Abstract
Objective
Intraoperative arterial hypotension (IOH) is associated with poor patient outcome. This study aims to compare the hemodynamic effects of Cafedrine/Theodrenaline (C/T) and Noradrenaline (NA) for the treatment of hypotension in patients who develop IOH after anesthesia induction.
Research design and methods
This is a national, randomized, parallel-group, multicenter, and open-label study. Adult patients (≥50 years, ASA-classification III–IV) who undergo elective surgery will be included. When IOH (MAP <70 mmHg) develops, C/T or NA will be given as a bolus injection (“bolus phase”, 0–20 min after initial application) and subsequently as continuous infusion (“infusion phase”, 21–40 min after initial application) to achieve MAP = 90 mmHg. Hemodynamic data are captured in real time by advanced hemodynamic monitoring.
Results
Primary endpoints, i.e. the treatment-related difference in average mean arterial pressure (MAP) during the “infusion phase” and the treatment-related difference in average cardiac index during the “bolus phase” are assessed (fixed-sequence method). Non-inferiority of C/T compared to NA in achieving 90 mmHg (MAP) when applied as continuous infusion is hypothesized. In addition, superiority of C/T over NA, applied as bolus injection, in increasing cardiac index is postulated. It is estimated that 172 patients are required to establish statistical significance with a power of 90%. After adjusting for ineligibility and dropout rate, 220 patients will be screened.
Conclusion
This clinical trial will yield evidence for marketing authorization of C/T applied as continuous infusion. Additionally, the effects of C/T compared to NA on cardiac index will be assessed. First results of the “HERO”-study are expected in 2024. DRKS identifier: DRKS00028589. EudraCT identifier: 2021-001954-76.
Introduction
More than 75% of surgical patients develop intraoperative arterial hypotension (IOH) with a mean arterial blood pressure (MAP) below 70 mmHgCitation1,Citation2. The extent and duration of hypotension are determinants of postoperative outcomeCitation3–5. Restoring and maintaining optimal blood pressure is paramount to securing perfusion and blunting organ damage, especially for the heart, kidney, and brain. In fact, a difference of 6.5 mmHg (mean SBP 123 mmHg vs 116 mmHg) throughout surgery was shown to increase postoperative organ dysfunction by 17% (46% vs 63% of patients) on postoperative day 30Citation6. Based on the available literature, it is assumed that an intraoperative MAP in a range of 80–100 mmHg is safe for all patientsCitation3.
In Germany, a 20:1 mixture of Cafedrine/Theodrenaline (C/T) has been widely used for the treatment of hypotension since 1963Citation7, while Noradrenaline (NA) was approved for treatment of hypotensive states only in 2016Citation8. Currently, 72% of German hospitals use C/T (ratiopharm GmbH, internal data 2021), which includes both short injection and off-label use as continuous infusion, which is currently not covered by the summary of product characteristics (SmPC)Citation9. Noradrenaline is administered as continuous infusion and off-label as bolus injection to treat IOHCitation10–13.
While both medicinal products effectively increase blood pressure, pharmacologic properties suggest a more pronounced increase of cardiac output in response to C/T when compared to NA. Cafedrine/Theodrenaline has been shown to effectively increase the MAP by combined effects on preload, contractility, and afterload, while heart rate is mostly unaffectedCitation14. Increase of cardiac index (CI) and MAP seem to be more pronounced in womenCitation15. Noradrenaline has potent alpha-adrenergic and slight beta-adrenergic effects resulting in potent vasoconstriction and less potent inotropy. The increase in blood pressure may cause reflex bradycardiaCitation16,Citation17. To date, no direct comparison between the two medicinal products is available.
The “HERO" study presented here compares C/T to NA for the treatment of intraoperative hypotensive states in a multicenter randomized, open label, parallel group clinical trial. This paper describes the design of the study, its rationale, and planned analyses.
Methods and results
Objective
The purpose of the study is to compare the hemodynamic effects of C/T and NA administered as bolus injection (“bolus phase”) and subsequent as continuous infusion (“infusion phase”) in a surgical cohort of at-risk adult patients who develop IOH. It is postulated that C/T is non-inferior to NA in the prolonged treatment of hypotensive states when administered as continuous infusion. It is further postulated that C/T has superior effects on cardiac index (CI) compared to NA at doses required to comparably stabilize blood pressure.
