Abstract
Background
Treatment guidelines recommend integrase strand transfer inhibitor (INSTI)-based antiretroviral therapy (ART) regimens for treatment naïve people living with HIV (PLWH) in the United States (US). This retrospective database study compared weight changes following initiation of INSTI-, non-nucleoside reverse transcriptase inhibitor (NNRTI)-, or protease inhibitor (PI)-based ART in treatment-naïve PLWH.
Methods
Adult (≥18 years) PLWH initiated on INSTI, NNRTI, or PI plus ≥2 nucleoside reverse transcriptase inhibitors (NRTI) between 1 January 2014 to 31 August 2019 were identified in IQVIA’s Ambulatory Electronic Medical Records (AEMR) linked to prescription drug claims (LRx). Weight changes over up to 36 months (M) of follow-up were compared among PLWH on INSTI- vs. NNRTI- and PI-based ART separately using non-linear mixed effect models, adjusting for demographics and baseline clinical characteristics.
Results
The INSTI, NNRTI, and PI cohorts included 931, 245, and 124 PLWH, respectively. For all three cohorts, the majority were male (78.2–81.2%) and overweight/obese (53.6–61.6%) at baseline; 40.8–45.2% of the groups were African American. The INSTI vs. NNRTI/PI cohorts were younger (median age: 38 years vs. 44 years/46 years), had lower weight at ART initiation (mean: 80.9 kg vs. 85.7 kg/85.0 kg), and had higher TAF usage during follow-up (55.6% vs. 24.1%/25.8%; all p < .05). Multivariate models showed higher weight gain among PLWH in INSTI vs. NNRTI and PI cohorts during treated follow-up (estimated weight gain after 36 M: 7.1 kg vs. 3.8 kg and 3.8 kg, both p < .05).
Conclusion
Study findings highlight the need to monitor an increase in weight and potential metabolic complications among PLWH starting ART with INSTI.
Introduction
Treatment guidelines for human immunodeficiency virus (HIV) in the United States (US) recommend initiating people living with HIV (PLWH) on an antiretroviral therapy (ART) of two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) in combination with a third active drug (“anchor agent”) from one of the following classes: non-nucleoside reverse transcriptase inhibitor (NNRTI), protease inhibitor (PI), or integrase strand transfer inhibitor (INSTI)Citation1. ARTs may be prescribed by physicians who are specialized in infectious disease, general/internal medicine (i.e. primary care), or obstetrics and gynecology (for women), a nurse practitioner, or a physician assistantCitation2. INSTI-based ART regimens are considered to be well tolerated, with fewer central nervous system, metabolic, or other side effects as compared to older NNRTI- or PI-based ART regimens;Citation1,Citation3 regimens containing INSTI have begun to eclipse NNRTI and PI in recent years due to better tolerability and reduced pill burdenCitation4.
Currently, four INSTI agents (raltegravir [RAL], elvitegravir [EVG], dolutegravir [DTG], and bictegravir [BIC]) are approved for use in ART for treatment-naïve PLWHCitation1,Citation5–8. BIC was approved by the US Food and Drug Administration (FDA) most recently in February 2018Citation8 and, along with DTG, are the recommended initial regimens for most PLWHCitation1. However, a growing body of studies have associated excess weight gain in treatment-naïve PLWH initiating INSTI-based regimens relative to NNRTI- or PI-based regimensCitation9. In a pooled analysis of clinical trials of treatment-naïve PLWH, DTG and BIC, were associated with significantly more weight gain than NNRTIs or PIsCitation10. The US treatment guidelines have noted greater weight gain among treatment-naïve PLWH receiving a INSTI-based ART compared to boosted PI- or NNRTI-based ARTCitation1. The prevalence of obesity, which contributes directly to incident cardiovascular risk factorsCitation11, is increasing among PLWH in the USCitation12. Given that cardiovascular disease is an important contributor to morbidity and mortality in PLWHCitation13,Citation14, it is imperative to gain a comprehensive view of the weight change trajectory of PLWH after initiating different ART regimens and to understand ART-related risk factors of weight gain.
Although the association between INSTI and weight gain in treatment-naïve PLWH has been examined in clinical trials and observational studies in multiple countriesCitation15–18, studies using real-world data on PLWH in the US, especially including those receiving BIC, remain scant. Two recent studies reported that the BIC-based regimen was associated with greater body mass index (BMI) and weight increase as compared to a darunavir-based single-tablet regimen among treatment naïve and virologically suppressed PLWHCitation19,Citation20. This study aimed to assess weight changes over time in treatment-naïve PLWH receiving NNRTI-, PI-, or INSTI-based ART using real-world data.
Methods
This was a retrospective observational study leveraging IQVIA’s Ambulatory Electronic Medical Records (AEMR) linked to a prescription drug claims database (LRx). Data from 1 January 2013 to 29 February 2020 (study period) were used. The study period was terminated on 29 February 2020 to avoid any impact of the Coronavirus 2019 (COVID-19) pandemic on study findings.
