ABSTRACT
Objective
Chronic obstructive pulmonary disease (COPD) is a leading cause of death worldwide. While two approved fixed-dose inhaled corticosteroid/long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) triple therapies reduce all-cause mortality (ACM) versus dual LAMA/LABA therapy in patients with COPD, head-to-head studies have not compared the effects of these therapies on ACM. We compared ACM in adults with moderate-to-very severe COPD receiving budesonide/glycopyrrolate/formoterol fumarate (BGF) in ETHOS versus fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) in IMPACT using a matching-adjusted indirect comparison (MAIC).
Methods
A systematic literature review identified two studies (ETHOS [NCT02465567]; IMPACT [NCT02164513]) of ≥52 weeks reporting ACM as an efficacy endpoint in patients receiving triple therapy. As ETHOS and IMPACT lack a common comparator, an unanchored MAIC compared ACM between licensed doses of BGF (320/18/9.6 μg) from ETHOS and FF/UMEC/VI (100/62.5/25 μg) from IMPACT in patients with moderate-to-very severe COPD. Using on- and off-treatment data from the final retrieved datasets of the intention-to-treat populations, BGF data were adjusted according to aggregate FF/UMEC/VI data using 11 baseline covariates; a supplementary unadjusted indirect treatment comparison was also conducted. P-values for these post-hoc analyses are not adjusted for Type I error.
Results
ACM over 52 weeks was statistically significantly reduced by 39% for BGF versus FF/UMEC/VI in the MAIC (hazard ratio [HR] [95% CI]: 0.61 [0.38, 0.95], p = 0.030) and unadjusted analysis (HR [95% CI]: 0.61 [0.41, 0.92], p = 0.019).
Conclusion
In this MAIC, which adjusted for population heterogeneity between ETHOS and IMPACT, ACM was significantly reduced with BGF versus FF/UMEC/VI in patients with moderate-to-very severe COPD.
Graphical Abstract

PLAIN LANGUAGE SUMMARY
Chronic obstructive pulmonary disease (known as COPD) is a leading cause of death worldwide, being responsible for over 3 million deaths in 2019. People living with COPD are more likely to die. Importantly, a sudden worsening of COPD symptoms (known as an exacerbation) is associated with a higher chance of death from heart-related and breathing-related problems. Therefore, reducing risk of death is an important treatment goal for COPD. Of the three medications approved for treating COPD that combine three drugs in a single-inhaler device, there are two—referred to generically as budesonide/glycopyrrolate/formoterol fumarate (BGF) and fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI)—that can reduce the risk of death in people living with COPD compared with treatments that combine two drugs. However, no studies have directly compared the risk of death in people living with COPD treated with these medicines. We compared the risk of death in people living with moderate-to-very severe COPD who received either BGF during a clinical trial called ETHOS or FF/UMEC/VI during a clinical trial called IMPACT. To make this comparison, we used a method called “matching-adjusted indirect comparison”, which used specific features (such as sex, breathing difficulty, and whether they were current smokers) to match patients from the two studies to ensure similar groups were examined. Our analysis showed a 39% decrease in the chance of death in patients who received BGF compared with patients who received FF/UMEC/VI. This finding may be important for doctors to improve patient health and reduce the risk of death in people living with COPD.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of death and disability worldwide, affecting over 200 million people and being responsible for an estimated 74 million disability-adjusted life years and over 3 million deaths annuallyCitation1–3. Patients with COPD are at risk of cardiopulmonary events, including exacerbations (i.e. an acute worsening of symptoms) of their COPD and myocardial infarctionCitation4–6. Cardiopulmonary-related death is the most common cause of mortality in patients with COPDCitation7,Citation8. Furthermore, exacerbations further amplify the risk of subsequent cardiovascular events and risk of all-cause, COPD-related, and cardiovascular-related mortalityCitation9–11. Though COPD is preventable and treatable, COPD-related mortality is projected to rise for the foreseeable futureCitation1,Citation12,Citation13. As such, reducing mortality is an important treatment goal for COPD.
