Abstract
Objective
Aripiprazole 2-month ready-to-use 960 mg (Ari 2MRTU 960) is a new long-acting injectable antipsychotic formulation for administration every 2 months. A 32-week trial evaluated the safety, tolerability, and pharmacokinetics of Ari 2MRTU 960 in clinically stable adults with schizophrenia or bipolar I disorder (BP-I) (per DSM-5 criteria). This secondary analysis evaluated the safety and efficacy of Ari 2MRTU 960 in the subpopulation of patients with BP-I.
Methods
Patients with BP-I were randomized to receive Ari 2MRTU 960 (n = 40) every 56 ± 2 days (4 injections scheduled) or aripiprazole once-monthly 400 mg (AOM 400; n = 41) every 28 ± 2 days (8 injections scheduled). Data were collected during August 2019–July 2020 across 16 US sites. Primary safety endpoints included reported adverse events (coded by the Medical Dictionary for Regulatory Activities preferred term), injection site reactions (assessments included a Visual Analog Scale [VAS] to evaluate patient-reported injection-site pain), and motoric symptoms. Secondary endpoints for efficacy included change from baseline at Week 32 in the Young Mania Rating Scale (YMRS), Montgomery–Åsberg Depression Rating Scale (MADRS), Clinical Global Impression – Bipolar Version (CGI-BP), and Subjective Well-being under Neuroleptic Treatment – Short Form (SWN-S) scores, and Clinical Global Impression – Improvement (CGI-I) at Week 32.
Results
The incidence of treatment-emergent adverse events (TEAEs) was similar between Ari 2MRTU 960 (82.5% [33/40]) and AOM 400 (87.8% [36/41]; p = .5468). The most frequently reported TEAE was increased weight (Ari 2MRTU 960: 25.0% [10/40]; AOM 400: 26.8% [11/41]; p = 1). Injection-site pain was experienced by more patients in the Ari 2MRTU 960 group (25% [10/40]) versus the AOM 400 group (7.3% [3/41]; p = .0622). Mean (standard deviation [SD]) VAS scores for patient-reported injection-site pain following the last injection were 1.2 (2.07) for Ari 2MRTU 960 group and 1.3 (2.19) for AOM 400 (p = .9479) (VAS scale range 0–100 [no pain–extreme pain]). No notable improvement or decline from baseline was observed in motoric symptoms in either treatment group. Patients in both treatment groups remained clinically stable for the entire 32-week trial duration, with minimal difference between treatment groups in the least squares (LS) mean change from baseline at Week 32 in the YMRS Total (p = .8995), MADRS Total (p = .3185), and CGI-BP scores (p = .8485), and in mean CGI-I score (p = .7960). LS mean change from baseline in SWN-S score was greater for Ari 2MRTU 960 than for AOM 400 at Week 32 (p = .0169).
Conclusions
Ari 2MRTU 960 was well tolerated in patients with BP-I, with efficacy similar to AOM 400.
Trial registration
ClinicalTrials.gov identifier: NCT04030143.
Introduction
Bipolar I disorder (BP-I) is a recurrent, life-long, episodic illness characterized by severe alterations in mood and behavior, manifesting through periods of mania, hypomania, and/or depressionCitation1–3. Antipsychotic treatment with or without mood stabilizing agents is recommended as a maintenance treatment for BP-ICitation4 to achieve symptom control and reduce recurrence rates in BP-ICitation3–5. However, adherence to oral medication is often poor in this patient population, with reported nonadherence rates of ∼50%Citation5.
Real-world evidence suggests that, compared with oral antipsychotics, long-acting injectable (LAI) formulations of antipsychotics are associated with a higher number of patients with BP-I achieving treatment adherence, a lower risk of treatment discontinuationCitation6, and a lower risk of psychiatric and all-cause hospitalizationCitation7. LAI formulations may be superior to oral antipsychotics in preventing relapse in patients with rapid-cycling bipolar disorderCitation8.
Aripiprazole once-monthly 400 mg (AOM 400) is an extended-release LAI formulation of aripiprazole monohydrate for administration every 28 days via intramuscular injectionCitation9,Citation10. In the US, AOM 400 has been approved by the Food and Drug Administration for the treatment of schizophrenia in adults and the maintenance monotherapy treatment of BP-I in adultsCitation9. AOM 400 has been found to be an effective and well-tolerated long-term maintenance treatment in patients with BP-ICitation11,Citation12. In Europe, AOM 400 has been approved for the maintenance treatment of schizophrenia in adult patients stabilized with oral aripiprazoleCitation10.