Study databases
The study dataset was created based on Health Insurance Portability and Accountability Act (HIPAA)-compliant linking processesCitation21,Citation22. IQVIA’s AEMR database comprises approximately 72 million patient records collected across >100,000 physicians from >800 large practices and physician networks across the US. LRx contains information on dispensed prescriptions with 92% coverage of prescriptions from the retail channel, 72% coverage of standard mail service, and 76% coverage of long-term care facilities in the US. All data were HIPAA-compliant to protect patient privacy. This study was conducted leveraging de-identified HIPAA-compliant data; therefore, Institutional Review Board (IRB) review was not required.
Patient selection and study cohorts
Inclusion and exclusion criteria for patient selection are detailed in . Briefly, prescriptions in AEMR were used to identify treatment naïve patients who initiated ART with NNRTI, PI, or INSTI during the selection window (i.e. index period) from 1 January 2014 to 31 August 2019 (index date: the earliest prescription) to allow for 12 months of baseline period and a minimum of 6 months of follow-up period. At least one diagnosis code for HIV was required (International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification [ICD-9/10-CM] code 042, V08, B20, or Z21) in AEMR during the study period. PLWH with AEMR prescription or LRx claims of NNRTI, PI, or INSTI during the 12-month pre-index (baseline) period were excluded. All PLWH had at least 6 months of continuous ART use after the index date (i.e. treated follow-up period). Follow-up ended with the earliest of treatment discontinuation, virologic failure (plasma HIV RNA ≥200 copies/ml) after 6 months of ART, switch to a different anchor agent class or a different INSTI agent, the last AEMR or LRx record in the study period, or the end of the study period (1 January 2013–29 February 2020). Treatment discontinuation was defined as a >90-day gap in medication supply for NNRTI, PI, or specific INSTI agentCitation23–25. During the treated follow-up period, PLWH were required to have at least two different NRTI backbones to ensure consistency with treatment recommendations.
Figure 1. Patient attrition. Abbreviations. AEMR, IQVIA’s Ambulatory Electronic Medical Record database; LRx, IQVIA’s prescription claims database; HIV, human immunodeficiency virus; INSTI, integrase strand inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; RNA, ribonucleic acid. aAEMR prescription data were prioritized when prescription claims in LRx and prescription records in AEMR were for the same medication (identified by the National Drug Code [NDC]) with the same days of supply on the same date. This was performed to avoid overestimation of treatment duration when the LRx and AEMR records were suspected to correspond to the same prescription. bData cleaning steps were taken to remove clinically implausible weight measurements (e.g. weight measurement that is beyond [previous measurement – 20 kg, previous measurement + 20 kg]). cConcurrent treatment was defined as ≥90-day overlap in days of supply for two different anchor agent classes or evidence of a fixed-dose combination medication containing two different anchor agents (e.g. NNRTI and INSTI). dMalignancy, pregnancy, and HIV-2 were identified using diagnosis codes. Gastric bypass was identified using procedure codes and diagnosis codes.
![Figure 1. Patient attrition. Abbreviations. AEMR, IQVIA’s Ambulatory Electronic Medical Record database; LRx, IQVIA’s prescription claims database; HIV, human immunodeficiency virus; INSTI, integrase strand inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; RNA, ribonucleic acid. aAEMR prescription data were prioritized when prescription claims in LRx and prescription records in AEMR were for the same medication (identified by the National Drug Code [NDC]) with the same days of supply on the same date. This was performed to avoid overestimation of treatment duration when the LRx and AEMR records were suspected to correspond to the same prescription. bData cleaning steps were taken to remove clinically implausible weight measurements (e.g. weight measurement that is beyond [previous measurement – 20 kg, previous measurement + 20 kg]). cConcurrent treatment was defined as ≥90-day overlap in days of supply for two different anchor agent classes or evidence of a fixed-dose combination medication containing two different anchor agents (e.g. NNRTI and INSTI). dMalignancy, pregnancy, and HIV-2 were identified using diagnosis codes. Gastric bypass was identified using procedure codes and diagnosis codes.](/cms/asset/769038e6-eec0-4f62-8e6e-c35ca6d6c9ab/icmo_a_2224165_f0001_b.jpg)
Treatment-naïve PLWH were stratified into three mutually exclusive cohorts, based on the anchor agent class of their index ART (NNRTI, PI, and INSTI cohorts). Subgroups initiating different INSTI agents (BIC, EVG, DTG) within the INSTI cohort were also identified. PLWH initiating RAL-based ART were not evaluated separately due to limited sample size (n = 41).