The availability of fixed-dose triple therapy, which combines an inhaled corticosteroid (ICS), a long-acting muscarinic antagonist (LAMA), and a long-acting β2-agonist (LABA), has improved treatment opportunities for patients diagnosed with COPD, with several robust clinical studies demonstrating improved lung function and reduced exacerbation rates with ICS/LAMA/LABA versus LAMA/LABA or ICS/LABA dual therapiesCitation14–19. Based on these findings, three ICS/LAMA/LABA triple therapies are currently marketed as maintenance treatment for COPD: budesonide/glycopyrrolate/formoterol fumarate (BGF) 320/18/9.6 µg (two actuations of 160/9/4.8 µg) twice daily, fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) 100/62.5/25 μg (one actuation) once daily, and beclomethasone dipropionate/glycopyrronium/formoterol fumarate (BDP/GLY/FF) 200/20/12 μg (two actuations of 100/10/6 µg) twice dailyCitation20–22.
As noted in the Global Initiative for Chronic Lung Disease (GOLD) 2023 reportCitation1, two important 52-week randomized controlled trials (ETHOS and IMPACT) have reported that fixed-dose ICS/LAMA/LABA triple therapy reduced all-cause mortality over LAMA/LABA dual therapyCitation14,Citation16,Citation23,Citation24. Descriptions of the study designs can be found in Supplementary Table 1. Although eligibility criteria differed slightly between the trials, baseline patient characteristics were broadly similar across the ETHOS and IMPACT study populations, with slight differences observed for sex, race, body mass index (BMI), COPD severity, and exacerbation history (Supplementary Table 2). An analysis of all-cause mortality from the ETHOS study in patients with moderate-to-very severe COPD and a history of exacerbations reported triple therapy with twice-daily BGF 320/18/9.6 µg reduced all-cause mortality by 49% versus dual LAMA/LABA therapy (30 deaths/2137 patients [1.4%] with BGF 320/18/9.6 µg vs 56 deaths/2120 patients [2.6%] with LAMA/LABA; hazard ratio [HR] [95% confidence interval; CI]: 0.51 [0.33, 0.80], unadjusted p = 0.0035) in the final retrieved dataset, corresponding to a number needed to treat of 80 (95% CI: 58, 198)Citation23. Similarly, in patients with moderate-to-very severe COPD and a history of exacerbations from the IMPACT study, triple therapy with FF/UMEC/VI 100/62.5/25 μg reduced all-cause mortality by 28% versus LAMA/LABA dual therapy (98 deaths/4151 patients [2.36%] with FF/UMEC/VI vs 66 deaths/2070 patients [3.19%] with LAMA/LABA; HR [95% CI]: 0.72 [0.53, 0.99], p = 0.042) in the final retrieved dataset (using on- and off-treatment data), corresponding to a number needed to treat of 121Citation24. Causes of death from both studies are summarized in Supplementary Table 3. All-cause mortality has not been examined as a prespecified efficacy endpoint for triple therapy with BDP/GLY/FF, but only in a post-hoc safety analysis. As highlighted by Vestbo et al.Citation25, this is not trivial, as the aim of mortality studies is to have follow-up for all patients until the end of the planned study period, therefore including on- and off-treatment data because patients may discontinue randomized treatment and/or study participation near the end of their lives. Safety analyses often only follow patients while on treatment, with follow-up for only a short period of time after treatment discontinuation, which can bias resultsCitation25. In this analysis, the risk of developing a fatal event was numerically but not statistically significantly reduced for BDP/GLY/FF versus therapies not containing ICSCitation25. Given that COPD continues to exert a considerable mortality burdenCitation3,Citation13, the importance of findings from studies examining all-cause mortality in COPD, particularly the mortality reductions observed in the ETHOS and IMPACT studies, where mortality was assessed as a prespecified efficacy endpoint, should not be underestimated.
