Abstract
Objective
Cytomegalovirus (CMV) can infect individuals at any age, including infants, who may contract it from infected mothers (congenital CMV [cCMV]). Whereas CMV infection is typically asymptomatic or causes mild illness in healthy individuals, infection can result in severe outcomes in immunocompromised individuals and in infants with cCMV. This systematic review aims to characterize the economic impact of CMV and cCMV infections.
Methods
Medline, Embase, and LILACS databases were searched for publications reporting the economic impact of cCMV and CMV infections across all age groups. Manuscripts published between 2010 and 2020 from Australia, Latin America, Canada, Europe, Israel, Japan, the United States, and global (international, worldwide) studies were included; congress materials were excluded. Outcomes of interest included cCMV- and CMV-attributable direct costs/charges, resource utilization, and indirect/societal costs.
Results
Of 751 records identified, 518 were excluded based on duplication, population, outcome, study design, or country. Overall, 55 articles were eligible for full-text review; 25 were further excluded due to population, outcome, study design, or congress abstract. Two publications were additionally identified, resulting in economic impact data compiled from 32 publications. Of these, 24 publications reported cost studies of cCMV or CMV, including evaluation of direct costs/charges, healthcare resource utilization, and indirect/societal costs, and 7 publications reported economic evaluations of interventions. The populations, methods and outcomes used across these studies varied widely.
Conclusions
CMV and cCMV infections impose a considerable economic impact on different countries, populations, and outcomes. There are substantial evidence gaps where further research is warranted.
PLAIN LANGUAGE SUMMARY
A plain language summary of this manuscript can be found here.
Introduction
Human cytomegalovirus (CMV), a member of the Herpesviridae family of viruses, is a common viral pathogen that can infect individuals of all agesCitation1,Citation2. An estimated 83% of the world’s population has been infected with CMVCitation3. In the United States, approximately 1 in 3 children will be infected with CMV by the age of 5 years, and more than half of adults will be infected by age 40 yearsCitation2.
CMV infection in healthy individuals is typically asymptomatic or results in mild illness; however, infection in immunocompromised individuals is more likely to lead to serious diseaseCitation2,Citation4,Citation5. Congenital CMV (cCMV) infection can result from vertical transmission during pregnancy in women who have had primary or reactivation of latent CMV infection, or have been reinfected while pregnantCitation2. cCMV infection occurs in approximately 0.5% to 1% of live births in the United States and Europe and is a leading viral cause of congenital birth defects worldwide, including neurodevelopmental delay and sensorineural hearing lossCitation5–8. Approximately 10% of infants with cCMV infection will exhibit symptoms at birth, such as rash, jaundice, retinitis, low birth weight, and microcephaly; 40% to 60% of these infants will have long-term complications such as hearing and vision loss, intellectual disability, or seizuresCitation2,Citation9. Among infants born with cCMV infection who are asymptomatic at birth, 10% to 15% will develop permanent sequelae such as sensorineural hearing loss (SNHL) by the time they begin schoolCitation7,Citation9,Citation10. Antiviral medications, such as oral valganciclovir, are approved for the prevention and/or treatment of CMV infection certain populations including newborns with moderate to severe symptomatic cCMV infectionCitation11, solid organ transplant recipientsCitation12, hematopoietic cell transplant recipientsCitation13, and those living with HIVCitation14. Although no approved vaccines are currently available to prevent infection, a CMV vaccine has been designated as a high priority by the Institute of Medicine, given the potential significant public health impactCitation15.
When assessing the value of preventive strategies, it is important to understand the economic impact and financial burden of CMV and cCMV infections on individuals and healthcare systems; however, it is unclear what data are available on the global economic impact of CMV and cCMV infections in different countries, in different populations, and for different economic outcomes. A comprehensive understanding of the economic impact of CMV and cCMV infections can provide guidance to decision makers developing and implementing strategies for prevention, including the use of vaccinations. Herein, we conducted a systematic literature review to describe data from 2010 to 2020 on the global economic impact of CMV and cCMV infections.
Methods
Literature search and information sources
The PICO (+) framework (Population, Intervention, Comparator, and Outcome with (+) indicating additional categories added to describe the research) was used to determine the scope of this review. A literature search on the economic impact of CMV infection was conducted on 24 December 2020, using the Medline, Embase, and the Latin American and Caribbean Health Sciences Literature (LILACS) databases. Searches were limited to the time frame of 2010–2020 for journal articles and 2017–2020 for congress abstracts, and restricted to English language publications from the following countries and regions: Australia, Latin America, Canada, Europe, Israel, Japan, the United States, and global (international, worldwide). A full list of free-text and controlled vocabulary terms utilized to conduct the searches is provided in Supplementary Tables S1–S3.
Study selection and data extraction
Titles of articles and abstracts identified in the literature search were assessed for relevance against predefined eligibility criteria in an initial screening, and then full texts were reviewed to further assess eligibility. Double independent record selection was performed during screenings of titles of articles and abstracts, as well as during full-text reviews. Discrepancies regarding inclusion or exclusion of records were resolved through discussions between reviewers or reconciled by a third reviewer. Following screening, data from the selected publications were abstracted into an evidence matrix.