Study measures
Patient demographic () and provider specialty for the index ART were reported from data on or closest to index; clinical characteristics (e.g. Quan’s Charlson Comorbidity Index [CCI] score;Citation26 ) were reported from the 12-month baseline period. Follow-up measures included duration of the index ART and type of NRTI backbone. Concomitant medications associated with weight gain or weight loss (Supplementary Table S1) in baseline and follow-up were also reported.
Table 1. Demographic clinical characteristics of the NNRTI, PI, and INSTI cohorts.
Table 2. Baseline clinical characteristics of the NNRTI, PI, and INSTI cohorts.
Baseline weight and BMI were reported from data up to 6 months before and 1 month after the index date, and over up to 36 (±3) months of follow-up. For PLWH without a BMI recorded in AEMR, BMI was calculated using height closest to the index date and the available weight measurements. BMI was categorized as underweight (BMI <18.5 kg/m2), normal (≥18.5 to <25.0 kg/m2), overweight (≥25.0 to <30 kg/m2), or obese (≥30 kg/m2).
Statistical analysis
Weight changes up to 36 months of follow-up were compared among PLWH on INSTI- vs. NNRTI- and PI-based ART separately using non-linear mixed effect models, which can account for the correlation between longitudinal repeated measurements of weight and generate unbiased estimates when data are missing at randomCitation27. The models adjusted for age at index, sex, race/ethnicity, Quan’s CCI score, payer type, and evidence of baseline comorbidities (i.e. anxiety or depression, diabetes, or dyslipidemia/hyperlipidemia) that significantly differed across the study cohorts and were anticipated to be associated with weight change (Supplementary Tables S2 and S3)Citation28. Covariate selection of the model was guided by observed differences between study cohorts based on bivariate analysis and literature on potential risk factors of weight gain among PLWH. Baseline cluster of differentiation 4 (CD4) T lymphocyte cell count and plasma HIV RNA levels were not adjusted in the model due to the high proportion of missing data in the study cohorts (). Baseline weight was adjusted in the model as it was better correlated with follow-up weight as compared to baseline BMI. To account for non-linear weight trajectories, time was modeled using restricted cubic splines with three knots. The first knot was positioned at 6 months of follow-up based on clinical expectation that most treatment-naïve PLWH are expected to reach an undetectable plasma HIV RNA from 3 to 6 months after ART initiationCitation29,Citation30, and the other two knots were positioned at the median and third quartile of follow-up and were selected based on model performance. Analyses of the impact of specific INSTIs (BIC, EVG, DTG) on weight change up to 18 months of follow-up were conducted among PLWH with ≥2 follow-up weight measurements (due to the recent approval of BIC). All analyses were conducted using SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 1,300 PLWH newly-treated with NNRTI (N = 245), PI (N = 124), or INSTI (N = 931) were included in the study population (). Within the INSTI cohort, 130, 356, 404, and 41 PLWH were indexed on BIC, DTG, EVG, and RAL, respectively.
Demographic and baseline clinical characteristics
The demographic and baseline clinical characteristics of the study cohorts are shown in and . All cohorts were predominantly male (79.9–81.2%), had similar racial/ethnic distributions (32.9–40.8% White, 40.8–45.2% Black, and 18.0–20.8% Other/unknown), and were primarily located in the Southern US (70.2–75.8%; all p >.05). The INSTI cohort was younger than the NNRTI and PI cohorts (median age: 38 years vs. 44 years and 46 years, p <.001) and their ART on the index date was more frequently prescribed by an infectious disease physician (46.9% vs. 41.6% and 37.1%, p = .01). The INSTI cohort was also more likely to be indexed in later years, with 30.0% indexed in 2018 or 2019 compared to 6.1–8.9% of the NNRTI and PI cohorts.
Across all cohorts, the most common baseline comorbidity was hypertension (12.9–14.7%) (). The INSTI cohort had a higher mean Quan’s CCI score (mean score: 0.26 vs. 0.16 vs. 0.17) and a higher prevalence of anxiety (9.1% vs. 6.1% vs. 2.4%) and depression (10.2% vs. 8.2% vs. 3.2%) compared to the NNRTI and PI cohorts (all p <.05). The INSTI cohort was more likely to have ≥1 plasma HIV RNA measurement during the baseline period in AEMR compared to the NNRTI and PI cohorts (39.6% vs. 26.5% vs. 21.0%, p <.001).
Within the INSTI cohort, baseline characteristics that differed included a higher median age for PLWH with BIC or DTG (40 years [BIC and DTG] vs. 36 years [EVG]), a higher proportion of PLWH located in the South for BIC (91.5% [BIC] vs. 74.2% [DTG] and 64.1% [EVG]), a higher proportion of PLWH with an infectious disease physician as their index ART prescriber for BIC or EVG (50.0% [BIC] vs. 44.1% [DTG] vs. 48.3% [EVG]) all p <.05). PLWH with DTG also had a higher mean Quan’s CCI score compared to BIC or EVG (0.32 vs. 0.25 vs. 0.17, p = .003). The distributions of specific comorbidities of interest were generally similar across the subgroups, with the exceptions that PLWH with BIC or DTG had a higher prevalence of hypertension (15.4% vs. 16.3% vs. 10.1%) and hepatitis C (5.4% vs. 4.2% vs. 1.2%) compared to PLWH with EVG (both p <.05). Among PLWH with available plasma HIV RNA data, the median plasma HIV RNA was similar across the subgroups (ranging from 50,700 to 61,100 copies/mL) (Supplementary Tables S4 and S5).