Due to differences in the components and delivery systems among ICS/LAMA/LABA triple therapiesCitation26, it is plausible that there may be differences in efficacy between treatments, which warrants further investigation. However, to date, no head-to head clinical studies have been performed to directly compare the effects of fixed-dose triple therapies on clinical endpoints, including mortality risk, in patients with COPD. Two previously published network meta-analyses (NMAs) by Lee et al.Citation27 and Rogliani et al.Citation28 have indirectly examined mortality in COPD and the use of triple therapies, reporting no significant differences in mortality reductions across different ICS/LAMA/LABA triple therapies. However, those analyses, which utilized a traditional Bayesian approach using aggregate-level data, did not only consider mortality as an efficacy outcome (all types of mortality events, e.g. adverse events, were evaluated within a single category) and/or included studies with a range of treatment durations <52 weeksCitation27,Citation28, introducing additional trial-specific heterogeneity to a comparison of already heterogenous patient populations and study designs. Given that ETHOS and IMPACT share similarities in study design and timelines, the exclusion of additional studies in further analyses may help avoid many of the limitations of previous analyses and better elucidate differences in efficacy between triple therapiesCitation14,Citation16.
Indirect treatment comparisons (ITCs) across separate trials are increasingly recognized as an essential form of evidence in developing healthcare guidanceCitation29,Citation30. Matching-adjusted indirect comparisons (MAICs) are a form of population-adjusted ITC that attempt to reduce bias in treatment comparisons by matching individual patient-level data (IPD) from clinical trials of one treatment to aggregate data reported for comparator trialsCitation30,Citation31. Here, following a systematic literature review (SLR), we report the results of a MAIC that assessed reductions in all-cause mortality risk in adults with moderate-to-very severe COPD receiving licensed doses of BGF (320/18/9.6 µg) in ETHOS versus FF/UMEC/VI (100/62.5/25 μg) in IMPACT.
Methods
Study selection & feasibility assessment
Systematic literature review
A systematic literature review of English language articles published before June 2022 was conducted to identify randomized controlled trials (RCTs) of ≥52-week duration that reported all-cause mortality as an efficacy endpoint in adult patients with moderate-to-very severe COPD receiving ICS/LAMA/LABA triple therapy (fixed-dose or open triple). A description of the SLR methodology and findings are provided in the Supplementary Material (see Supplementary SLR Methods and Results), which reports descriptions of included study characteristics (Supplementary Table 1) and patient characteristics (Supplementary Table 2), and describes study inclusion criteria (Supplementary Table 4), the SLR search strategies (Supplementary Table 5) and the PRISMA flow diagram (Supplementary Figure 1).
Feasibility of indirect comparison
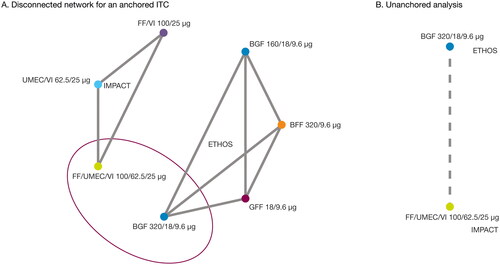
Two studies (ETHOS, NCT02465567Citation14,Citation23; IMPACT, NCT02164513Citation16,Citation24) met the SLR inclusion criteria, and the feasibility of an ITC between these studies was assessed. Both studies were conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki, both studies received approval from local institutional review boards or independent ethics committees, and all patients provided written informed consentCitation14,Citation16. According to the National Institute for Health and Care Excellence (NICE) Decision Support Unit Technical Support Document 18Citation30, MAICs can be used to carry out either an “anchored” ITC, where there is a common comparator arm in each trial, or an “unanchored” ITC, where there is a disconnected treatment network or single-arm studiesCitation29,Citation30. Due to the lack of a common comparator arm (same treatment) in the ETHOS and IMPACT studies (disconnected network; ), an anchored analysis was deemed infeasible. As such, an unanchored ITC method () was selected as the primary approach. A MAIC was utilized as the primary unanchored ITC method, and an unadjusted ITC was conducted as a supplementary analysis to support the primary MAIC.