Aripiprazole 2-month ready-to-use 960 mg (Ari 2MRTU 960) is a new LAI formulation containing 960 mg of aripiprazole monohydrate, which will be provided in a pre-filled syringe for gluteal administration once every 2 months. Ari 2MRTU 960 is currently in development for the treatment of schizophrenia and BP-I. Offering LAI formulations with a variety of dosing intervals may increase the range of treatment options available for patients with BP-ICitation13–16.
The safety, tolerability, and pharmacokinetics of multiple doses of Ari 2MRTU 960 was compared with AOM 400 in clinically stable adult patients with schizophrenia or BP-I in a randomized, multiple-dose, parallel-arm, pivotal trial. The outcomes of this trial in the full patient population have been published elsewhereCitation17. Data are reported here for the safety, tolerability, and efficacy of multiple doses of Ari 2MRTU 960 versus AOM 400 for the subpopulation of patients with BP-I, to help inform clinical decisions concerning the treatment of adult patients with BP-I. Data for the subpopulation of patients with schizophrenia are reported elsewhereCitation18.
Methods
Study design and the subpopulation of patients with BP-I
This was an open-label, multiple-dose, randomized, parallel-arm, multicenter trialCitation17. The trial population comprised patients with schizophrenia or BP-I, who were enrolled across 16 sites in the United States (ClinicalTrials.gov identifier: NCT04030143)Citation17. The trial started on 1 August 2019 and was completed on 8 July 2020Citation17. The study was conducted in accordance with the International Council for Harmonization Good Clinical Practice guidelines and local regulatory requirements. The study protocol was approved by the governing institutional review board or independent ethics committee for each investigational site. Participating sites were within the United States and utilized the central IRB Advarra. Advarra IRB approved each site by issuing a dated approval letter. Each site was approved by Advarra IRB prior to the site initiation visit and prior to screening potential participants. All patients provided written informed consent prior to the start of the study.
Key inclusion criteria for the subpopulation of patients with BP-I were: age 18–64 years; current diagnosis of BP-I (as defined by Diagnostic and Statistical Manual of Mental Disorders, 5th edition [DSM-5] criteria)Citation19; body mass index of 18–35 kg/m2; good physical health; clinical stability on an atypical antipsychotic medication (except for clozapine, which was not allowed) for ≥2 months prior to screening (continued concomitant mood stabilizer and antidepressant treatment was allowed); and prior history of tolerating oral aripiprazole and/or AOM 400 (based on the investigator’s judgment). Patients with no history of tolerating aripiprazole received 10 mg oral aripiprazole on 3 consecutive days (30 mg in total, in addition to their current oral antipsychotic) during the screening period to establish tolerability.
Key exclusion criteria for the subpopulation of patients with BP-I were: substance use disorder (as defined by DSM-5 criteria)Citation19 within the past 180 days, or a positive test for drugs of abuse (excluding nicotine, alcohol, and marijuana if sufficient clinical rationale was provided, which must have included rationale/indication for use, review of patterns and frequency of use, and justification to support compliance with the protocol for the intended duration of treatment; prior to attaining clearance to proceed with the enrolment of such a patient, the investigator must have provided sufficient written rationale and discussed these with the medical monitor); use of any cytochrome P450 (CYP)2D6 and CYP3A4 inhibitors or CYP3A4 inducers within 14 days (i.e. fluoxetine, if administered on its own, or in combination with olanzapine within 28 days) prior to dosing, for the duration of the trial, and 30 days after the last study drug dose; current DSM-5 diagnosis other than BP-ICitation19; a significant risk of committing suicide (based on history, routine psychiatric status examination, investigator’s judgment, or a “yes” answer to questions 4 or 5 on the Columbia Suicide Severity Rating Scale [C-SSRS] questionnaire [active suicidal ideation with some intent to act, without specific plan, or with specific plan and intent, currently or over the last 6 months])Citation20; treatment resistance to an atypical antipsychotic medication; and history of neuroleptic malignant syndrome or clinically significant tardive dyskinesia (based on assessment by the investigator).
The study design is shown in Supplementary Figure 1; a full description of the study design has been published elsewhereCitation17.