ART treatment patterns during follow-up
The median durations of the index anchor agents were 15.2, 14.5, and 18.0 months for the NNRTI, PI, and INSTI cohorts, respectively (p = .09). In the NNRTI cohort, the most common anchor agents on the index date were efavirenz (58.4%) and rilpivirine (38.4%). For the PI cohort, the index anchor agents were primarily darunavir (46.8%) and atazanavir (33.9%). On the index date and anytime during follow-up, tenofovir alafenamide (TAF)-based NRTI backbones were more commonly observed in PLWH treated with INSTI compared to PLWH treated with NNRTI or PI (55.6% vs. 24.1% vs. 25.8%, p <.0001). For specific INSTIs, the usage of TAF was more frequently observed with BIC or EVG compared to DTG (100.0% vs. 74.8% vs. 22.8%), while non-TAF NRTIs were more commonly used with DTG (Supplementary Table S6). Compared to the NNRTI or INSTI cohort, the PI cohort also had a higher proportion of PLWH using medications associated with weight gain (62.9% vs. 50.2% vs. 49.6%, p = .02) during follow-up.
Changes in weight and BMI categories during follow-up
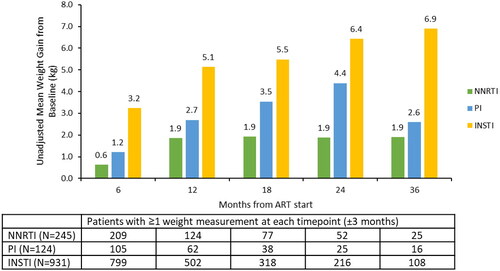
At ART initiation, the majority of PLWH in all cohorts had BMIs categorized as overweight or obese (53.3–61.6%), and the mean weight was the lowest for INSTI, followed by NNRTI and PI (80.9 kg vs. 85.7 kg vs. 85.0 kg, p = .002) (). Most patients had ≥3 follow-up weight measurements (69.0–75.5%). Among PLWH with available weight measurements at pre-specified timepoints, significantly greater weight gains were observed in the INSTI cohort compared to the NNRTI or PI cohorts during follow-up. For example, at 12 (±3) months of follow-up, the INSTI cohort had a mean weight gain of 5.1 kg vs. 1.9 kg for the NNRTI and 2.7 kg for the PI cohort (p <.001) (). Among PLWH who had BMI measurements at baseline and 12 (±3) months of follow-up (501, 124, and 62 PLWH in the INSTI, NNRTI, and PI cohorts, respectively), most PLWH in all three cohorts remained in their baseline BMI category at 12 months of follow-up. The highest proportion of PLWH transitioning from under/normal weight to overweight/obese BMI was observed in the PI cohort (17.7%), followed by the INSTI cohort (10.2%) and the NNRTI cohort (6.5%). However, the proportions of transition did not significantly differ across the cohorts (p = .06) (Supplementary Figure S1).
Figure 2. Unadjusted changes in weight from baseline to follow-up for the NNRTI, PI, and INSTI cohorts. Abbreviations. ART, antiretroviral therapy; INSTI, integrase strand inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. The comparison of mean weight change from baseline across the NNRTI, PI, and INSTI cohorts was significantly different (p <.05) at all timepoints.
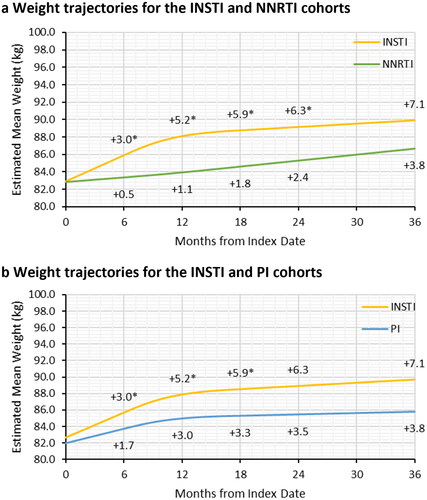
In the adjusted analyses, PLWH who initiated treatment with INSTI gained significantly more weight at 6, 12, 18, and 24 months of follow-up compared to PLWH on NNRTI (). For specific INSTIs, DTG (6.7 kg) was associated with the largest absolute weight gain from index to 18 months, followed by BIC (5.9 kg) and EVG (5.8 kg) (Supplementary Figure S2). PLWH with INSTI also had significantly more weight gain compared to those who initiated PI-based ART at 6, 12, and 18 months of follow-up, but not at 24 months (). PLWH with DTG had the greatest weight gain at 18 months of treatment (6.7 kg); however, the difference in weight gained between PLWH with DTG and those with PI (6.7 kg vs. 4.3 kg, p = .17) did not reach statistical significance (Supplementary Figure S2).