Study design
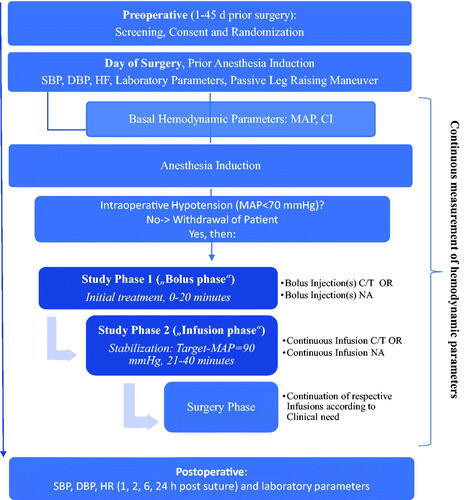
This is a national, multicenter, open label randomized reference medicinal product-controlled clinical trial set in Germany that evaluates the efficacy, safety, and hemodynamic effects of C/T compared to NA administered initially in boluses followed by continuous infusion, in predefined adult patients who develop IOH (MAP <70 mmHg) after induction of general anesthesia (). This study will be conducted in full accordance with the ICH Harmonized Tripartite Guideline, Guideline for Good Clinical Practice E6.
Figure 1. Schematic flow diagram of study. Intraoperative hypotension is defined as MAP <70 mmHg. The dosages of C/T or NA are adjusted according to body weight (study phase 1) or follows the clinical effect (Target MAP = 90 mmHg) (study phase 2). Additional bolus injections of study medication are given at MAP <70 mmHg in study phases 1 and 2. Measures for hemodynamic stabilization (volume, administration of catecholamines, positioning, etc.) will be taken as required. The hemodynamic effects will be captured in real time by advanced monitoring: Mean Arterial Pressure, Systolic Blood Pressure, Diastolic Blood Pressure, Stroke Volume (Index), Heart rate, Systemic Vascular Resistance (Index), dP/dtmax. Stroke Volume Variation. Laboratory parameters are: creatinine, GFR (calculated), troponin; blood gas analysis: pH, base excess, lactate (blood), potassium. Abbreviations: C/T, Cafedrine/Theodrenaline; NA, Noradrenaline; PLR, passive leg raising maneuver; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
The leading ethic committee of the Medical Faculty of the Philipps-University Marburg (chairman: Prof. Dr. med. C. Seifart) approved this trial on February 23, 2022 (Az.202/21 A-ff). All participating hospitals will obtain approval by their responsible ethics committees. The “HERO” study was registered at German Clinical Trials Register (DRKS) identifier: DRKS00028589 and EudraCT identifier: 2021-001954-76.
Study population
This study includes adult patients with comorbidities (ASA III/IV) who undergo elective vascular surgery and require antihypotensive drug treatment due to arterial hypotension (MAP <70 mmHg) after induction of anesthesia. Written informed consent will be obtained from each patient before any study specific procedures or assessments are done.
Inclusion criteria
The consentable patients are at least 50 years of age with chronic cardiac failure (NYHA-stages: II–III), of ASA-Classification 3 or 4, and have an indication for arterial cannulation and advanced hemodynamic monitoring prior to induction of general anesthesia using Propofol/Fentanyl or Propofol/Sufentanil. According to the NYHA-Classification, NYHA I is defined as no limitation of physical activity (ordinary physical activity does not cause undue breathlessness, fatigue, or palpitations), NYHA II as a slight limitation of physical activity (comfortable at rest, but ordinary physical activity results in undue breathlessness, fatigue, or palpitations), NYHA III as a marked limitation of physical activity (comfortable at rest, but less than ordinary physical activity results in undue breathlessness, fatigue, or palpitations), and NYHA IV as unable to carry out any physical activity without discomfort (symptoms at rest can be present; if any activity is undertaken, discomfort is increased).
Exclusion criteria
Patients are not included if there is any known hypersensitivity to Sodium Metabisulfite or any of the components of medicinal products as per their SmpCs and/or bronchial asthma with sulfite hypersensitivity. In addition, patients are excluded with underlying conditions (clinically relevant mitral stenosis, clinically relevant aortic stenosis, hyperthyroidism, pheochromocytoma, uncontrolled hypertension) or undergoing treatments with cardiac sensitizing agents or co-medications that negatively affect the effectiveness of the medicinal products (ACE-Inhibitor/AT2-Antagonists or MAO-Inhibitors on the day of surgery). Furthermore, patients are excluded if they have sepsis, septic shock, or systemic inflammatory response syndrome, if there is use of regional or inhalation anesthesia during bolus- and/or infusion phase, and/or if a female patient cannot exclude pregnancy.