Figure 1. Network of studies for ETHOS and IMPACT.
BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; FF, fluticasone furoate; GFF, glycopyrrolate/formoterol fumarate; ITC, indirect treatment comparison; MAIC, matching-indirect treatment comparison; UMEC, umeclidinium; VI, vilanterol
Indirect treatment comparisons
Primary analysis: MAIC
The primary analysis compared mortality risk reduction in patients treated with a licensed dose of BGF (320/18/9.6 µg) from ETHOS versus mortality risk reduction in patients treated with a licensed dose of FF/UMEC/VI (100/62.5/25 µg) from IMPACT using a MAIC, which mitigated the impact of interstudy population heterogeneity. The analysis utilized on- and off-treatment data from the final retrieved dataset from the intention-to-treat (ITT) populations of both studies over 52 weeks, using published mortality analyses for ETHOS and IMPACTCitation23,Citation24. The final retrieved datasets included the original datasets (the datasets established at database lock) plus additional data retrieved for patients missing Week 52 vital status, resulting in data for 99.6% of the ITT populations for each study.
For BGF, IPD from ETHOS was adjusted according to aggregate FF/UMEC/VI data from IMPACT, following the general steps described by Signorovitch et al.Citation32 and Phillippo et al.Citation29. In brief, to re-weight the BGF arm from ETHOS so it matched the population characteristics of the FF/UMEC/VI arm from IMPACT, balancing weights were derived from a propensity score-type logistic regression equation that predicted whether a given patient type originated from the index study (ETHOS) or the comparator study (IMPACT) as a function of baseline characteristics. The weights were used to calculate the effective study sample size, and the weighted average of baseline characteristics was compared with target values from the relevant comparator study arm.
A listing of 11 weighted baseline covariates (sex, BMI, smoking status, race [Asian, White, Other], percent bronchodilator reversibility, severe exacerbation history in the past 12 months, and COPD severity [moderate, severe, very severe]; see Supplementary Table 6) was derived and selected by clinical expert opinion and statistical measures of large deviation. These covariates were used for adjustment if there was a standardized mean difference (SMD) >0.1 between the ETHOS and IMPACT populationsCitation33. Balancing weights were applied to derive adjusted outcome estimates.
Web plot DigitizerCitation34 converted a Kaplan–Meier curve image for FF/UMEC/VI from the IMPACT study into x- and y-coordinates (i.e. time and survival probabilities), with the digitized curve overlaid and compared to the original image to ensure accuracy. Following methods described by Guyot et al.Citation35, pseudo-IPD were generated from the coordinates for each curve and checked for accuracy by plotting the resulting Kaplan–Meier curves against the published plot. Relative effects on mortality between BGF and FF/UMEC/VI were quantified using HRs with 95% CIs. As the balancing weights were not case weights, robust standard errors were used for HRs.
For the unadjusted supplementary analysis, IPD for BGF from ETHOS were directly compared with pseudo-IPD extracted from a Kaplan–Meier curve of FF/UMEC/VI from IMPACT.
Sensitivity analyses
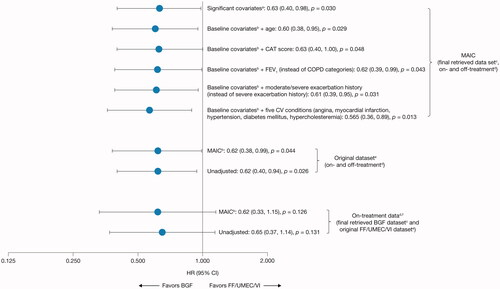
Several sensitivity analyses were performed to test the robustness of the primary analysis (). First, sensitivity analyses considered the final retrieved dataset (on- and off-treatment data) with different covariates to the primary analysis, namely: only the significantly imbalanced baseline covariates (as measured by a standardized mean difference >0.1) of sex, BMI, bronchodilator reversibility, and categorical COPD severity; 11 baseline covariates plus age; 11 baseline covariates plus COPD Assessment Test (CAT) score; 11 baseline covariates with forced expiratory volume in 1 s (FEV1) included instead of categorical COPD severity; 11 baseline covariates with moderate/severe exacerbation history included instead of severe exacerbation history; and 11 baseline covariates plus five cardiovascular (CV) conditions (angina, myocardial infarction, hypertension, diabetes mellitus, and hypercholesterolemia). Second, sensitivity analyses considered the original dataset (the dataset established at database lock; on- and off-treatment data). Third, sensitivity analyses considered only on-treatment mortality outcomes with the final retrieved dataset for BGF and the original dataset for FF/UMEC/VI.