Data included in the evidence matrix included study identification variables (i.e. reference identifier, author and year, and full reference), study characteristics (i.e. study design, study period, country, data type, and other information), and population characteristics (i.e. general information, sample sizes, subgroup analyses, age distribution, age range, sex, race/ethnicity, social status, educational level, and other information). Extracted economic outcomes of interest were direct costs/charges (i.e. direct costs/charges related to CMV infection and other direct costs); indirect costs/charges (i.e. productivity losses for patients and caregivers, and other); and resource utilization, including drug utilization, hospitalizations (i.e. hospital admission, readmission, discharge, length of stay, frequency of hospitalization, and other), emergency department visits, outpatient/ambulatory visits (i.e. outpatient/ambulatory visits, language/speech therapy, frequency, and other types of outpatient visits), and other resource utilization (i.e. laboratory tests, examinations, equipment related to CMV infection, palliative care or terminal care, and other).
Eligibility criteria
Each record identified during the initial screening and full-text review was assessed for relevance against predefined eligibility criteria for study types, populations, and outcomes. Populations included in this review were the general population at all ages; toddlers and children up to 5 years of age; adolescents and adults 9 through 40 years of age; mothers and babies infected with HIV; and other specific subpopulations or immunocompromised groups. These subpopulations included patients with autoimmune diseases (i.e. rheumatoid arthritis, systemic lupus erythematosus, Crohn’s disease, multiple sclerosis, Graves’ disease, Still’s disease, and polymyalgia rheumatica), cCMV-related inflammatory bowel disease (IBD), cancer, childhood leukemia, glioblastoma multiforme, cCMV-related subacute sclerosing panencephalitis and other neurologic disorders, hearing loss, ventilator-associated pneumonia (for babies with CMV in intensive care units [ICUs]), cardiovascular disease (i.e. atherosclerosis, coronary artery disease, hypertension), mononucleosis-like syndrome, and cCMV-related viral encephalitis. Sub-populations excluded from this study included blood and solid organ donors, transplant recipients, other high-risk and immunosuppressed groups (including people with HIV who were neither pregnant nor babies), and patients with the following diagnoses: diabetes mellitus, hepatitis or renal disorders, peripheral artery disease, systemic sclerosis, primary immunodeficiency such as autosomal recessive hyperimmunoglobulin E syndrome, Parkinson’s disease, Alzheimer’s disease, ulcerative colitis (UC; considered for inclusion only when presented as an irritable bowel syndrome subpopulation) or necrotizing enterocolitis, respiratory disease, dermatologic conditions, or Down syndrome.
Study types included in this review were epidemiologic studies, seroepidemiologic studies, cross-sectional studies, longitudinal studies, surveillance studies, registries, mathematical models, economic studies, claims analyses, systematic reviews, and meta-analyses; clinical trials were excluded. Inclusion criteria for the economic impact outcomes of CMV and cCMV infections were direct costs or charges related to disease, including treatment of related comorbidities, and lifetime cost; indirect/societal costs or charges, including loss of productivity and childcare outcomes; and resource utilization. Following review, congress materials such as abstracts and presentations were excluded to enhance the quality of the data.
Results
Study selection
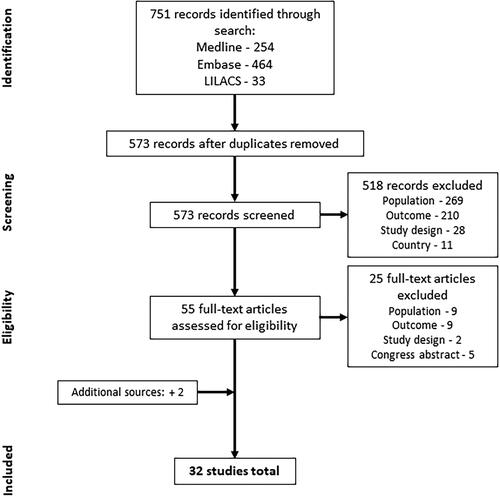
A total of 751 citations were identified through the systematic literature search (). After removing duplicates, 573 references were screened. Of these, 518 references were excluded because they did not meet the inclusion criteria for the study population (n = 269), economic outcome (n = 210), study design (n = 28), or country (n = 11). After the initial screening, 55 citations were eligible for full-text review. Of these, 25 were excluded because they did not satisfy the study population (n = 9), economic outcome (n = 9), or study design (n = 2), or because they were congress abstracts (n = 5). Two additional publications were identified and added following analysis of a systematic literature review published after the predefined search timeframeCitation16,Citation17. A total of 32 publications were included in the data extraction stage and are included in this review.
Cost studies
The characteristics of studies (n = 24) reporting CMV- and cCMV-related direct healthcare costs/charges, resource utilization, and indirect/societal costs to patients, caregivers, and the general population are summarized below and in , and extracted results are shown in Supplementary Table S4.
