Dr Adrienne Csutak;Ingeborg Stalmans and Gerhard Garhofer were investigators of the study and received financial compensation.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors participated in the conduct of the study and in the review and approval of the manuscript.
Ethical approval
This study involved human participants and was approved by independent Ethics Committee(s) in each participating country.
Acknowledgements
The authors thank Richard Allan of Laboratoires Théa for medical writing services.
Notes
i Ganfort (Allergan, Ireland)
ii Geltim (Laboratoires Théa, France)
iii Azopt (Novartis Pharma, Switzerland)
References
- Tham Y-C, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology. 2014;121(11):2081–2090.
- Lu LJ, Tsai JC, Liu J. Novel pharmacologic candidates for treatment of primary Open-Angle glaucoma. Yale J Biol Med. 2017;90(1):111–118.
- Russo A, Riva I, Pizzolante T, et al. Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: a review. Clin Ophthalmol. 2008;2(4):897–905.
- Sambhara D, Aref AA. Glaucoma management: relative value and place in therapy of available drug treatments. Ther Adv Chronic Dis. 2014;5(1):30–43.
- Costagliola C, Prete AD, Incorvaia C, et al. Ocular surface changes induced by topical application of latanoprost and timolol: a short-term study in glaucomatous patients with and without allergic conjunctivitis. Graefes Arch Clin Exp Ophthalmol. 2001;239(11):809–814.
- Cvenkel B, Kolko M. Current medical therapy and future trends in the management of glaucoma treatment. J Ophthalmol. 2020;2020:6138132.
- European Glaucoma Society. 2014. Terminology and guidelines for glaucoma. 4th edn. Savona: PubliComm.
- Brandt JD, Cantor LB, Katz LJ, et al. Bimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2008;17(3):211–216.
- Brief G, Lammich T, Nagel E, et al. Fixed combination of bimatoprost and timolol in patients with primary open-angle glaucoma or ocular hypertension with inadequate IOP adjustment. Clin Ophthalmol. 2010;4:1125–1129.
- Hommer A, Ganfort Investigators Group I A double-masked, randomized, parallel comparison of a fixed combination of bimatoprost 0.03%/timolol 0.5% with non-fixed combination use in patients with glaucoma or ocular hypertension. Eur J Ophthalmol. 2007;17(1):53–62.
- Feuerhake C, Buchholz P, Kimmich F. Efficacy, tolerability and safety of the fixed combination of bimatoprost 0.03% and timolol 0.5% in a broad patient population: multicenter, open-label observational study. Curr Med Res Opin. 2009;25(4):1037–1043.
- Higginbotham EJ. Considerations in glaucoma therapy: fixed combinations versus their component medications. lin Ophthalmol. 2009;4:1–9.
- Gutierrez-Diaz E, Silva Cotta J, Muñoz-Negrete FJ, et al. Bimatoprost/timolol fixed combination versus latanoprost in treatment-naïve glaucoma patients at high risk of progression: a pilot study. Clin Ophthalmol. 2014;8:725–732.
- Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin-timolol fixed combinations: a meta-analysis of randomized clinical trials. Eur J Ophthalmol. 2012;22(1):5–18.
- Volotinen M, Hakkola J, Pelkonen O, et al. Metabolism of ophthalmic timolol: new aspects of an old drug. Basic Clin Pharmacol Toxicol. 2011;108(5):297–303.
- Pratt NL, Ramsay EN, Kalisch Ellett LM, et al. Association between ophthalmic timolol and hospitalisation for bradycardia. J Ophthalmol. 2015;2015:567387.
- Quaranta L, Katsanos A, Floriani I, et al. Circadian intraocular pressure and blood pressure reduction with timolol 0.5% solution and timogel 0.1% in patients with primary open-angle glaucoma. J Clin Pharmacol. 2012;52(10):1552–1557.
- Rolle T, Curto D, Alovisi C, et al. Timogel® vs timolol 0.5% ophthalmic solution: efficacy, safety, and acceptance. Eur J Ophthalmol. 2012;22(1):28–33.
- Uusitalo H, Niño J, Tahvanainen K, et al. Efficacy and systemic side-effects of topical 0.5% timolol aqueous solution and 0.1% timolol hydrogel. Acta Ophthalmol Scand. 2005;83(6):723–728.
- Chiou SH, Hsu WM, Liu JH, et al. Comparative study of timolol gel versus timolol solution for patients with glaucoma. Zhonghua Yi Xue Za Zhi (Taipei). 2000;63(10):737–743.
- Ling Z, Zhang M, Hu Y, et al. Safety and efficacy of bimatoprost/timolol fixed combination in chinese patients with open-angle glaucoma or ocular hypertension. Chin Med J (Engl). 2014;127(5):905–910.
- Allergan. GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution, in single-dose container; 2018.
- Shim SH, Kim JM, Choi CY, et al. Diurnal intraocular pressure with bimatoprost/timolol fixed combination versus latanoprost/timolol fixed combination in healthy subjects. Korean J Ophthalmol. 2014;28(1):39–48.
- Rigollet JPK, Ondategui JA, Pasto A, et al. Randomized trial comparing three fixed combinations of prostaglandins/prostamide with timolol maleate. Clin Ophthalmol. 2011;5:187–191.
- Woodward DF, Krauss AH-P, Chen J, et al. The pharmacology of bimatoprost (lumigan™). Survey of Ophthalmology. 2001;45: s337–S345.
- Nieminen T, Lehtimäki T, Mäenpää J, et al. Ophthalmic timolol: plasma concentration and systemic cardiopulmonary effects. Scand J Clin Lab Invest. 2007;67(2):237–245.
- Niño J, Tahvanainen K, Uusitalo H, et al. Cardiovascular effects of ophthalmic 0.5% timolol aqueous solution and 0.1% timolol hydrogel. Clin Physiol Funct Imaging. 2002;22(4):271–278.
- Rouland J-F, Morel-Mandrino P, Elena P-P, et al. Timolol 0.1% gel (nyogel 0.1% once daily versus conventional timolol 0.5% solution twice daily: a comparison of efficacy and safety. Ophthalmologica. 2002;216(6):449–454.
- Martinez A, Sanchez M. Bimatoprost/timolol fixed combination vs latanoprost/timolol fixed combination in open-angle glaucoma patients. Eye (Lond). 2009;23(4):810–818.
- Katz LJ, Cohen JS, Batoosingh AL, et al. Twelve-month, randomized, controlled trial of bimatoprost 0.01%, 0.0125%, and 0.03% in patients with glaucoma or ocular hypertension. Am J Ophthalmol. 2010;149(4):661–671.e1.