Study interventions
Study interventions were delivered as a single injection in the gluteal muscle, administered every 56 ± 2 days for Ari 2MRTU 960 (4 injections scheduled in total), or every 28 ± 2 days for AOM 400 (8 injections scheduled in total) over the course of 32 weeks. A one-time dose decrease to 660 mg for Ari 2MRTU 960 and to 300 mg for AOM 400 was allowed, along with a one-time subsequent increase back to 960 mg for Ari 2MRTU 960 and to 400 mg for AOM 400, in case of possible safety and tolerability issues. All study treatments were administered by the investigators at the clinical trial sites. All investigators and trial site clinical personnel underwent initial and ongoing training specific to the trial.
For patients stabilized on oral antipsychotic treatment, overlapping oral antipsychotic treatment was administered for 7 days after the first administration of Ari 2MRTU 960 or for 14 days after the first administration of AOM 400. Overlapping oral antipsychotic treatment was administered for a shorter period for patients randomized to Ari 2MRTU 960, taking into account the higher dose of aripiprazole monohydrate received by injection on Day 1. For patients stabilized on a non-aripiprazole oral antipsychotic, they either continued to receive their current oral antipsychotic for the period of overlapping oral antipsychotic treatment, or they switched to 10–20 mg oral aripiprazole per day, depending on which pharmacokinetic sampling schedule they were assigned to (sparse or robust). There was no oral overlap for participants stabilized on AOM 400. For additional detail on the study design and interventions, see Supplementary Figure 1.
Endpoints
Safety endpoints were assessed as a primary objective of the study. They were: reported adverse events (AEs; coded by system organ class and the Medical Dictionary for Regulatory Activities (MedDRA) preferred term); investigator’s assessment of the most recent injection site for symptoms of pain, swelling, redness, and induration; Visual Analog Scale (VAS)Citation21 scores for the patient-reported rating of pain at the most recent injection site (scale range 0–100 [no pain to extreme pain]); motoric (extrapyramidal) symptoms, assessed by the Simpson–Angus Scale (SAS)Citation22, Abnormal Involuntary Movement Scale (AIMS)Citation23, and Barnes Akathisia Rating Scale (BARS)Citation24; vital signs, electrocardiograms (ECGs), clinical laboratory monitoring (serum chemistry, hematology, and urinalysis), and physical examinations; and suicidality (assessed by the C-SSRS)Citation20.
Efficacy was evaluated as a secondary objective of the study, to establish whether patients remained clinically stable throughout the study. Scales used for patients with BP-I included the Young Mania Rating Scale (YMRS)Citation25, Montgomery–Åsberg Depression Rating Scale (MADRS)Citation26 and Clinical Global Impression – Bipolar Version (CGI-BP)Citation27. Patients were also evaluated using the Clinical Global Impression – Improvement (CGI-I)Citation23 and Subjective Well-being under Neuroleptic Treatment – Short Form (SWN-S)Citation28. Change from baseline at Week 32 was evaluated for all scales, except for the CGI-I; CGI-I score was evaluated at Week 32. For more detail on the scales used to evaluate study treatment efficacy, see Supplementary Table 1. A schedule of all assessments is shown in Supplementary Table 2.
Statistical analysis
This was an ad hoc exploratory analysis to evaluate the safety, tolerability, and efficacy of multiple administrations of Ari 2MRTU 960 versus AOM 400 in the subpopulation of patients with BP-I from an open-label, multiple-dose, randomized trialCitation17.
All randomized patients with BP-I who received at least one dose of the study drug, regardless of any protocol violation, were included in the safety analysis. The statistical significance of between-group differences in the incidence of adverse events was calculated using Fisher’s exact test. The last observation carried forward (LOCF) method was used to impute missing data for the motoric assessment scales at postbaseline visits.
All randomized patients with BP-I who received at least one dose of the study drug and had at least one efficacy assessment were included in the efficacy analysis. As the study enrolled clinically stable patients, minimal change was expected from baseline in efficacy outcomes, and the study was not designed to show statistical significance of any such change. Change from baseline in YMRS, MADRS, CGI-BP and SWN-S score was evaluated using a mixed model for repeated measures (MMRM), including categorically fixed effects of treatment, trial week, and treatment-by-trial week interaction, pharmacokinetic sampling schedule (sparse or robust) for determining the concentration of aripiprazole in patients’ plasma, as well as the covariates of baseline-score-by-week interaction in addition to patient as random effect.