Withdrawal criteria
Patients are withdrawn from the study if they revoke consent or request withdrawal or if they experience a medical condition which indicates that continued participation is not in their best interest. Furthermore, patients receiving another anti-hypotensive agent prior to the initial application of the study medication or not developing IOH (MAP <70 mmHg) after anesthesia induction are withdrawn. In cases of missed recordings of basal parameters (CI, MAP) or recording of less than 80% of hemodynamic values (CI, MAP) in bolus- or infusion phase (e.g., due to technical failure) or f surgery (cut) starts prior to or during bolus- or infusion phase, the patients are withdrawn.
Study medication
C/T (Cafedrine hydrochloride 200 mg/Theodrenaline hydrochloride 10 mg per 2 ml solution for injection) (ratiopharm GmbH, Ulm, Germany) and NA (2 mg Noradrenaline tartrate corresponding to 1 mg Noradrenaline base per 1 ml solution for infusion) (Sintetica GmbH, Münster, Germany) are used in this study.
Study procedure
After consenting, patients will be randomly assigned to one of two treatment groups using a gender-stratified approach aiming for a 1:1 ratio of female and male (but allowing for an imbalance between genders up to 2:3). The total duration of participation in the study for each patient is from the time point of consenting to collection of postoperative data 24 h after the end of surgery (suture).
The test period is prepared prior to anesthesia by placing an arterial line for advanced hemodynamic monitoring and attaching electrodes for Bispectral index (BIS) monitoring. Basal hemodynamic parameters are collected and a passive leg raising maneuver is performed to assess preload responsiveness. A stroke volume index (SVI) ≥10% induced is an indicator of fluid responsiveness. Anesthesia induction will be performed using Propofol/Sufentanil or Propofol/Fentanyl. Anesthesia will be maintained during bolus- and infusion phase by administering Propofol by continuous infusion or by repeated bolus injections and single bolus administration of Fentanyl/Sufentanil at the discretion of the anesthetist (target BIS-range = 40–60). Tidal volume is 7–8 ml/kg predicted body weight.
A given screen view of advanced hemodynamic monitoring platform for bolus- and infusion phase includes the hemodynamic parameters systolic and diastolic blood pressure, MAP, and pulse rate only.
Study phase 1: “bolus phase”
When IOH develops after anesthesia induction (MAP <70 mmHg), C/T (1.0 mg Cafedrine/kg body weight (idealized)) or NA (0.1 mcg Noradrenaline/kg body weight (idealized)) (depending on the study arm) are applied as bolus injection and the “bolus phase” (20 min) begins. Repeated bolus administrations of C/T or NA (depending on study arm) are applied if MAP is <70 mmHg. Additional measures for hemodynamic stabilization may be taken (e.g., volume/positioning/atropine) according to clinical demand at the discretion of the anesthetist.
Study phase 2: “infusion phase”
The “infusion phase” lasts from 21 min until 40 min after the initial bolus application. C/T or NA (depending on the study arm) are applied as continuous infusion. The initial infusion rate of C/T or NA is chosen commensurate with MAP at the beginning of this study phase (Supplementary Table S1). Once infusion has been established, the dose is titrated, targeting a MAP of 90 mmHg.
“Surgery phase”
The “surgery phase “is defined as the end of study phase 2 until the end of surgery (measured as the time point of suturing), where the treatment arms will continue. Posology is at the anesthetist’s discretion based on the effect on MAP that is captured in real time by advanced hemodynamic monitoring. Screen appearance of the advanced hemodynamic monitoring platform may be changed according to clinical demand at the discretion of the anesthetist. Additional measures (e.g., volume/fluid administration, other catecholamines, positioning, atropine, etc.) may be necessary to maintain hemodynamic stability according to clinical demand. Concomitant regional or inhalational anesthesia may be administered according to clinical demand at the discretion of the anesthetist.
Postoperative phase and end of the study
The study duration refers from induction of anesthesia until the end of surgery and subsequent monitoring of vital parameters until 24 h after the end of surgery (measured as the time point of suturing). The end of study is defined as the last collection of vital parameters 24 h after the end of surgery (measured as the time point of suturing).