Table 1. Summary of primary MAIC and sensitivity analyses.
Statistical analyses
Statistical analyses were performed using SAS version 9.4 (https://support.sas.com/software/94/), and figures were generated using R version 4.0.2 (https://cran.r-project.org/bin/windows/base/old/4.0.2/). Significance testing was defined using a two-tailed p-value of <0.05, and all between-group comparisons are reported using HRs with Wald-type 95% CIs and p-values. All analyses were conducted post-hoc and are not adjusted for the potential inflation of Type I error rate due to multiple testing.
Results
Indirect mortality risk reduction comparison
Primary MAIC and unadjusted analyses
The primary MAIC analysis demonstrated a statistically significant 39% reduction for on- and off-treatment-specific all-cause mortality with BGF versus FF/UMEC/VI in the final retrieved dataset (HR [95% CI]: 0.61 [0.38, 0.95], p = 0.030; ). Supporting the primary analysis results, the unadjusted analysis also demonstrated a statistically significant 39% reduction for on- and off-treatment specific all-cause mortality with BGF versus FF/UMEC/VI in the final retrieved data set (HR [95% CI]: 0.61 [0.41, 0.92], p = 0.019; ).
Figure 2. Kaplan–meier curves and hazard ratios for all-cause mortality (MAICa,b and unadjusted analyses) for BGF from ETHOS versus FF/UMEC/VI from IMPACT over 52 weeks (ITT populationc).
aBGF was adjusted according to aggregate FF/UMEC/VI data from IMPACT for sex, body mass index, smoking status, race (Asian, White, Other), severe exacerbation history in the last 12 months, bronchodilator reversibility, and COPD severity (moderate, severe, very severe).
bIn the MAIC analysis, absolute risk reduction of BGF versus FF/UMEC/VI at week 52 was 0.009 (95% CI [Greenwood SE]: 0.0016, 0.0164), corresponding to a number needed to treat of 112. MAIC weights were scaled to the original sample size when computing the SE so that they are representative of the quantity of data available.
cBoth analyses used on- and off-treatment data in the final retrieved data, which included 99.6% of data from the ITT populations of both ETHOS and IMPACT.
BGF, budesonide/glycopyrrolate/formoterol fumarate 320/18/9.6 μg; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FF/UMEC/VI, fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 μg; HR, hazard ratio; ITT, intention-to-treat; MAIC, matching-adjusted indirect comparison; SE, standard error.