Results
Patients
Following the screening of 394 patients, 266 were enrolled into the study, including 81 patients with BP-I. Patient disposition is shown in . Data for the subpopulation of patients with schizophrenia (n = 185) are reported elsewhereCitation18. Patients with BP-I were randomized to receive Ari 2MRTU 960 (n = 40) or AOM 400 (n = 41).
Figure 1. Patient disposition. aIncludes patients with schizophrenia or BP-I. Data for the subpopulation of patients with schizophrenia will be reported elsewhereCitation18. bCompleted visit on Day 225. Abbreviations. AE, adverse event; AOM 400, aripiprazole once-monthly 400 mg; Ari 2MRTU 960, aripiprazole 2-month ready-to-use 960 mg; BP-I, bipolar I disorder.
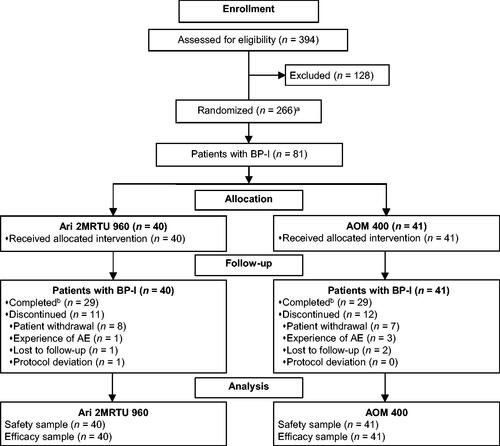
Study completion rate among patients with BP-I was comparable between groups: 72.5% (29/40 patients) for Ari 2MRTU 960 and 70.7% (29/41 patients) for AOM 400 (p = 1). Patient withdrawal was the most common reason for study discontinuation in both treatment groups, observed in 20.0% (8/40) of patients in the Ari 2MRTU 960 group and in 17.1% (7/41) of patients in the AOM 400 group ().
Demographic and baseline disease characteristics were well balanced between groups ().
Table 1. Demographics and baseline disease characteristics in patients with BP-I.
Safety
All 81 randomized patients with BP-I received at least one study drug dose and were included in the safety analyses. Multiple doses of Ari 2MRTU 960 into the gluteal muscle were generally well tolerated; only 2.5% of patients in the Ari 2MRTU 960 group (1/40) and 4.9% of patients in the AOM 400 group (2/41) required a dose adjustment during the trial.
Overall, 85.2% of patients with BP-I (69/81) experienced treatment-emergent AEs (TEAEs; 82.5% [33/40] in the Ari 2MRTU 960 group and 87.8% [36/41] in the AOM 400 group). The incidence of TEAEs and serious TEAEs was similar overall between the two treatment groups (p = .5468 and p = .6156, respectively). Most TEAEs in either treatment group occurred following the first injection, with a lower incidence of TEAEs with subsequent injections of Ari 2MRTU 960 or AOM 400. Most TEAEs were mild or moderate in severity. A summary of TEAEs is presented in .
Table 2. Summary of TEAEs in patients with BP-I.
Serious TEAEs (i.e. TEAEs resulting in hospitalization, prolonged hospitalization, life endangerment, persistent or significant disability, or death)Citation30 occurred in 1 patient in the Ari 2MRTU 960 group (auditory hallucination) and 3 patients in the AOM 400 group (akathisia, depression, and depressive symptoms, respectively). No serious TEAE occurred in more than one patient, and no trends were observed with respect to the timing of serious TEAE onset and the number of study drug injections received.
The most frequently reported TEAEs were increased weight, injection site pain, akathisia, and anxiety (). Increased weight, akathisia, and anxiety had a comparable incidence rate between treatment groups. None of the cases of increased weight, injection site pain, or anxiety were considered severe or serious by the investigator. At the end of the study, potentially clinically significant weight gain of ≥7% was reported in 41.4% of patients in each treatment group (12 of the 29 patients in each treatment group for whom post-baseline weight assessment data were available).
Injection-site pain was experienced by slightly more patients in the Ari 2MRTU 960 group (25.0% [10/40]) than in the AOM 400 group (7.3% [3/41]; p = .0622). All events of injection site pain appeared within 2 days of the injection, with most events in both treatment groups occurring after the first injection. No symptoms of swelling or induration were observed by the investigators after any injection. In ≥90% of patients, the investigator’s assessment of the most recent injection site (reported using a 4-point categorical scale [absent, mild, moderate, severe]) was rated as “absent” for symptoms of pain and redness after the first and last injections in either treatment group; any pain or redness experienced was rated as “mild” or “moderate” for either treatment.