Table 1. Summary of included studies (n = 24) reporting direct healthcare costs/charges, resource utilization and indirect costs related to CMV or cCMV.
United States
Six studies of cCMV and CMV infections in infants, children, adolescents, and adults in the United States were included ()Citation18–23. Of these, four studies evaluated direct healthcare costs or healthcare charges associated with drug utilization; visits to inpatient, outpatient, and emergency facilities; equipment; laboratory tests; and/or physical and occupational therapy related to cCMV (three studies) or CMV (one study) infectionCitation18–21. These reports consistently found additional/higher healthcare costs associated with cCMV infectionCitation18,Citation19,Citation21 and in immunocompromised patients with UC and CMV infectionCitation20. These results align with the reported increase in the length of hospital stay in five of the six US-based cost studiesCitation18–20,Citation22,Citation23. Four of the studies evaluated length of hospital stay in infants with cCMV infectionCitation18,Citation19,Citation22,Citation23 and one study assessed hospitalization in immunocompromised adults with IBD and CMV infectionCitation20. Overall, the studies suggest that CMV infections in infants and immunocompromised adults are associated with increased length of hospital stay and increased healthcare cost.
Europe
Ten publications reporting cost studies of cCMV or CMV infection among infants, children, adolescents, and adults from eight European countries were identifiedCitation17,Citation24–32. Countries with available data include France (n = 2), the United Kingdom (n = 2), Germany (n = 1), Italy (n = 1), Poland (n = 1), Slovenia (n = 1), Spain (n = 1), and the Netherlands (n = 1).
Direct healthcare costs of cCMV or CMV infection were assessed in three of the studiesCitation17,Citation24,Citation25. The studies from Europe were more diverse in observation periods and scope, reporting data for costs up to six years after birth, direct costs incurred by the government for health, social and educational services and costs for children with cCMV, stratified by long-term impairments and remote damagesCitation17,Citation24,Citation25. Mean total costs incurred due to long-term impairments, such as hearing loss, were more than double in children with cCMV infection than in those without in the NetherlandsCitation24. Direct healthcare costs/charges due to cCMV infection were estimated at 324 million British pound sterling (GBP) in the United Kingdom in 2016Citation25, and 70.5 million EUR per annum for all children affected by cCMV in GermanyCitation17. Notably, direct costs comprised a minor proportion of the total cost in both the United Kingdom (40%)Citation25 and Germany (8%)Citation17.
Resource utilization: hospitalization and other
Seven studies reported length of stay in hospital associated with CMV or cCMV infection in EuropeCitation24,Citation26,Citation27,Citation29–32. Results were diverse for adult patients, with length of hospital stays in Slovenia and France not significantly different between CMV-positive and CMV-negative patients while in Spain, average length of stay in hospital was longer for patients with CMV infectionCitation27,Citation29,Citation30. Although this variation in length of hospital stay is notable, it is important to consider that the populations reported in these studies were heterogenous with respect to the presence of comorbidities and the use of treatment regimens. Length of stay in hospital were similar for infants with and without cCMVCitation24; however, among infants with cCMV infection, length of stay varied between depending on the type of antiviral therapy administered (6 days with oral valganciclovir [VGCG] and 38 days for IV ganciclovir [GC] monotherapy)Citation31. In adult patients with inflammatory bowel disease and CMV colitis, an intravenous regimen of GC was associated with a superior clinical response, including a shorter hospital stay compared with oral VGCVCitation26. Despite the heterogeneity of these populations, these reports suggest an overall trend of increased length of hospital stay in patients with CMV infection.
Indirect costs
In contrast to the absence of data on indirect costs in the United States, two publications reported these costs in EuropeCitation17,Citation25. In Germany, indirect costs contribute 92% of the total costs associated cCMV infection; caregivers to children with cCMV infection experience changes in employment status and careers while costs of education were as high as 44,835 EUR for children with cCMV-related disabilitiesCitation17. By contrast, 60% of cCMV-associated costs in the United Kingdom were estimated to be indirect costs, amounting to 408 million GBP incurred by families and society as a result of productivity losses and costs of SNHL, cerebral palsy, epilepsy, and autism spectrum disordersCitation25. Taken together, these reports suggest that the indirect costs constitute a major proportion of the overall economic burden associated with CMV infections.
Japan
Three studies reported on CMV- and cCMV-related direct healthcare costs/charges, resource utilization, and/or indirect costs in JapanCitation33–35.
Direct and indirect healthcare costs/charges
Two publications providing data on direct healthcare costs/chargesCitation33,Citation34. One of these publications suggests that the economic impact of CMV infection in adult patients with T-cell leukemia in Japan is substantialCitation34. The second study estimated the overall costs of cCMV infection in the 2019 Japanese birth cohort at approximately 27.6 billion Japanese Yen (JPY), of which only 4% represented the direct healthcare costs and those associated with hospitalizationCitation33.