Figure 3. Adjusted weight trajectories after initiation of NNRTI-based ART (a) and PI-based ART (b) compared to INSTI-based ART. Abbreviations. ART, antiretroviral therapy; INSTI, integrase strand inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor. *Significant difference between the INSTI and NNRTI or PI cohorts in gained weight relative to the baseline weight at the specified follow-up time. p-values and estimated mean weight were obtained from multivariable linear mixed model with random intercept and restricted cubic splines with three knots to adjusted for non-linear trend of weight change trajectory. The first knot was placed at 6 months of follow-up and the remaining two knots were placed at median and third quartile of follow-up. The models adjusted for age at index, sex, race/ethnicity, Charlson comorbidity index, payer type, evidence of baseline anxiety or depression, and evidence of baseline diabetes or dyslipidemia/hyperlipidemia.
Discussion
This retrospective study analyzed real-world electronic medical records linked to prescription claims data and evaluated the association between INSTI- vs. NNRTI- or PI-based ART and weight gain in treatment naïve PLWH. While previously published studies have reported greater weight gain during treatment with INSTI- compared to NNRTI- or PI-based regimens, this is one of the first to leverage real-world data in the US to report weight changes in PLWH treated with the most recently approved INSTI, BIC.Citation1
Consistent with literature reporting, our results demonstrated that the proportion of PLWH initiating an INSTI-based ART regimen increased significantly between 2014 and 2019. Like other HIV cohort studies, most PLWH in our real-world cohort were overweight or obese at baseline and weight gain was observed over the course of treatmentCitation10,Citation12,Citation16,Citation17. Furthermore, the proportion of PLWH who initiated an INSTI-based regimen appeared to be lower among PLWH with a baseline BMI category of overweight or obese vs. those with a BMI category of underweight or normal (p = .05), likely reflecting the emerging concern of greater weight gain associated with INSTI-based regimens vs. PI- or NNRTI-based regimens. In adjusted analyses where time was flexibly modeled using restricted cubic splines, the INSTI cohort gained an additional 4.1 kg compared to the NNRTI cohort and 2.2 kg compared to the PI cohort in the first year of treatment, which is similar to the 2–5 kg range reported in other observational studies using data from a single clinic or HIV cohortCitation16,Citation17,Citation31 and using EMR dataCitation19. Similar to other studies that reported greater short-term weight gain, this study found greater rates of weight gain during the first 0–12 months of treatment compared to 12–36 months, which may be reflective of the “return-to-health” stage of PLWH in response to ART treatmentCitation16,Citation17. Among the INSTI agents, DTG was associated with the greatest weight gain after 18 months of treatment in this study. However, evidence from randomized trials suggests similar or greater weight gains for treatment-naïve PLWH using BIC compared to DTG, depending on the NRTI backboneCitation10. In a phase 3 randomized clinical trial (RCT), weight gain was similar between PLWH treated with DTG (3.9 kg) and BIC (3.5 kg), both with TAF, from baseline to week 96Citation32. Similar weight gains at week 96 were also reported for BIC (4.2 kg) and DTG (4.1 kg) by a meta-analysis of eight phase 3 RCTs, comprising >5,000 participants and 10,000 person-years of follow-up. The meta-analysis also reported greater weight gains for TAF as compared to abacavir (ABC) and tenofovir disoprozil fumarate (TDF)Citation10. However, in another phase 3 trial, PLWH using DTG with ABC had smaller weight gains compared to those using BIC with TAF (3.6 kg vs. 2.4 kg) at week 96Citation33. Most PLWH receiving DTG had ABC (73.0%) in the regimen during follow-up in this study, while BIC was almost exclusively used with TAF, due to its single-tablet formulation. Therefore, the greater weight gain observed in these PLWH with DTG as compared to PLWH receiving BIC may be due to unmeasured differences between the two groups, such as treatment response and lifestyle factors.