Following the first and last injection, mean (standard deviation [SD]) and median VAS scores for pain were low and similar between the groups (VAS range: 0 to 100, where 0 represents no pain and 100 the worst possible pain). Mean (SD) VAS scores were 3.2 (6.75) following the first injection and 1.2 (2.07) following the last injection in the Ari 2MRTU 960 group, and 3.2 (5.04) following the first injection and 1.3 (2.19) following the last injection in the AOM 400 group. Median VAS scores were 1 in both treatment groups following the last injection. There was no significant difference between the two treatment groups in mean VAS score after the first injection (p = .9698) or after the last injection (p = .9479).
Motoric TEAEs were reported in 25.0% of patients (10/40) in the Ari 2MRTU 960 group and in 17.1% of patients (7/41) in the AOM 400 group. The most frequently observed motoric adverse event was akathisia, reported in 5 patients in each treatment group (). In total, 7.5% of patients (3/40) in the Ari 2MRTU 960 group and 7.3% of patients (3/41) in the AOM 400 group received anticholinergic treatments for motoric TEAEs. No notable improvement or decline from baseline was observed in motoric rating scale scores in either treatment group (Supplementary Table 3).
No notable differences between groups were observed in laboratory test results, vital signs, or ECG parameters.
Suicidality, suicidal ideation, and emergence of suicidal ideation were reported in 1 patient in the Ari 2MRTU 960 group and in 2 patients in the AOM 400 group.
Efficacy
The study population evaluated in this secondary analysis consisted of patients with BP-I who were clinically stable at baseline. Efficacy assessments at Week 32 showed that, on average, patients in both treatment arms remained clinically stable throughout the treatment period of this open-label study. Minimal change from baseline, and the minimal difference between treatment groups, was observed in the YMRS Total, MADRS Total, and CGI-BP scores ( and ). Greater improvement in the SWN-S score was observed in the Ari 2MRTU 960 group at Week 32 compared with the AOM 400 treatment group (p = .0169; and ). At Week 32, the mean (SD) CGI-I score was similar in the two groups, with a score of 3.1 (1.2) reported in the Ari 2MRTU 960 group and 3.2 (1.5) in the AOM 400 group (p = .7960; ).
Figure 2. Efficacy outcomes at Week 32. Data shown are from the efficacy analysis sample (MMRM analysis). Mean (SD) YMRS Total score at baseline was 6.6 (7.4) in the Ari 2MRTU 960 group and 9.4 (8.3) in the AOM 400 group. Mean (SD) MADRS Total score at baseline was 11.0 (9.5) in the Ari 2MRTU 960 group and 13.0 (9.3) in the AOM 400 group. Mean (SD) CGI-BP score at baseline was 2.3 (1.2) in the Ari 2MRTU 960 group and 2.8 (1.2) in the AOM 400 group. Mean (SD) SWN-S score at baseline was 91.9 (17.3) in the Ari 2MRTU 960 group and 89.2 (18.5) in the AOM 400 group. ap-values for between-group comparison were derived from an MMRM analysis with fixed effects of treatment, pharmacokinetic sampling schedule for determining the concentration of aripiprazole in patients’ plasma, week, treatment-by-week interaction, and baseline-by-week interaction as covariate. Abbreviations. AOM 400, aripiprazole once-monthly 400 mg; Ari 2MRTU 960, aripiprazole 2-month ready-to-use 960 mg; CGI-BP, Clinical Global Impression – Bipolar Version; LS, least squares; MADRS, Montgomery–Åsberg Depression Rating Scale; MMRM, mixed model for repeated measures; SD, standard deviation; SE, standard error; SWN-S, Subjective Well-being under Neuroleptic Treatment – Short Form; YMRS, Young Mania Rating Scale.
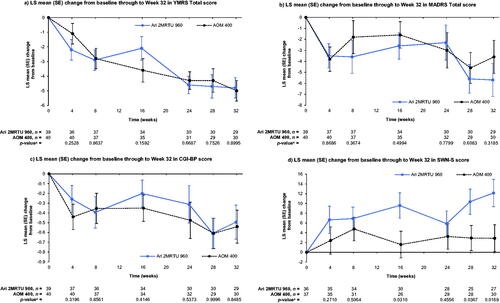
Table 3. Efficacy outcomes at Week 32 in patients with BP-I.