Resource utilization: hospitalization and other
Two publications reported data on cCMV- or CMV-related hospitalizations in JapanCitation34,Citation35. Although 47% of patients with cCMV-related symptoms at birth were admitted to the neonatal ICU (NICU), this proportion was not significantly higher than that for infants without cCMV symptoms at birth (18%)Citation35. This longitudinal study of medical claims also reported that the proportion of neonates, <6 months of age, receiving antiviral treatment in Japan (26%) is similar to that reported for the United States. Similar with publications from the United States and Europe reporting longer hospital stays among patients with comorbid CMV infection, stays in hospital among immunocompromised adults with T-cell leukemia were longer for those with CMV infection compared with other opportunistic infectionsCitation34.
Indirect costs
As observed in the case of the United Kingdom and Germany, indirect costs represented a major proportion of the total cost burden of CMV, approximately 96% of costs incurred due to cCMV infection in Japan in 2019 comprised indirect costs, such as education and productivity losses for both patients and parentsCitation33.
Brazil
Three publications from Brazil reported direct healthcare costs/charges and resource utilization associated with CMV and cCMV infectionCitation36–38. One of these studies reported the number of infants with cCMV infection who received mechanical ventilation (23%) following admission to a neonatal ICUCitation36. Another reported on the use of auditory brainstem response assessments during early childhood (84%) and the observed frequency of SNHLCitation37.
Global
Two systematic literature reviews provided data on resource utilization among adults with CMV infection in multiple countries, one of which reported a mean increase of eight days in length of ICU stay and an increased duration of mechanical ventilation among immunocompetent critically ill patients with CMV reactivation versus patients without CMV reactivation (this review did not report countries of included studies)Citation39. Similarly, a second review of resource utilization across various countries, including the United States, Europe, Egypt, and New Zealand, reported mean increases of 12 days for length of stay in ICU and 9 days for the duration of mechanical ventilation between patients with and without CMV infectionCitation40.
Economic evaluations of interventions
The seven economic evaluation studies that reported on interventions for CMV and cCMV infection are presented in , and a summary of the extracted results is below and in Supplementary Table S5. As described previouslyCitation47, economic evaluations were of two types, cost-effectiveness and cost-benefit analyses that predict the effect of preventive or therapeutic interventions on health outcomes or costs.
Table 2. Summary of included economic evaluations of interventions related to CMV or cCMV (n = 7).
United States
Of the four economic evaluations from the United States, one cost-benefit analysis predicted that net benefits and societal savings would result from early screening and treatment of cCMV infection in infants who fail newborn hearing screening assessments, based on the assumption that antiviral therapy would reduce the need for cochlear implantsCitation41. The other three studies estimated the cost-effectiveness of maternal CMV screening, neonatal cCMV screening, and female vaccination before first pregnancies using decision models to compare costs versus outcomes associated with these interventionsCitation16,Citation42,Citation43. The cost-effectiveness of universal prenatal serum screening outweighed that of maternal risk-based screening for CMV infection during pregnancy and was cost-effective in 63% of modeled scenarios according to a 2019 studyCitation16. However, the cost-effectiveness of universal prenatal screening was significantly influenced by the assumed incidence of primary CMV infection during pregnancy. Costs of identifying one cCMV infection were estimated to be substantially higher for universal screening than targeted screening of newbornsCitation42. This study also modeled direct costs related to antiviral treatment for symptomatic newborns and estimated moderate costs of 11 USD (universal screening) to net savings of 1 USD (targeted screening) per screened newbornCitation42. Modeling a scenario in which a CMV vaccine was available, savings were estimated at approximately 32 million USD per 100,000 adolescent females vaccinated against CMV infection before their first pregnancies; in this scenario, the number of infants affected by long-term sequelae was also reduced as the vaccination strategy avoided eight infant deaths as well as reductions in the number of infants affected with hearing loss, vision loss, and mental retardation compared to no vaccination (14/100,000 versus 66/100,000, 1/100,000 versus 6/100,000, 5/100,000 versus 23/100,000, respectively)Citation43.
Europe
Two studies used decision modeling to evaluate interventions for CMV and cCMV infection in EuropeCitation44,Citation45. Various strategies were modeled for CMV vaccination in France; vaccination of all eligible adolescent females, as in the cost-effectiveness evaluation from United States, or of all CMV seronegative women were among the most beneficial strategies against cCMV infection in infantsCitation44. Similar to the estimated benefits of broader newborn cCMV screening in the United States, costs incurred as a result of targeted screening of infants who did not pass their universal newborn hearing screening assessment in the United Kingdom were low (14.000 GBP per case) and could potentially result in significant cost benefit over the lifetime of the individualCitation45.
Australia
A single cost-effectiveness analysis of cCMV-related costs in Australia used decision tree modeling to evaluate the costs of targeted salivary cCMV testing into a newborn hearing screening programCitation46. In contrast to the potential savings arising from the introduction of targeted newborn cCMV screening in the United Kingdom, this economic evaluation projected negligible cost differences as a result of implementing targeted cCMV screening for infants who do not pass newborn hearing screening, with authors concluding that the inclusion of cCMV screening would be practically and financially achievable.