Due to the relatively recent approval, there have been few real-world studies describing the impact of BIC on weight gain in treatment-naïve populations;Citation19,Citation20 this is one of the first studies to do so. Another notable strength of this study is the statistical methodology. Based on prior studies reporting greater rates of short-term weight gain following ART initiationCitation16,Citation17, this study leveraged models with restricted cubic splines, rather than simple linear regression models, to model weight more flexibly and account for non-linear weight change trajectories. However, our study findings should be interpreted in the context of limitations inherent to the data and study design. First, prescription data in the AEMR database and dispensation claims in the LRx database were used to assess the duration of ART treatment. However, since there may be care outside of the AEMR system and the LRx database is an open claims database where continuous enrollment cannot be confirmed, continuous observation of PLWH and their treatments during the study period cannot be guaranteed; therefore, the impact of ART on weight gain may be underestimated. Furthermore, our study identified treatment-naïve PLWH by requiring no evidence of baseline ART and plasma HIV RNA <1,000 copies/ml. To that end, there might be misclassification of treatment experienced PLWH into the study if their baseline ART and/or plasma HIV RNA were not captured by the study data. Second, weight is inconsistently measured and recorded in real-world data; PLWH with more frequent weight measurements during the follow-up period may have been more closely monitored by their physicians for reasons which may confound the association between ART and weight change. Given that PLWH without any follow-up weight measurements and those on ART for less than 6 months were excluded, the study sample may be biased towards PLWH who had more weight gain following ART initiation (i.e. physician more likely to record if weight changed). In terms of study design, there was likely residual confounding after adjusting for observed patient characteristics in multivariable regression models due to unmeasured confounders such as reasons for discontinuing ART (e.g. adverse effects) and lifestyle factors. Baseline plasma HIV RNA and CD4 T lymphocyte cell count were also not adjusted in the models as both variables were missing for most patients in the study. The low capture of these virological measurements may be due to the receipt of testing in other settings (e.g. public sector), which was not integrated into the AEMR system. Given that patients with high baseline plasma HIV RNA and those with low CD4 T lymphocyte cell count have a greater return-to-health effect when initiating ARTCitation34, the estimates of short-term weight gain in this study should be interpreted carefully. Similarly, the use of TAF in the index regimens was also not adjusted in the models due to the small number of patients using TAF in the NNRTI and PI cohorts (<60 patients). However, the high usage of TAF in the INSTI cohort was primarily driven by PLWH initiating on BIC- and EVG-based regimens; the proportion of PLWH using TAF was similar between PLWH on DTG-based regimens (22.8%) and those on NNRTI- (24.1%) or PI-based (25.8%) regimens. Thus, no major difference on main study findings is expected by adding adjustment of TAF use in the analysis. The study also did not adjust for any comorbid conditions that may have developed over time of follow-up (e.g. thyroid dysfunction) and may play a role in weight change of PLWH after ART initiation.
Although the findings of this study and previous studies highlighted INSTI-associated weight gain in treatment-naïve PLWH, further research is warranted to elucidate the underlying mechanisms for the observed weight gain and their long-term clinical implications. Given that cardiovascular disease is a large contributor to mortality in PLWHCitation13,Citation14 and HIV has been identified as a risk factor for several cardiovascular diseases (e.g. acute myocardial infarction, heart failure, stroke)Citation35–38, minimizing ART-associated weight gain can help reduce the burden of noncommunicable diseases on PLWH and healthcare systems. Due to limited sample sizes, the association between INSTI and weight gain in patient subgroups that have been reported to be at risk for greater weight gain (e.g. female gender, Black race, and older ageCitation10,Citation39–41) was not investigated in this study; research examining how patient and other ART regimen characteristics mediate the association between INSTI and weight gain would be valuable in better informing HIV care in different populations. In addition, studies using real-world data have reported a higher risk of developing Type 2 diabetes mellitus (T2DM) or dyslipidemia in PLWH treated with INSTI compared to NNRTI or PICitation42,Citation43, yet it remains inconclusive how much of the increased risk can be attributed to gained weight. However, joint HIV/specialty clinics and personalized ART decisions are needed for PLWH who have pre-existing obesity-related multimorbidity (e.g. T2DM, chronic kidney disease, hypertension)Citation44. Furthermore, it is important to note that not all fat contributes equally to the increased risk of metabolic disorder; thus, studies assessing lipodystrophy weight gain, which is characterized by visceral fat gain and a higher risk for metabolic disorders, in PLWH treated with ART are warrantedCitation42,Citation45.
Conclusions
This study suggests that treatment with INSTI, particularly DTG and BIC, is associated with more rapid weight gain in treatment-naïve PLWH compared to treatment with non-INSTI regimens. Considering the existing burden from noncommunicable disease in PLWH, weight gain and related metabolic health of PLWH treated with INSTI should be closely monitored by clinicians. Further research is needed to identify patient subgroups that are at higher risk of weight gain and associated metabolic complications, as well as identify effective risk-reducing interventions.
Transparency
Author contributions
Conception and design of study: Girish Prajapati, Xiaohui Zhao, Jenny Tse, and Aimee M. Near. Acquisition of data, analysis, and interpretation of data: Girish Prajapati, Xiaohui Zhao, Jenny Tse, Aimee M. Near, and Princy N. Kumar. Drafting and revising the manuscript: Girish Prajapati, Xiaohui Zhao, Jenny Tse, Aimee M. Near, and Princy N. Kumar. All authors approved the final manuscript version to be submitted.