Discussion
In this study, Ari 2MRTU 960 was well tolerated by patients with BP-I, with a rate of discontinuation due to TEAEs lower than those observed for AOM 400 in previous trials (8.7–17.4%)Citation11,Citation12. The study completion rate was similar in both groups (72.5% [29/40]) in the Ari 2MRTU 960 group and 70.7% [29/41] in the AOM 400 group) and higher than that observed in previous studies evaluating the use of AOM 400 in patients with BP-I (48.1–63.0%)Citation11,Citation12. The overall incidence rates of TEAEs and serious TEAEs were comparable with those reported for patients with schizophrenia treated with Ari 2MRTU 960Citation17,Citation18, as well as with those observed in patients with BP-I in the AOM 400 treatment group and in previous trials evaluating AOM 400Citation11,Citation12. No notable change from baseline was observed in motoric rating scale scores in either treatment group, an encouraging finding given the previously observed increased vulnerability to motoric adverse events in patients with mood disorders receiving long-term treatment with antipsychoticsCitation31.
Minimal change from baseline was observed at the end of the study in most of the efficacy endpoints assessing BP-I symptoms, which was the expected outcome given that the study population consisted of clinically stable patients with BP-I. A greater change from baseline in SWN-S score was observed at Week 32 for Ari 2MRTU 960 than for AOM 400. SWN-S was included among the efficacy endpoints to provide an outcome measure assessing patients’ health-related quality of life and treatment satisfactionCitation28,Citation32,Citation33, as patients’ satisfaction with various aspects of treatment has been found to be a determinant of treatment non-adherence in bipolar disorderCitation34. SWN-S scores of ∼90 observed in either treatment group at baseline indicate that patients with BP-I included in this secondary analysis had a positive attitude towards their prior treatmentCitation33.
Adherence to treatment in patients with BP-I is crucial for the maintenance of their long-term clinical and mood stability, which are, in turn, key for achieving the ultimate goal of BP-I management: optimum (or pre-morbid) levels of functioningCitation35. LAI antipsychotic formulations are considered a well-tolerated maintenance treatment for patients with bipolar disordersCitation36, and are recommended by clinical guidelines for the management of bipolar disorder as a therapeutic tool for improving treatment adherenceCitation4. They also help improve treatment convenience for patients who prefer to receive an injection every few weeks instead of having to take oral medication dailyCitation37. Overall, the results of the present secondary analysis suggest that Ari 2MRTU 960 and AOM 400 are comparable in their safety, tolerability, and efficacy in the treatment of clinically stable patients with BP-I. Ari 2MRTU 960 may be more convenient for patients who would prefer a longer dosing interval and may reduce any discomfort associated with injection for patients scared of needles or injection site painCitation37.
The symptomatic stability observed throughout the study in the Ari 2MRTU 960 and AOM 400 treatment groups is paralleled by the similar pharmacokinetic profiles reported for the treatments in the overall population of patients with schizophrenia or BP-ICitation17. Throughout the study period, mean aripiprazole plasma exposure was comparable between the Ari 2MRTU 960 and the AOM 400 treatment groupsCitation17, and mean aripiprazole plasma concentrations remained within the therapeutic reference range for aripiprazole (100–350 ng/mL)Citation38.
Study limitations and strengths
It is important to emphasize that this study was open-label in design, which is a limitation for the assessment of treatment efficacy due to possible expectation biasCitation39, and that its predominant focus was on the comparison of the safety, tolerability, and pharmacokinetic profiles of Ari 2MRTU 960 and AOM 400; it was not designed to show statistical significance in change from baseline in the efficacy outcomes. As such, the study sample size, whilst large for the evaluation of the pharmacokinetic profile in the full trial population including patients with schizophrenia or BP-ICitation17, was small for the present evaluation of safety, tolerability, and efficacy in a subpopulation of patients with BP-I. Moreover, it was conducted in the US only. Study strengths include the detailed assessment of the safety and tolerability of Ari 2MRTU 960.
Conclusion
Multiple doses of Ari 2MRTU 960 were well tolerated in patients with BP-I, with a safety profile consistent with that previously demonstrated for AOM 400 in this patient population. The efficacy of Ari 2MRTU 960 and AOM 400 in maintaining symptomatic stability in clinically stable patients with BP-I was similar.