![Figure 2. Kaplan–meier curves and hazard ratios for all-cause mortality (MAICa,b and unadjusted analyses) for BGF from ETHOS versus FF/UMEC/VI from IMPACT over 52 weeks (ITT populationc).aBGF was adjusted according to aggregate FF/UMEC/VI data from IMPACT for sex, body mass index, smoking status, race (Asian, White, Other), severe exacerbation history in the last 12 months, bronchodilator reversibility, and COPD severity (moderate, severe, very severe).bIn the MAIC analysis, absolute risk reduction of BGF versus FF/UMEC/VI at week 52 was 0.009 (95% CI [Greenwood SE]: 0.0016, 0.0164), corresponding to a number needed to treat of 112. MAIC weights were scaled to the original sample size when computing the SE so that they are representative of the quantity of data available.cBoth analyses used on- and off-treatment data in the final retrieved data, which included 99.6% of data from the ITT populations of both ETHOS and IMPACT.BGF, budesonide/glycopyrrolate/formoterol fumarate 320/18/9.6 μg; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FF/UMEC/VI, fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 μg; HR, hazard ratio; ITT, intention-to-treat; MAIC, matching-adjusted indirect comparison; SE, standard error.](/cms/asset/8aeaf035-fe21-4fd4-8472-313912ea97bb/icmo_a_2247969_f0002_c.jpg)
Sensitivity analyses
Overall, the sensitivity analyses were highly consistent with, and supportive of, the primary analyses (). The MAIC analysis including significantly imbalanced univariate variables (SMD >0.1) showed a 37% reduction for on- and off-treatment specific all-cause mortality for BGF versus FF/UMEC/VI in the final retrieved dataset (), and MAIC analyses adding age, FEV1 (instead of COPD severity), moderate/severe exacerbation history (instead of severe exacerbation history), CAT score, or CV conditions to the primary baseline covariates demonstrated reductions ranging from 37–43.5% for on- and off-treatment specific all-cause mortality for BGF versus FF/UMEC/VI in the final retrieved dataset (). MAIC and unadjusted analyses using the original dataset only or the original dataset for FF/UMEC/VI and the final retrieved dataset for BGF demonstrated on- and off-treatment or on-treatment specific reductions in all-cause mortality ranging from 35–38% for BGF versus FF/UMEC/VI ().
Figure 3. Hazard ratios for all-cause mortality (MAIC and unadjusted sensitivity analyses) for BGF from ETHOS versus FF/UMEC/VI from IMPACT (ITT population).
aSignificant covariates (as measured by a standardized mean difference >0.1): sex, body mass index, bronchodilator reversibility, and COPD severity category (moderate, severe, very severe).
bBGF was adjusted according to aggregate FF/UMEC/VI data from IMPACT for sex, race (Asian, White, Other), body mass index, smoking status, severe exacerbation history in the last 12 months, bronchodilator reversibility, and COPD severity (moderate, severe, very severe).
cThe final retrieved datasets included the original datasets plus additional data retrieved for patients missing Week 52 vital status, resulting in data for 99.6% of the ITT populations for each study.
dA death was defined as “on-treatment” in IMPACT if the date of death occurred ≤7 days after the last treatment day and was considered “off-treatment” if the date of death occurred >7 days after the last treatment day and up to within 7 days of the projected Week 52 dateCitation24. In ETHOS, time to all-cause death was a prespecified secondary endpoint and was assessed in the ITT population using the treatment policy estimand, which included all randomized patients who received any amount of study drug and all observed data within 52 weeks of randomization regardless of whether patients remained on randomized treatmentCitation23. Data from within 52 weeks of randomization was used for the on- and off-treatment analyses.
eDataset established at database lock.
fA 7-day data cut-off from ETHOS for on-treatment sensitivity analysis was used to be consistent with the definition in IMPACT.
BGF, budesonide/glycopyrrolate/formoterol fumarate 320/18/9.6 μg; CAT, COPD Assessment Test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FF/UMEC/VI, fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 μg; HR, hazard ratio; ITT, intention-to-treat; MAIC, matching-adjusted indirect comparison.
Discussion
To date, three fixed-dose triple combination therapies have been approved for the treatment of COPDCitation20–22. In well-conducted RCTs, two of these therapies, BGF in ETHOSCitation23 and FF/UMEC/VI in IMPACTCitation24, demonstrated evidence of reduced all-cause mortality risk versus LAMA/LABA dual therapy in patients with COPD. For the third approved triple therapy (i.e. BDP/GLY/FF), all-cause mortality was not examined as a prespecified efficacy endpoint, and a post-hoc safety analysis of the risk of developing a fatal event reported a numerical, but not statistically significant, risk reduction for fatal events versus therapies not containing ICSCitation25.