Ethics statement
Not applicable. This was a retrospective database study using de-identified data compliant with the US Health Insurance Portability and Accountability Act of 1996. Therefore, ethics approval from the Institutional Review Board (IRB) was not required for this study.
Supplemental_materials.docx
Download MS Word (876.6 KB)Acknowledgements
The authors thank Wei-Ti Huang and Hangcheng Liu of IQVIA for statistical expertise during the analysis of data of this study.
Declaration of funding
This work was funded by Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc, Rahway, NJ, USA.
Declaration of financial/other relationships
Girish Prajapati is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc, Rahway, NJ, USA. Aimee Near, Jenny Tse, and Xiaohui Zhao are employed by IQVIA, which received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc, Rahway, NJ, USA to conduct this study. Princy Kumar reports grant/research support from GSK, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc, Rahway, NJ, USA, and Gilead; stock ownership with Merck & Co., Inc., Rahway, NJ, USA, Pfizer, Johnson & Johnson, GSK, and Gilead; and service as consultant/advisory board member with AMGEN, GSK, Merck & Co., Inc., Rahway, NJ, USA, and Gilead.
Results in part were presented at the 2022 AIDS meeting, Montreal, Canada, 29 July–2 August 2022.
A reviewer on this manuscript has disclosed that they have received a research grant from Gilead Science. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Data availability statement
The data that support the findings of this study are available from IQVIA but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
References
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV: Department of Health and Human Services 2023 [updated March 23, 2023; cited 2023 June 6]. Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf.
- HIV.gov. Types of providers 2018 [updated May 21, 2018; cited 2023 May 16]. Available from: https://www.hiv.gov/hiv-basics/starting-hiv-care/find-a-provider/types-of-providers/.
- Kolakowska A, Maresca AF, Collins IJ, et al. Update on adverse effects of HIV integrase inhibitors. Curr Treat Options Infect Dis. 2019;11(4):372–387. doi: 10.1007/s40506-019-00203-7.
- Eaton EF, Tamhane A, Davy-Mendez T, et al. Trends in antiretroviral therapy prescription, durability and modification: new drugs, more changes, but less failure. AIDS. 2018;32(3):347–355. doi: 10.1097/QAD.0000000000001708.
- Merck Sharp & Dohme Corp. Isentress package insert [package insert] 2019; [cited 2020 May 22]. Available from: https://www.merck.com/product/usa/pi_circulars/i/isentress/isentress_pi.pdf.
- Gilead Sciences Inc. Vitekta package insert [package insert] 2014; [cited 2020 May 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/203093s000lbl.pdf.
- GlaxoSmithKline. Tivicay package insert [package insert] 2013; [cited 2020 May 22]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf.
- Gilead Sciences Inc. Biktarvy package insert [package insert] 2018; [cited 2022 April 5]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210251s000lbl.pdf.
- Shah S, Hindley L, Hill A. Are new antiretroviral treatments increasing the risk of weight gain? Drugs. 2021;81(3):299–315. doi: 10.1007/s40265-020-01457-y.
- Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Diseases. 2020;71(6):1379–1389. doi: 10.1093/cid/ciz999.
- Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973.
- Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5(4):e10106. doi: 10.1371/journal.pone.0010106.
- Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D: A: D): a multicohort collaboration. Lancet. 2014;384(9939):241–248. doi: 10.1016/S0140-6736(14)60604-8.
- Feinstein MJ, Bahiru E, Achenbach C, et al. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117(2):214–220. doi: 10.1016/j.amjcard.2015.10.030.
- Hester EK, Greenlee S, Durham SH. Weight changes with integrase strand transfer inhibitor therapy in the management of HIV infection: a systematic review. Ann Pharmacother. 2022;56(11):1237–1249. doi: 10.1177/10600280211073321.
- Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4):e25484. doi: 10.1002/jia2.25484.
- Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267–1274. doi: 10.1093/cid/ciz407.
- Ruderman SA, Crane HM, Nance RM, et al. Brief report: weight gain following ART initiation in ART-Naïve people living with HIV in the current treatment era. J Acquir Immune Defic Syndr. 2021;86(3):339–343. doi: 10.1097/QAI.0000000000002556.
- Emond B, Rossi C, Côté-Sergent A, et al. Body mass index increase and weight gain among people living with HIV-1 initiated on single-tablet darunavir/cobicistat/emtricitabine/tenofovir alafenamide or bictegravir/emtricitabine/tenofovir alafenamide in the United States. Curr Med Res Opin. 2022;38(2):287–298. doi: 10.1080/03007995.2021.2007006.