A compilation of dosage, route of administration, and administration rate are available in Supplementary Table S1.
Trial Endpoints
Primary Endpoints
Primary Endpoint (1)
Treatment related difference in average MAP (DaMAP) during the infusion phase is calculated as the mean difference between areas below the target MAP value (90 mmHg). Non-inferiority is assumed if DaMAP can be shown to be lower than the non-inferiority margin (5 mmHg) at a one-sided alpha-level of 0.025.
Primary Endpoint (2)
Treatment-related difference in average cardiac index (DaCI) during the bolus phase is calculated as the difference in means of individual areas above the reference cardiac index (CIref), where CIref is the CI-measurement obtained at the time of the first bolus administration. Superiority is concluded if a positive DaCI can be shown at a one-sided alpha-level of 0.025.
Secondary Endpoints
Secondary Endpoint (1)
Treatment-related difference in average MAP (DaMAP) during the bolus phase. The estimated DaMAP will be presented along with its two-sided 95% confidence interval.
Secondary Endpoint (2)
Treatment related differences in change of heart rate (HR), change of stroke volume index (SVI), change of systemic vascular resistance index (SVRI), and change of maximal rate of ventricular pressure rise (dP/dtmax) during the bolus phase. The estimated treatment related differences will be presented along with their two-sided 95% confidence intervals.
Secondary Endpoint (3)
Ratio of treatment specific incidence rates of overshooting blood pressure (MAP >110 mmHg) events during the bolus phase. The estimated incidence rate ratio will be presented along with its two-sided 95% confidence interval.
Secondary Endpoint (4)
Ratio of treatment-specific incidence rates of low blood pressure (MAP <80 mmHg) events during the bolus phase. The estimated incidence rate ratio will be presented along with its two-sided 95% confidence interval.
Secondary Endpoint (5)
Treatment-related differences in the number of additional bolus-injections (at MAP <70 mmHg) and additional measures (volume/fluid administration, position changes, use of additional catecholamins, etc.) necessary to stabilize blood pressure during the bolus phase, where both entities and corresponding contrasts between treatments will be presented along with their two-sided 95% confidence intervals.
Exploratory Endpoints
Exploratory Endpoint (1)
Treatment-related differences in: change of HR, change of SVI, change of SVRI, change of dP/dtmax, and change of SVV during the infusion phase. The estimated treatment-related differences will be presented along with their two-sided 95% confidence intervals.
Exploratory Endpoint (2)
Ratio of treatment-specific incidence rates of overshooting blood pressure (MAP >110 mmHg) events during the infusion phase. The estimated incidence rate ratio will be presented along with its two-sided 95% confidence interval.
Subgroup analysis
Subgroup analyses will be performed for the primary endpoints (1) and (2) by means of exploratory analyses:
grouping by gender [male/female];
grouping by beta-blocking agents taken as concomitant medication at the day of surgery [yes/no];
grouping by preload responsiveness (Passive Leg Raising Maneuver) [yes/no];
grouping by age [<75years/≥75 years].
Statistical calculations
Sample size and power calculations
To conduct the test of non-inferiority, namely that the average MAP under infusion with C/T is non-inferior to that under infusion with NA during the infusion phase, the following sample size estimations were performed:
An average difference in MAP of 5 mmHg over a 20-minute window (i.e., an area under target MAP-level related difference of 5 mmHg × 20 min = 100 mmHg*min) is deemed to be medically important when assessing the duration and risk of unfavorable outcomes. Assuming no difference between test and reference IMP (difference: 0 mmHg [or 0 mmHg*min, respectively]), a non-inferiority margin of 5 mmHg (MAP) [or 100 mmHg*min (MAP-AUC over a 20-min window), respectively] and standard deviation of 3.0 mmHg [or 60 mmHg*min for the 20-min period, respectively] and a 1-sided alpha level of 0.025, 10 completers per treatment group are needed to achieve a statistical power of 90%. Adjusting for an approximately 20% ineligibility and dropout rate, especially due to not developing hypotension under anesthesia, 12 patients per treatment group are needed for this hypothesis testing.