For these analyses, ETHOSCitation14,Citation23 and IMPACTCitation16,Citation24 were identified through a clinical SLR as two studies that reported all-cause mortality as an efficacy endpoint in large randomized sample sizes (8588 and 10,355 patients, respectively) with a 52-week study duration; the eligibility criteria for the studies were broadly similar, with nuanced differences for prior COPD maintenance therapies, FEV1%, and exacerbation history (Supplementary Table 1). Both studies enrolled broadly similar populations and demonstrated reduced mortality for ICS/LAMA/LABA triple therapy versus LAMA/LABA dual therapies in patients with COPD, and both datasets comprehensively included 99.6% of the ITT populationsCitation23,Citation24.
The current ITCs utilized two unanchored methods: a MAIC with 11 covariates compared IPD for BGF from ETHOS with aggregate FF/UMEC/VI pseudo-IPD from IMPACT generated from digitized Kaplan–Meier curves as the primary analysis, and an unadjusted supplementary analysis. Both methods suggest statistically significant reductions of 39% in all-cause mortality risk with BGF versus FF/UMEC/VI in patients with moderate-to-very severe COPD (MAIC analysis: HR [95% CI]: 0.61 [0.38, 0.95], p = 0.030; unadjusted analysis: HR [95% CI]: 0.61 [0.41, 0.92], p = 0.019; ). The findings of several sensitivity analyses supported the primary MAIC analysis, with estimated reductions in all-cause mortality ranging from 35% to 43.5% for BGF versus FF/UMEC/VI ().
Given the pathophysiological interrelatedness of COPD and cardiac eventsCitation36, we examined the prevalence of CV conditions in the ETHOS and IMPACT populations, even though patients with significant cardiac risk were mostly excluded from both studies. Although not all CV conditions were defined/classified in the same way in ETHOS and IMPACT, both study populations had similarly high percentages of patients with ≥1 CV condition (70% vs 67% in ETHOS and IMPACT, respectively; Supplementary Table 7), and the frequency of the most frequently reported CV comorbidities (hypertension: ETHOS, 59%, IMPACT, 51%; hypercholesterolemia: ETHOS, 36%, IMPACT, 33%; diabetes mellitus: ETHOS, 19%, IMPACT, 15%) were similar. Additionally, the percentage of patients with a history of myocardial infarction, an important CV conditionCitation37, was similar in ETHOS (7%) and IMPACT (7%). Also, although New York Heart Association class III heart failure was exclusionary in ETHOS and not in IMPACT, no congestive heart failure-related mortalities were reported in patients treated with FF/UMEC/VI in IMPACTCitation16,Citation24. To test the robustness of the findings from the primary analysis with regard to CV conditions, an additional sensitivity analysis of the MAIC was conducted through population adjustment for five CV conditions that were similarly defined in ETHOS and IMPACT (angina, hypertension, hypercholesterolemia, diabetes mellitus, and myocardial infarction). The results of this analysis () were consistent with those of the primary MAIC analysis.
To the best of our knowledge, these analyses represent the first MAIC of a fixed-dose triple therapy with a primary focus on reductions in all-cause mortality. However, two published studies lend support for the differences in mortality rates between BGF and FF/UMEC/VI observed in this MAICCitation38,Citation39. First, in a real-world comparative effectiveness and safety study of fluticasone-based versus budesonide-based triple therapies, budesonide-based therapy was associated with a lower incidence of all-cause mortality than fluticasone-based therapy (HR [95% CI], 0.80 [0.66, 0.98]) in patients with COPD who did not have a history of exacerbationsCitation39. Second, in a meta-analysis of the association of ICS with all-cause mortality in patients with COPD, budesonide was associated with a reduction in all-cause mortality risk compared with therapies that did not include ICS (Peto odds ratio [OR] [95% CI]: 0.75 [0.59, 0.94]). In contrast, fluticasone propionate (OR [95% CI]: 0.96 [0.86, 1.08]), fluticasone furoate (OR [95% CI]: 0.91 [0.81, 1.01]), mometasone furoate (OR [95% CI]: 0.84 [0.46, 1.51]), or beclomethasone dipropionate (OR [95% CI]: 0.75 [0.49, 1.14]) were not associated with significant reductions in all-cause mortality riskCitation38. The authors speculated that differences in safety profiles of budesonide compared with other ICSs, for example the reduced risk of pneumonia with budesonide use versus other ICSsCitation40, may be associated with the comparatively greater mortality risk reduction.