The extended dosing interval of Ari 2MRTU 960 compared with that of AOM 400 has the potential to reduce antipsychotic treatment burden on patients with BP-I as well as clinicians. Ari 2MRTU 960 will be the only LAI with a 2-month dosing interval approved for the treatment of BP-I and, thus, will provide clinicians with a new therapeutic option for BP-I.
Transparency
Author contributions
Matthew Harlin and Frank Larsen participated in trial conception and design. All authors were involved in data collection and/or analysis. All authors participated in the drafting or the critical review of the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgements
Writing support was provided by Babette Jamieson, MSc, assisted by her colleagues at Cambridge – a Prime Global Agency (Knutsford, UK), and funded by Otsuka Pharmaceutical Development & Commercialization Inc. and H. Lundbeck A/S.
The authors would like to thank Calvin Liu for his programming support in data analysis, and Suzanne Watkin for her support in clinical management.
This is a secondary analysis of study drug safety and efficacy outcomes from study NCT04030143, in a subpopulation of patients with bipolar I disorder; results for the full study population, comprising patients with schizophrenia or bipolar I disorder, have been published in Harlin et al. CNS Drugs 2023. Data for the subpopulation of patients with schizophrenia will be reported elsewhere. Some of the data in this manuscript have previously been reported in a poster presented at the NEI Congress 2022, Colorado Springs, CO, USA, 3–6 November 2022; Poster 113.
McIntyre_et_al._Supplementary_material_5-May-23.pdf
Download PDF (361.9 KB)Declaration of funding
This work was sponsored by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, NJ, USA) and H. Lundbeck A/S (Valby, Denmark). The sponsors were involved in the design of the study, the collection, analysis and interpretation of data, the writing and reviewing of this article, and the decision to submit the article for publication.
Declaration of financial/other relationships
Roger S. McIntyre has received research grant support from the Canadian Institutes of Health Research, Global Alliance for Chronic Diseases, the Milken Institute and the National Natural Science Foundation of China, and speaker/consultation fees from AbbVie, Alkermes, Atai Life Sciences, Axsome Therapeutics, Bausch Health, Biogen, Boehringer Ingelheim, Eisai, Intra-Cellular Therapies, Janssen, Kris Pharma, Lundbeck, Mitsubishi Tanabe Pharma, Neumora Therapeutics, Neurocrine Biosciences, NewBridge Pharmaceuticals, Novo Nordisk, Otsuka Pharmaceutical, Pfizer, Purdue Pharma, Sage Therapeutics, Sanofi, Sunovion, Takeda Pharmaceutical Company, Viatris. Roger S. McIntyre is the CEO of Braxia Scientific Corp. Frank Larsen, Pedro Such, and Murat Yildirim are full-time employees of H. Lundbeck A/S. Jessica Madera-McDonough, Zhen Zhang, and Matthew Harlin are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
D ata availability statement
To submit inquiries related to Otsuka clinical research, or to request access to individual participant data (IPD) associated with any Otsuka clinical trial, please visit https://clinical-trials.otsuka.com/. For all approved IPD access requests, Otsuka will share anonymized IPD on a remotely accessible data sharing platform.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. text rev. Washington (DC): American Psychiatric Association; 2022.
- McIntyre RS, Berk M, Brietzke E, et al. Bipolar disorders. Lancet. 2020;396(10265):1841–1856.
- Vázquez GH, Holtzman JN, Lolich M, et al. Recurrence rates in bipolar disorder: systematic comparison of long-term prospective, naturalistic studies versus randomized controlled trials. Eur Neuropsychopharmacol. 2015;25(10):1501–1512.
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian network for mood and anxiety treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170.
- Jawad I, Watson S, Haddad PM, et al. Medication nonadherence in bipolar disorder: a narrative review. Ther Adv Psychopharmacol. 2018;8(12):349–363.
- Yan T, Greene M, Chang E, et al. Medication adherence and discontinuation of aripiprazole once-monthly 400 mg (AOM 400) versus oral antipsychotics in patients with schizophrenia or bipolar I disorder: a real-world study using US claims data. Adv Ther. 2018;35(10):1612–1625.
- Lähteenvuo M, Tanskanen A, Taipale H, et al. Real-world effectiveness of pharmacologic treatments for the prevention of rehospitalization in a Finnish nationwide cohort of patients with bipolar disorder. JAMA Psychiatry. 2018;75(4):347–355.
- Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analyses of randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(9):pyw038.
- Abilify Maintena® (aripiprazole). Prescribing Information. Rockville (MD): Otsuka America Pharmaceutical, Inc.; 2020.
- Abilify Maintena® (aripiprazole). Summary of product characteristics. Amsterdam (Netherlands): Otsuka Pharmaceutical Netherlands B.V.; 2021.
- Calabrese JR, Jin N, Johnson B, et al. Aripiprazole once-monthly as maintenance treatment for bipolar I disorder: a 52-week, multicenter, open-label study. Int J Bipolar Disord. 2018;6(1):14.
- Calabrese JR, Sanchez R, Jin N, et al. Efficacy and safety of aripiprazole once-monthly in the maintenance treatment of bipolar I disorder: a double-blind, placebo-controlled, 52-week randomized withdrawal study. J Clin Psychiatry. 2017;78(3):324–331.
- Citrome L. Long-acting injectable antipsychotics update: lengthening the dosing interval and expanding the diagnostic indications. Expert Rev Neurother. 2017;17(10):1029–1043.
- Citrome L. Long-acting injectable antipsychotics: what, when, and how. CNS Spectr. 2021;26(2):118–129.
- Jawad MY, McIntyre RS. Barriers to managing bipolar I disorder with long-acting injectable antipsychotics. Eur Neuropsychopharmacol. 2022;64:4–6.
- Tohen M, Goldberg JF, Hassoun Y, et al. Identifying profiles of patients with bipolar I disorder who would benefit from maintenance therapy with a long-acting injectable antipsychotic. J Clin Psychiatry. 2020;81(4):OT19046AH1.
- Harlin M, Yildirim M, Such P, et al. A randomized, open-label, multiple-dose, parallel-arm, pivotal study to evaluate the safety, tolerability, and pharmacokinetics of aripiprazole 2-month long-acting injectable in adults with schizophrenia or bipolar I disorder. CNS Drugs. 2023;37(4):337–350.
- Citrome L, Such P, Yildirim M, et al. Safety and efficacy of aripiprazole 2-month ready-to-use 960 mg: secondary analysis of outcomes in adult patients with schizophrenia in a randomized, open-label, parallel-arm, pivotal study. 2023. 23 p.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277.
- Delgado DA, Lambert BS, Boutris N, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2(3):e088.
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19.
- Guy W. ECDEU assessment manual for psychopharmacology. Rockville (MD): National Institute of Mental Health; 1976.
- Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672–676.
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–435.
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389.
- Spearing MK, Post RM, Leverich GS, et al. Modification of the clinical global impressions (CGI) scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159–171.
- Naber D, Moritz S, Lambert M, et al. Improvement of schizophrenic patients’ subjective well-being under atypical antipsychotic drugs. Schizophr Res. 2001;50(1–2):79–88.
- Altman DG. Comparability of randomised groups. Statistician. 1985;34(1):125–136.
- Food & Drug Administration. What is a serious adverse event? 2016. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event
- Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51–S66.
- Boyer L, Baumstarck K, Boucekine M, et al. Measuring quality of life in patients with schizophrenia: an overview. Expert Rev Pharmacoecon Outcomes Res. 2013;13(3):343–349.
- Mauriño J, Cordero L, Ballesteros J. The subjective well-being under neuroleptic scale – short version (SWN-K) and the SF-36 health survey as quality of life measures in patients with schizophrenia. Patient Prefer Adherence. 2012;6:83–85.
- Chakrabarti S. Treatment-adherence in bipolar disorder: a patient-centred approach. World J Psychiatry. 2016;6(4):399–409.
- Malhi GS, McAulay C, Das P, et al. Maintaining mood stability in bipolar disorder: a clinical perspective on pharmacotherapy. Evid Based Ment Health. 2015;18(1):1–6.
- Pacchiarotti I, Tiihonen J, Kotzalidis GD, et al. Long-acting injectable antipsychotics (LAIs) for maintenance treatment of bipolar and schizoaffective disorders: a systematic review. Eur Neuropsychopharmacol. 2019;29(4):457–470.
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24.
- Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–2):9–62.
- Manja V, Lakshminrusimha S. Epidemiology and clinical research design, part 1: study types. NeoReviews. 2014;15(12):e558–e569.