Other studies
One other publication identified in our systematic literature review was neither a cost study, nor an economic evaluation of interventions ()Citation48. This observational study reported an increase in annual discharge rates of infants with cCMV infection from 4.6 per 100,000 infant population during 1979–1985 to 22.3 per 100,000 in 2016 in the United Kingdom.
Table 3. Summary of other studies reporting the burden of cCMV (n = 1).
Discussion
The goal of this systematic literature review was to summarize the economic impact of CMV and cCMV infections across a broad selection of outcomes, populations, and countries, as well as to identify any gaps in the current body of literature. The heterogeneity of the outcomes reported in the available data limited the ability to succinctly collate and draw conclusions on economic impact. Of the 24 cost studies identified, 15 studies reported outcomes for cCMV, and 10 on CMV. Limited analyses of direct healthcare costs were available for cCMV (n = 6) and CMV (n = 3). Data on hospitalization and resource utilization were available from 11 reports in cCMV, and in all 10 reports on acquired CMV infection. Increased costs and resource utilization related to medical care were reported for other specific conditions in people with CMV, such as IBD. Of the seven economic evaluation studies identified, the cost-effectiveness or cost-benefit of a wide range of preventative measures or therapies for cCMV or acquired CMV infection, including prenatal screening, newborn screening, and adolescent vaccination, were assessed. There was less heterogeneity with respect to economic perspective, with most publications evaluating costs and or charges from a healthcare perspective (n = 30 publications), societal impact (n = 18), and the least from a patient/family centric perspective (n = 7). Economic impact data compiled for this systematic literature review stemmed from 32 publications from the United States, Europe, Brazil, Australia, Japan, and global (international, worldwide); however, most studies were conducted in the United States (n = 10).
The heterogeneity of outcomes and study populations, and in many cases, the small sample sizes, analyzed in the publications included in this review, highlights the need for more comprehensive and carefully designed studies to fully understand the economic impact of cCMV and acquired CMV infection both on a global and region scale. Nonetheless, taken together, these data suggest that CMV and cCMV infections have a substantial economic impact on healthcare systems, patients, and society as a whole. For example, multiple studies show that the mean all-cause healthcare costs per infant are higher for those with cCMV infection compared with those without cCMV infection, and mean total costs per child are higher for cCMV positive children with symptomatic cCMV infection at birth compared to children with asymptomatic cCMV infection at birthCitation18,Citation24. Mean lengths of hospital stays for infants with cCMV infection were longer than for infants without cCMV infection, with increased lengths of stays for newborns with low birth weights and other cCMV-related symptomsCitation22–24. Similar findings were noted for length of hospitalization and ICU stays in CMV-infected individuals in immunocompromised populations (leukemia or IBD)Citation20,Citation30,Citation34,Citation39. In contrast to these studies, analyses from 2 other studies suggest that CMV infection did not have a significant impact on the lengths of hospital stays for patients treated for IBD or community-acquired pneumoniaCitation27,Citation29.
Three studies included in this review obtained data via diagnostic codes associated with cCMV from hospital and insurance claims databases in their respective countriesCitation18,Citation19,Citation35. A recent review of cCMV identification in healthcare administrative databases found that although the prevalence of cCMV infection in developed countries is estimated to be 30 to 70 per 10,000 records, only 1 to 3 per 10,000 records in these countries are coded for cCMVCitation49. It is therefore likely that these per child total costs for cCMV diagnosis and treatment may not extend to the whole population of infants and children with cCMV infectionCitation49. However, information from these records can still provide insight into the economic costs and charges of cCMV infection and associated neurodevelopmental sequelaeCitation49.
The economic impact of CMV infection remains a key consideration when assessing the value of interventions and preventive strategies, such as vaccines. Five publications presented mathematical models that estimated either the direct and indirect costs or direct costs/charges of CMV infection in four countriesCitation17,Citation21,Citation25,Citation33,Citation45. A total of seven studies provided cost-effectiveness or cost-benefit analyses for CMV vaccination and screening strategies: vaccinating adolescent girls in both the United States and France before their first pregnancies was predicted to be more cost-effective than not vaccinating themCitation43,Citation44, as was providing universal screening of pregnant women in the United States (versus targeted screening)Citation16and providing universal/targeted newborn screening (versus no screening) in the United States, United Kingdom, and AustraliaCitation41,Citation42,Citation45,Citation46.
However, modeling of hypothetical interventions, as was the case with the majority of the economic evaluations reported here, requires a large number of assumptions to be made. Certain assumptions may have a large influence on the cost-effectiveness or cost-benefit results; for example, the cost-effectiveness of adolescent vaccination strategies were sensitive to variations in CMV seroprevalence and vaccine efficacyCitation42,Citation43, whereas the cost-effectiveness of universal prenatal screening for CMV was sensitive to the estimated incidence of primary CMV in pregnancyCitation16. In addition, model inputs regarding the benefit of antiviral treatment in reducing hearing loss as well as the costs associated with management of hearing loss, including prevention of cochlear implants, varied across studies that evaluated the cost-effectiveness or cost-benefit of newborn cCMV screening programs, making comparisons difficultCitation41,Citation44–46. Further research is needed to further quantify the economic burden of CMV infection, particularly for indirect/societal costs.