To conduct the test of superiority, namely a higher average cardiac index in the C/T group compared to the NA group during study phase 1, the following sample size estimations were performed:
Assuming the C/T related treatment effect yields an average (over a 20-min window) cardiac index that is better by 0.5 L/min/m2 compared to the NA arm (i.e., an area above CIref-level related improvement of 0.5 × 20 = 10 L/min/m2*min), standard deviation of 1.0 L/min/m2 (or 20 L/min/m2*min for the 20-min period, respectively) and 1-sided alpha level of 0.025, 86 completers per treatment group (a total of 172 patients) are needed to achieve a statistical power of 90%. Adjusting for 20% ineligibility and dropout rate, especially due to not developing hypotension under anesthesia, 108 patients per treatment arm are needed (giving a total of 216 patients). Rounding up to 110 patients per group, a total of 220 patients is sufficient to demonstrate superiority with a power of 90% under these assumptions with a 1-sided type I error of 2.5%.
In summary, 110 patients per group (220 patients in total) are to be enrolled in this study (enrolled study population). The evaluation study population will comprise 176 patients.
Analysis sets
Analysis sets are defined in Supplementary Table S2.
Analysis of primary Endpoints
To address multiple testing, the endpoints are tested in a hierarchical order (fixed-sequence method): If non-inferiority in MAP-related assessment, as analyzed by the primary endpoint (1) can be demonstrated, assessment of superiority regarding Cardiac Index related measurements [primary endpoint (2)] will be performed. If not, the primary endpoint (2) related data will be analyzed descriptively only.
Primary Endpoint (1) difference in average MAP
For each Modified Intent to Treat (mITT) patient, the individual phase 2 related area under the targeted MAP (90 mmHg) curve (AUCMAP90) will be calculated using the trapezoidal rule. The individual average MAP below 90 mmHg (aMAP) represents the AUCMAP90 value divided by the observational time. Descriptive statistics for AUCMAP90 and aMAP will be presented by treatment arm. The treatment related difference in aMAP [DaMAP; DaMAP = aMAP(C/T) – aMAP(NA)] will be statistically assessed against a non-inferiority margin of 5 mmHg. The following hypothesis is tested: H0: DaMAP ≥ 5 mmHg versus H1: DaMAP <5 mmHg.
The primary endpoint (1) will be considered a “success” if H0 is rejected at a one-sided alpha level of 0.025. If the primary endpoint (1) is a “success”, primary endpoint (2) will be evaluated as follows, otherwise the primary objective is considered “failed”.
Primary Endpoint (2) difference in average CI
For each mITT patient, the individual phase 1 related area above the CIref curve (AUCCIref) will be calculated using the trapezoidal rule. The individual average CI above CIref (aCI) represents the AUCCIref value divided by the observational time. Descriptive statistics for AUCCIref and aCI will be presented by treatment arm. The treatment-related difference in aCI [DaCI; DaCI = aCI(C/T) – aCI(NA)] will be statistically assessed for superiority. The following hypothesis is tested: H0: DaCI ≤ 0 [units] versus H1: DaCI > 0 [units].
The primary endpoint (2) will be considered a “success” if H0 is rejected at a one-sided alpha level of 0.025.
Data collection
Hemodynamic data will be recorded by advanced hemodynamic monitoring using the HemoSphere advanced monitoring platform (Edwards Lifesciences Services GmbH) from the time point of placing the arterial line prior to anesthesia induction until the end of surgery (suture). Individual average MAP and CI will be collected as basal parameters prior to anesthesia. Hemodynamic parameters are collected in real-time during bolus-, infusion- and surgery phases.
Time of anesthesia induction, intubation, and placement of central venous catheter (CVC) (defined as first measurement) are recorded. Until the CVC-placement or in the case of no central venous access being attained, a central venous pressure of 6 mmHg is manually entered in the HemoSphere advanced monitoring platform.
Cumulative doses of Propofol/Fentanyl or Propofol/Sufentanil, method of Propofol-administration, type of muscle relaxants, and cumulative volume of crystalloid solution (applied as “basal fluid”) applied from anesthesia induction until the start of the bolus-phase, and during the bolus-, infusion-, and surgery phases are recorded.
Time of initial and repeated bolus administrations of the study medication (at MAP <70 mmHg) during the bolus phase as well as time of first application of continuous infusion (start of the infusion phase) and application of rescue boluses (at MAP <70 mmHg) of the study medication within the infusion phase are recorded. Furthermore, time and type of additional measures for hemodynamic stabilization (e.g., volume/fluid administration, positioning, catecholamine, etc.) during the bolus- and infusion phase are collected.