- Emond B, Rossi C, Côté-Sergent A, et al. Weight change and predictors of weight change among patients initiated on darunavir/cobicistat/emtricitabine/tenofovir alafenamide or bictegravir/emtricitabine/tenofovir alafenamide: a Real-World retrospective study. J Health Econ Outcomes Res. 2021;8(1):88–98. doi: 10.36469/jheor.2021.24535.
- Ober NS, Grubmuller J, Farrell M, et al. System and method for generating de-identified health care data. Google Patents. 2008.
- Zubeldia K, Romney GW. Anonymously linking a plurality of data records. Google Patents. 2002.
- Hines DM, Ding Y, Wade RL, et al. Treatment adherence and persistence among HIV-1 patients newly starting treatment. Patient Prefer Adherence. 2019;13:1927–1939. doi: 10.2147/PPA.S207908.
- Rosenblatt L, Buikema AR, Seare J, et al. Economic outcomes of First-Line regimen switching among stable patients with HIV. J Manag Care Spec Pharm. 2017;23(7):725–734. Juldoi: 10.18553/jmcp.2017.16403.
- Sax PE, Meyers JL, Mugavero M, et al. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One. 2012;7(2):e31591. doi: 10.1371/journal.pone.0031591.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. Novdoi: 10.1097/01.mlr.0000182534.19832.83.
- Lindstrom MJ, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46(3):673–687. doi: 10.2307/2532087.
- Mazzitelli M, Isabel Pereira B, Moyle G, et al. Factors associated with overweight/obesity in a cohort of people living with HIV over 50 years of age. AIDS Care. 2022;34(4):542–544. Aprdoi: 10.1080/09540121.2021.1935438.
- Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384(9939):258–271. doi: 10.1016/S0140-6736(14)60164-1.
- Shoko C, Chikobvu D. A superiority of viral load over CD4 cell count when predicting mortality in HIV patients on therapy. BMC Infect Dis. 2019;19(1):169. doi: 10.1186/s12879-019-3781-1.
- Ruderman S, Nance R, Whitney B, et al. editors. Dolutegravir-based regimens are associated with weight gain over two years following ART-initiation in ART-naïve people living with HIV (PLWH). 17th European AIDS Conference; 2019; Basel, Switzerland.
- Stellbrink HJ, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e364–e372. doi: 10.1016/S2352-3018(19)30080-3.
- Wohl DA, Yazdanpanah Y, Baumgarten A, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e355–e363. doi: 10.1016/S2352-3018(19)30077-3.
- Yuh B, Tate J, Butt AA, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60(12):1852–1859. doi: 10.1093/cid/civ192.
- Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728.
- Barnes RP, Lacson JCA, Bahrami H. HIV infection and risk of cardiovascular diseases beyond coronary artery disease. Curr Atheroscler Rep. 2017;19(5):20–20. doi: 10.1007/s11883-017-0652-3.
- Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the veterans aging cohort study. JAMA Cardiol. 2017;2(5):536–546. doi: 10.1001/jamacardio.2017.0264.
- Beckman JA, Duncan MS, Alcorn CW, et al. Association of human immunodeficiency virus infection and risk of peripheral artery disease. Circulation. 2018;138(3):255–265. doi: 10.1161/CIRCULATIONAHA.117.032647.
- Lake JE, Wu K, Erlandson KM, et al. editors. Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy. Conference on Retroviruses and Opportunistic Infections; 2019 Feb 26; Seattle, Washington2020/02/27).
- Koethe J, Bian A, Rebeiro PF, et al. editors. Greater weight gain after switch to InSTI-based regimen from NNRTI vs PI regimens. Conference on Retroviruses and Opportunistic Infections; 2020; Boston, MA.
- Menard A, Meddeb L, Tissot-Dupont H, et al. Dolutegravir and weight gain: an unexpected bothering side effect? AIDS. 2017;31(10):1499–1500. doi: 10.1097/QAD.0000000000001495.
- Rebeiro PF, Jenkins CA, Bian A, et al. Risk of incident diabetes mellitus, weight gain, and their relationships with integrase inhibitor-based initial antiretroviral therapy among persons with HIV in the US and Canada. Clin Infect Dis. 2021;73(7):e2234–e2242.
- Hindley L, McCann K, Sokhela S, et al. Predicted 10-year risks of cardiovascular disease and diabetes in the ADVANCE trial. CROI 2021, Conference on Retroviruses and Opportunistic Infections, March 6–10, 2021.
- Pereira B, Mazzitelli M, Milinkovic A, et al. Evaluation of a clinic dedicated to people aging with HIV at chelsea and westminster hospital: results of a 10-Year experience. AIDS Res Hum Retroviruses. 2022;38(3):188–197. doi: 10.1089/AID.2021.0083.
- O’Halloran JS, Olsen M, Powderly W. Incident diabetes associated with integrase strand transfer inhibitor initiation. CROI 2021, Conference on Retroviruses and Opportunistic Infections, March 6–10, 2021. 2021.