A strength of this review is the inclusion of multiple populations such as infants, children, adolescents, adults, and other specific subpopulations (including patients with comorbidities that impair their immune competence such as IBD and T-cell leukemia). Examination of multiple subpopulations provides data on the disease not only in the general population but also in groups where the burden of CMV infection may be significant. Another strength of our review is the wide array of outcomes included, namely direct costs, indirect and societal costs, and resource utilization related to disease.
Limitations of this review include variations in the quality of studies reported and the lack of data from countries regarding certain economic outcomes. Additionally, the geographical search was based within the literature search; therefore, studies that were not geographically indexed (MeSH, Entree) or did not contain the country or territory in title or abstract could have been missed. This review also revealed a lack of consistent data on the economic burden of CMV infection worldwide, as well as heterogeneity among existing studies. We did not adjust cost data for inflation, as the heterogeneity among outcomes made direct comparisons across studies all but impossible. In addition, this review included only English-language publications. Exclusion of other languages may have precluded local or regional literature with pertinent information; however, two included systematic literature reviews did not limit their searches to English-only publications (one review included studies in FrenchCitation39 and another review did not set language restrictionsCitation40). This review mainly focused on developed countries and information on the economic burden of CMV and cCMV infections in developing regions or countries is not provided. Meta-analyses or other aggregate analyses were not performed, and the individual studies were not assessed or ranked according to methodology or data quality; these types of evaluations and were not feasible given the limited and heterogeneous nature of the data. However, presentation of the individual results descriptively enables evaluation of the available data for each country, population, subpopulation, and outcome to identify knowledge gaps and facilitate further research. Despite the limitations, our descriptive compilation of economic impact studies provides broad high-level perspective toward the current and future CMV and cCMV infection landscape, further complementing narrower critical reviews already publishedCitation49.
Conclusions
CMV and cCMV infections are globally prevalent and impose a substantial economic burden. This systematic literature review provides information on the economic impact of CMV and cCMV infections for different countries, populations, and outcomes, which can be used for further analysis and to identify gaps in data where further research may be beneficial. The majority of identified economic studies stemmed from the United States and Europe, with a minimal number of studies in Australia and Latin America. This review highlighted an overall dearth of research on indirect/societal costs, with data from only three publications identified. Additional country-level research is needed to further quantify the economic burden of CMV and cCMV infections. Finally, this comprehensive economic impact overview can provide guidance in developing, implementing, and assessing strategies to treat and prevent CMV and cCMV infections, including vaccination.
Transparency
Declaration of funding
This study was funded by Moderna, Inc.
Declaration of financial/other relationships
JDD, AN, and POB are employees of Moderna, Inc. and hold stock/stock options in the company. CT was an employee of Moderna, Inc. at the time of the study and held stock/stock options in the company. JM, MN, WL, MK, and ES are employees of Certara and were paid consultants for Moderna, Inc., for conduct of this research. EM is a consultant for Moderna, Inc. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
MN, AN, CT and POB were involved in the study design. Data collection was performed by JM, MN, WL, MK and ES. Analysis and interpretation of the data were undertaken by JDD, EM, JM, WL, MK, ES, CT, and POB. JDD, EM, JM, MN, ES, CT, and POB were involved in the drafting and critical review of this manuscript. All authors give final approval of the published version and agree to be accountable for all aspects of the work.
Supplemental Material
Download PDF (302.9 KB)Buck_Supplemental_Material.docx
Download MS Word (153.7 KB)Acknowledgements
Medical writing and editorial assistance were provided by Kate Russin, PhD, Jessica Nepomuceno, PhD, and Clare Lee, PhD, of MEDiSTRAVA in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, Inc., and under the direction of the authors.
Data availability statement
The data summarized from this review are from published articles and are publicly available.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Taylor GH. Cytomegalovirus. Am Fam Physician. 2003;67:519–524.
- Centers for Disease Control and Prevention. Congenital CMV infection. [cited 2022 Sep 19]. Available from: https://www.cdc.gov/cmv/clinical/congenital-cmv.html.
- Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034. doi: 10.1002/rmv.2034.
- Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol. 2011;21(4):240–255. doi: 10.1002/rmv.695.
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–276. doi: 10.1002/rmv.535.
- Manicklal S, Emery VC, Lazzarotto T, et al. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26(1):86–102. doi: 10.1128/CMR.00062-12.
- Kabani N, Ross SA. Congenital cytomegalovirus infection. J Infect Dis. 2020;221(Suppl 1):S9–S14. doi: 10.1093/infdis/jiz446.
- Pass RF, Arav-Boger R. Maternal and fetal cytomegalovirus infection: diagnosis, management, and prevention. F1000Res. 2018;7:255. doi: 10.12688/f1000research.12517.1.
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis. 2013;57(suppl 4):S178–S181. doi: 10.1093/cid/cit629.