Blood samples for BGA (pH, base excess (blood), lactate, potassium) are obtained prior to anesthesia induction and at the beginning and at the end of the surgery phase. Blood samples for clinical laboratory tests (creatinine, GFR (calculated), troponin) are obtained prior to anesthesia induction and on the first post-operative day.
Other collected data will include vital parameters (systolic blood pressure (SBP), diastolic blood pressure (DBP), and HR) prior to anesthesia and on the first post-operative day and use of concomitant regional or inhalational anesthesia during the surgery-phase.
Data, which also include baseline demographics, comorbidities, concomitant medication, pre-medication on the day of surgery and relevant anesthesia and surgical parameters will be collected using an electronic, web-based case report form (eCRF).
Missing values
In general, a complete case analysis is foreseen. The number of randomized patients, which do not belong to ITT, will be reported. Univariable logistic regression approaches will be used to analyze whether missingness is related to treatment as well as to baseline variables. In the case of related findings, the primary endpoint analyses will be adjusted to these factors (sensitivity analysis).
Safety
In this study, safety will be assessed by qualified study personnel by evaluating reported adverse events (any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with this treatment), adverse drug reactions (any harmful and unintended effect arising from the use of the medication), and any special situations (medication error, overdose, misuse, abuse, occupational exposure).
Monitoring of the study
All physicians and clinical personnel participating in the study will be trained in every aspect of the study prior to recruitment of patients. Online and offline screening of the documentation, as well as monitoring of each site before, during, and after the study to ensure compliance with Good Clinical Practice (GCP) guidelines and adherence to the protocol will be carried out.
Discussion
Intraoperative hypotension is a common complication of anesthesiaCitation1,Citation2. In a recent expert consensus statement, even brief durations of MAP below 60–70 mmHg were identified to be associated with poor patient outcome but there was no recommendation on best treatments rather a call for more randomized controlled trialsCitation4. Restoring and maintaining individualized blood pressure has been shown to reduce organ damageCitation6, and the relevance of pharmacotherapy is considered to be high as non-pharmaceutical interventions are often insufficient to resolve hypotensive episodesCitation18. A number of pharmacotherapies are currently available, including Phenylephrine, Ephedrine, C/T, and NA. The hemodynamic effects and the pharmacokinetics of the substances were shown to be differentCitation18. However, there is a lack of meaningful head-to-head studies.
This study will compare hemodynamic effects of C/T versus NA for the treatment of IOH after anesthesia induction in a patient population with a high risk of developing IOH. Risk factors that have been identified to be associated with IOH include age, ASA status, and propofol used for inductionCitation19,Citation20.
The primary purpose of this study is to test whether C/T (compared to NA), administered as a continuous infusion following initial treatment with (repeated) bolus injections is non-inferior in increasing MAP and maintaining normotension (MAP = 90mmHg). Additionally, the primary purpose is to test whether C/T (compared to NA), administered as a bolus injection, leads to a higher increase of cardiac index.
In Germany, C/T are widely used for the treatment of IOH. For the history of its use, we refer to a systematic literature reviewCitation7. Use of C/T includes both bolus injectionCitation9 and off-label use as continuous infusion. Cafedrine/Theodrenaline solution for infusion (Akrinor® pro infusione) was available from 1971 until 2005. In 2005, solution for infusion was taken off the market because the requirements for subsequent approval could not be met within the required timeframeCitation21. Clinical practice and recent data show that a majority of the patients treated with bolus injection C/T (or Ephedrine) require repetitive doses of the drugCitation22. Owing to the frequency of repetitive bolus injections of C/T – whilst retaining a good safety profileCitation22 – it seems reasonable to provide clinicians with the option to administer the drug not only via short bolus injection but also via continuous infusion. This clinical trial aims for a marketing approval of C/T as continuous infusion. Providing clinicians with the option to administer C/T not only via short bolus injection but also via continuous infusion is likely to be advantageous (1) when trying to achieve stable hemodynamics as fluctuations of arterial blood pressure would be avoided, (2) as switching from one catecholamine to another, any ensuing potential drug interaction would be avoided, and (3) as augmenting the pharmacological therapeutic options with different pharmacologic properties for clinicians is meaningful.
The 20:1 C/T is a mixture of Noradrenaline and Norephedrine, both covalently bound to TheophyllineCitation7. Previous data indicate that the rapid onset of action seems to be based on Noradrenaline-mediated vasoconstriction, which is subsequently reduced by the delayed effect of Cafedrine, while Norephedrine leads to a positive inotropic effect, which is enhanced and prolonged by phosphodiesterase 3 inhibition mediated by TheophyllineCitation7. In sum, C/T exerts inotropic and moderate vasopressor effects, meaning the term “inopressor” best describes its mechanism of action and differentiates this medicinal product from other sympathomimetic agents. Inopressors may be particularly suitable for treatment of hypotension that occurs in association with Propofol use as they counteract both cardiac impairment and vasodilationCitation14,Citation15,Citation18. The effect of C/T is long-lastingCitation7. Noradrenaline, a well-established alternative agent for the treatment of acute hypotensionCitation8 with a short elimination half-life, has potent alpha-adrenergic and slight beta-adrenergic effects, resulting in potent vasoconstriction and less potent inotropy. Reflex bradycardia can occurCitation16,Citation17. The effect of NA on cardiac output has been shown to be highly variableCitation23. In summary, the pharmacodynamic and -kinetic profiles of C/T compared to NA are described to be different.
There are some limitations in our study. Due to a lack of dose-equivalence studies, the posology of study drugs is based on published data and clinical practiceCitation11,Citation14,Citation22,Citation24,Citation25. In addition, initial posology of continuous administration of C/T was developed using a pharmacometric modelCitation26 to simulate dosing schedules of C/T as continuous infusion based on data of the HYPOTENS study (ratiopharm, internal data). This is an open label clinical trial which therefore poses some risk of bias when compared to blinded clinical trials.
Initial results from this clinical trial will be available in 2024. It is expected to provide valuable information about hemodynamic effects of C/T versus NA. Furthermore, the estimates of equipotent doses during this study may guide the design of future studies.
Conclusion
This clinical trial will yield evidence for marketing authorization of C/T applied as continuous infusion. Additionally, the effects of C/T compared to NA on cardiac index will be assessed. The first results are expected in 2024.
Transparency
Declaration of financial/other relationships
The authors received no financial support for the authorship and/or publication of this article. L. Eberhart reports honoraria from Baxter Deutschland GmbH, Fresenius Kabi Deutschland GmbH, Grünenthal GmbH, Sintetica GmbH, and ratiopharm GmbH. B. Vojnar has received honoraria from ratiopharm GmbH. G. Geldner has received honoraria from ratiopharm GmbH. S. Huljic-Lankinen is an employee of ratiopharm GmbH. M. Murst is an employee of Teva GmbH. S. Weber is an employee of ACOMED statistik. T. Keller, ACOMED statistik, receives payments from the sponsor for statistical services (design and analysis) as part of this this study. T. Koch received honoraria for consulting from ratiopharm GmbH. P. Kranke has received honoraria for consulting from ratiopharm GmbH, Acacia Ltd, Amicus Clinical Development Ltd, BBraun, and Sintetica. He has received honoraria for lecturing from Fresenius Kabi Deutschland GmbH, Grünenthal GmbH, Baxter Deutschland GmbH, CSL Behring, Medtronic GmbH, Vifor Pharma, Pharmacosmos, and Sintetica. A. Weyland has received honoraria from ratiopharm GmbH. S. Kreuer is a shareholder of Saarmetrics GmbH, Saarbrücken, Germany. C. Gaik has received no payments. D. Chappell has received honoraria from ratiopharm GmbH, BBraun, Fresenius Kabi, Edwards Lifesciences, and Sintetica. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (35.3 KB)Acknowledgements
The HERO study is funded by ratiopharm GmbH, Ulm, Germany. Editorial assistance for manuscript preparation was provided by CRO Dr. med. Kottmann GmbH & Co. KG, Hamm, Germany, with financial support from ratiopharm GmbH, Ulm, Germany.
Additional information
Funding
References
- Bijker JB, van Klei WA, Kappen TH, et al. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107(2):213–220.
- Wickham AJ, Highton DT, Clark S, et al. Treatment threshold for intra-operative hypotension in clinical practice-a prospective cohort study in older patients in the UK. Anaesthesia. 2022;77(2):153–163.
- Wesselink EM, Kappen TH, Torn HM, et al. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121(4):706–721.
- Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563–574.