- Gantt S, Bitnun A, Renaud C, et al. Diagnosis and management of infants with congenital cytomegalovirus infection. Paediatr Child Health. 2017;22(2):72–74. doi: 10.1093/pch/pxx002.
- Rawlinson WD, Boppana SB, Fowler KB, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17(6):e177–e188. doi: 10.1016/S1473-3099(17)30143-3.
- Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102(6):900–931. doi: 10.1097/TP.0000000000002191.
- Hakki M, Aitken SL, Danziger-Isakov L, et al. American Society for Transplantation and Cellular Therapy Series: #3-Prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27(9):707–719. doi: 10.1016/j.jtct.2021.05.001.
- Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. [cited 2022 Sep 19]. Available from: https://clinicalinfo.hiv.gov/en/guidelines/adult-andadolescent-opportunistic-infection
- Institute of Medicine (US). Committee to study priorities for vaccine development: vaccines for the 21st century: a tool for decisionmaking. Stratton KR, Durch JS, Lawrence RS, editor. Washington (DC): National Academies Press; 2000.
- Albright CM, Werner EF, Hughes BL. Cytomegalovirus screening in pregnancy: a cost-effectiveness and threshold analysis. Am J Perinatol. 2019;36(7):678–687. doi: 10.1055/s-0038-1676495.
- Walter E, Brennig C, Schöllbauer V, et al. How to save money: congenital CMV infection and the economy. In: Halwachs-Baumann G, editor. Congenital cytomegalovirus infection: epidemiology, diagnosis, therapy. Cham (Switzerland): Springer International Publishing; 2018. p. 121–144.
- Meyers J, Sinha A, Samant S, et al. The economic burden of congenital cytomegalovirus disease in the first year of life: a retrospective analysis of health insurance claims data in the United States. Clin Ther. 2019;41(6):1040 e1043–1056 e1043. doi: 10.1016/j.clinthera.2019.04.022.
- Inagaki K, Blackshear C, Palmer A, et al. Risk factors, geographic distribution, and healthcare burden of symptomatic congenital cytomegalovirus infection in the United States: analysis of a nationally representative database, 2000-2012. J Pediatr. 2018;199:118.e111–123.e111. doi: 10.1016/j.jpeds.2018.03.036.
- Grossberg LB, Ezaz G, Grunwald D, et al. A national survey of the prevalence and impact of cytomegalovirus infection among hospitalized patients with ulcerative colitis. J Clin Gastroenterol. 2018;52(3):241–245. doi: 10.1097/MCG.0000000000000736.
- Lucas A, Sinha A, Fowler KB, et al. A framework for assessing the lifetime economic burden of congenital cytomegalovirus in the United States. Cost Eff Resour Alloc. 2019;17:21. doi: 10.1186/s12962-019-0189-0.
- Lanzieri TM, Bialek SR, Bennett MV, et al. Cytomegalovirus infection among infants in California neonatal intensive care units, 2005–2010. J Perinat Med. 2014;42(3):393–399. doi: 10.1515/jpm-2013-0183.
- Tran C, Bennett MV, Gould JB, et al. Cytomegalovirus infection among infants in neonatal intensive care units, California, 2005 to 2016. Am J Perinatol. 2020;37(2):146–150. doi: 10.1055/s-0039-1683958.
- Korndewal MJ, Weltevrede M, van den Akker-van Marle ME, et al. Healthcare costs attributable to congenital cytomegalovirus infection. Arch Dis Child. 2018;103(5):452–457. doi: 10.1136/archdischild-2017-312805.
- Retzler J, Hex N, Bartlett C, et al. Economic cost of congenital CMV in the UK. Arch Dis Child. 2019;104(6):559–563. doi: 10.1136/archdischild-2018-316010.
- Ahmed I, Kassem W, Salam Y, et al. Outcome of cytomegalovirus colitis in inflammatory bowel disease with different regimes of ganciclovir. Middle East J Dig Dis. 2018;10(4):220–229. doi: 10.15171/mejdd.2018.114.
- Delvincourt M, Lopez A, Pillet S, et al. The impact of cytomegalovirus reactivation and its treatment on the course of inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39(7):712–720. doi: 10.1111/apt.12650.
- Touhami S, Qu L, Angi M, et al. Cytomegalovirus anterior uveitis: clinical characteristics and long-term outcomes in a French series. Am J Ophthalmol. 2018;194:134–142. doi: 10.1016/j.ajo.2018.07.021.
- Saletinger R, Poljak M, Strle F. Presence of human cytomegalovirus DNA in blood of patients with community-acquired pneumonia. Clin Microbiol Infect. 2015;21(1):97–102. doi: 10.1016/j.cmi.2014.09.001.
- Vergara A, Cilloniz C, Luque N, et al. Detection of human cytomegalovirus in bronchoalveolar lavage of intensive care unit patients. Eur Respir J. 2018;51(2):1701332. doi: 10.1183/13993003.01332-2017.