- Wijnberge M, Schenk J, Bulle E, et al. Association of intraoperative hypotension with postoperative morbidity and mortality: systematic review and meta-analysis. BJS Open. 2021;5(1):zraa018.
- Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction Among High-Risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–1357.
- Bein B, Christ T, Eberhart LH, et al. Cafedrine/Theodrenaline (20:1) Is an established alternative for the management of arterial hypotension in Germany-a review based on a systematic literature search [review. Front Pharmacol. 2017 Feb 21;8(68):68.
- Sintetica GmbH. Fachinformation/SMPC Sinora. 2020.
- ratiopharm GmbH. Fachinformation/SMPC Akrinor. 2019.
- Onwochei DN, Ngan Kee WD, Fung L, et al. Norepinephrine intermittent intravenous boluses to prevent hypotension During spinal anesthesia for cesarean delivery: a sequential allocation Dose-Finding study. Anesth Analg. 2017;125(1):212–218.
- Vallée F, Passouant O, Le Gall A, et al. Norepinephrine reduces arterial compliance less than phenylephrine when treating general anesthesia-induced arterial hypotension. Acta Anaesthesiol Scand. 2017;61(6):590–600.
- Mohta M, Dubey M, Malhotra RK, et al. Comparison of the potency of phenylephrine and norepinephrine bolus doses used to treat post-spinal hypotension during elective caesarean section. Int J Obstet Anesth. 2019;38:25–31.
- Wang X, Mao M, Zhang SS, et al. Bolus norepinephrine and phenylephrine for maternal hypotension during elective cesarean section with spinal anesthesia: a randomized, double-blinded study. Chin Med J (Engl). 2020;133(5):509–516.
- Weitzel M, Hammels P, Schorer C, et al. Hämodynamisches wirkungsspektrum von cafedrin/theodrenalin bei anästhesieassoziierter hypotension [hemodynamic effects of cafedrine/theodrenaline on anesthesia-induced hypotension]. Anaesthesist. 2018;67(10):766–772. German.
- Weyland J, Weyland A, Günther U. Kinetik der hämodynamischen differentialeffekte von cafedrin/theodrenalin (akrinor®) bei anästhesie-assoziierter hypotension. S141 [abstract]. Anästh intensivmed. 2021;62:s116–S166. German.
- Kanter J, DeBlieux P. Pressors and inotropes. Emerg Med Clin North Am. 2014;32(4):823–834.
- Kee VR. Hemodynamic pharmacology of intravenous vasopressors. Crit Care Nurse. 2003;23(4):79–82.
- Eberhart LH, Bein B. Intraoperative hypotonie: therapie. Anasthesiol Intensivmed Notfallmed Schmerzther. 2017;52(1):45–54. German.
- Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101(3):622–628.
- Taffé P, Sicard N, Pittet V, et al. The occurrence of intra-operative hypotension varies between hospitals: observational analysis of more than 147,000 anaesthesia. Acta Anaesthesiol Scand. 2009;53(8):995–1005. Sep
- Koch T, Wenzel V. Alte medikamente und neue zulassungsverfahren – akrinor® bleibt verkehrsfähig und ein nachzulassungsantrag für arginin vasopressin ist gestellt. Anaesthesist. 2006;6:708–710. German.
- Eberhart L, Geldner G, Kowark A, et al. Treatment of intraoperative hypotension with cafedrine/theodrenaline versus ephedrine: a prospective, national, multicenter, non-interventional study-the HYPOTENS trial. Anaesthesist. 2021;70(4):298–307.
- Maas JJ, Pinsky MR, de Wilde RB, et al. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013;41(1):143–150.
- Haas A, Schürholz T, Reuter D. Perioperative medikamentöse kreislaufunterstützung in der täglichen routine [perioperative pharmacological circulatory support in daily clinical routine]. Anaesthesist. 2020;69(11):781–792. German.
- Poterman M, Vos JJ, Vereecke HEM, et al. Differential effects of phenylephrine and norepinephrine on peripheral tissue oxygenation during general anaesthesia: a randomised controlled trial. Eur J Anaesthesiol. 2015;32(8):571–580.
- Dings C, Lehr T, Koch T, et al. Entwicklung eines pharmakometrischen modells für cafedrin/theodrenalin (akrinor) und analyse von einflussfaktoren auf die wirkung [abstract]. Anästh Intensivmed. 2021;62:S239–S261. German.