- Jedlińska-Pijanowska D, Czech-Kowalska J, Kłodzińska M, et al. Antiviral treatment in congenital HCMV infection: the six-year experience of a single neonatal center in Poland. Adv Clin Exp Med. 2020;29(10):1161–1167. doi: 10.17219/acem/125427.
- Maconi G, Lombardini M, Furfaro F, et al. Long-term outcome of inflammatory bowel diseases with cytomegalovirus colitis: effect of antiviral treatment. Eur J Gastroenterol Hepatol. 2014;26(10):1146–1151. doi: 10.1097/MEG.0000000000000175.
- Aoki H, Kitano T, Kitagawa D. Disease burden of congenital cytomegalovirus infection in Japan. J Infect Chemother. 2021;27(2):161–164. doi: 10.1016/j.jiac.2020.08.018.
- Maeda T, Babazono A, Nishi T, et al. The impact of opportunistic infections on clinical outcome and healthcare resource uses for adult T cell leukaemia. PLoS One. 2015;10(8):e0135042. doi: 10.1371/journal.pone.0135042.
- Lin C, Tomio J, Tanaka H, et al. Diagnosis and medical care for congenital cytomegalovirus infection: an observational study using claims data in Japan, 2010 to 2017. Medicine. 2020;99(10):e19419. doi: 10.1097/MD.0000000000019419.
- Niz Xavier PC, Gonçalves Vieira P, de Souza Arantes T, et al. Cytomegalovirus identification in blood and urine of newborns by nested polymerase chain reaction. West Indian Med J. 2015;65(2):291–294. doi: 10.7727/wimj.2014.378.
- Yamamoto AY, Mussi-Pinhata MM, Isaac Mde L, et al. Congenital cytomegalovirus infection as a cause of sensorineural hearing loss in a highly immune population. Pediatr Infect Dis J. 2011;30(12):1043–1046. doi: 10.1097/INF.0b013e31822d9640.
- Marin LJ, Santos de Carvalho Cardoso E, Bispo Sousa SM, et al. Prevalence and clinical aspects of CMV congenital infection in a low-income population. Virol J. 2016;13(1):148–148. doi: 10.1186/s12985-016-0604-5.
- Lachance P, Chen J, Featherstone R, et al. Association between cytomegalovirus reactivation and clinical outcomes in immunocompetent critically ill patients: a systematic review and Meta-Analysis. Open Forum Infect Dis. 2017;4(2):ofx029. doi: 10.1093/ofid/ofx029.
- Li X, Huang Y, Xu Z, et al. Cytomegalovirus infection and outcome in immunocompetent patients in the intensive care unit: a systematic review and meta-analysis. BMC Infect Dis. 2018;18(1):289. doi: 10.1186/s12879-018-3195-5.
- Bergevin A, Zick CD, McVicar SB, et al. Cost-benefit analysis of targeted hearing directed early testing for congenital cytomegalovirus infection. Int J Pediatr Otorhinolaryngol. 2015;79(12):2090–2093. doi: 10.1016/j.ijporl.2015.09.019.
- Gantt S, Dionne F, Kozak FK, et al. Cost-effectiveness of universal and targeted newborn screening for congenital cytomegalovirus infection. JAMA Pediatr. 2016;170(12):1173–1180. doi: 10.1001/jamapediatrics.2016.2016.
- Dempsey AF, Pangborn HM, Prosser LA. Cost-effectiveness of routine vaccination of adolescent females against cytomegalovirus. Vaccine. 2012;30(27):4060–4066. doi: 10.1016/j.vaccine.2012.04.011.
- N’Diaye DS, Launay O, Picone O, et al. Cost-effectiveness of vaccination against cytomegalovirus (CMV) in adolescent girls to prevent infections in pregnant women living in France. Vaccine. 2018;36(10):1285–1296. doi: 10.1016/j.vaccine.2018.01.042.
- Williams EJ, Gray J, Luck S, et al. First estimates of the potential cost and cost saving of protecting childhood hearing from damage caused by congenital CMV infection. Arch Dis Child Fetal Neonatal Ed. 2015;100(6):F501–506. doi: 10.1136/archdischild-2014-306756.
- Beswick R, David M, Higashi H, et al. Integration of congenital cytomegalovirus screening within a newborn hearing screening programme. J Paediatr Child Health. 2019;55(11):1381–1388. doi: 10.1111/jpc.14428.
- Grosse SD, Dollard SC, Ortega-Sanchez IR. Economic assessments of the burden of congenital cytomegalovirus infection and the cost-effectiveness of prevention strategies. Semin Perinatol. 2021;45(3):151393. doi: 10.1016/j.semperi.2021.151393.
- Kadambari S, Pollard AJ, Goldacre MJ, et al. Congenital viral infections in England over five decades: a population-based observational study. Lancet Infect Dis. 2020;20(2):220–229. doi: 10.1016/S1473-3099(19)30416-5.
- Grosse SD, Leung J, Lanzieri TM. Identification of congenital CMV cases in administrative databases and implications for monitoring prevalence, healthcare utilization, and costs. Curr Med Res Opin. 2021;37(5):769–779. doi: 10.1080/03007995.2021.1890556.