Two previously published NMAs by Lee et al.Citation27 and Rogliani et al.Citation28, both utilizing a traditional Bayesian approach using aggregate-level data, reported no significant differences in mortality reductions across various ICS/LAMA/LABA treatmentsCitation27,Citation28. However, those analyses did not only consider mortality as an efficacy outcome (all types of mortality events, e.g. adverse events, were evaluated within a single category) and/or included studies with durations of <52 weeksCitation27,Citation28, which can be limitations when measuring mortality outcomes. Further, the studies included in those analyses are heterogenous in terms of study designs, durations, and populations. For example, Rogliani et al. considered four studies of ≥24 weeks duration (ETHOS, IMPACT, KRONOS, and TRILOGY). In terms of study populations, ETHOS, KRONOS, and IMPACT included patients with moderate-to-very-severe COPD, whereas TRILOGY included patients with severe or very severe COPD (<50% FEV1 predicted). Furthermore, ETHOS, IMPACT, and TRILOGY also included patients with a history of COPD exacerbations within the last 12-months, but this was not an inclusion criterion in KRONOS (in which 74% of patients had no history of recent exacerbations). Lastly, Lee et al. did not include the ETHOS study, one of the two largest 52-week studies providing new evidence on mortality risk reductions in COPD. The current analyses overcame these limitations by focusing on two large 52-week randomized controlled studies that had all-cause mortality reduction as a prespecified efficacy outcome and by adjusting data from ETHOS at the individual patient level to match the patient characteristics of the IMPACT study, providing a more homogenous pair of comparator populations. Importantly, the comparable results from the adjusted and unadjusted analyses indicate low heterogeneity between ETHOS and IMPACT, which is not surprising given the relative similarity in design and timelines of these studies. Overall, given the different approaches used to account for heterogeneity and the information sources and assumptions used, it is perhaps not unexpected that the results from the current analyses differ from previous reports.
However, to compare our results with anchored methods used by Lee et al.Citation27 and Rogliani et al.Citation28, we further explored an anchored MAIC using the ETHOS and IMPACT studies (see Supplementary Methods – Anchored MAIC Analysis for a brief summary). Due to the lack of a connected network given the different comparator arms in the ETHOS and IMPACT studies, an additional assumption, which was not required in the unanchored analyses, was made to connect the network via a LAMA/LABA (class effect) arm. Under this assumption, LAMA/LABAs are considered as common comparators (Supplementary Figure 2). However, it should be noted that while the LAMA/LABA arms had similar exacerbation rates in a head-to-head studyCitation41, they had different estimated mortality rates in ETHOS and IMPACT (approximately a 20% relative difference in the patients with all-cause mortality event rates)Citation23,Citation24, and the comparative efficacy between LAMA/LABA fixed-dose combinations on mortality reported in the literature is still inconclusiveCitation42,Citation43. The results of this anchored MAIC analysis were supportive of the unanchored MAIC, with a 35% reduction in all-cause mortality for BGF versus FF/UMEC/VI (Supplementary Table 8).
As the current data point to improved all-cause mortality outcomes with BGF versus FF/UMEC/VI, it is important to consider what may account for this observed difference, i.e. the different active components included in each therapy. Previous reports suggest budesonide-based therapy may be more efficacious in reducing exacerbation rates and/or mortality risk than fluticasone-based therapiesCitation39,Citation44,Citation45. It can be postulated that these differences arise from budesonide’s pharmacodynamic and pharmacokinetic properties; for example, compared with fluticasone furoate, budesonide may be less locally immunosuppressive, and thus potentially less likely to facilitate infection-induced exacerbations, and less lipophilicCitation45. Additionally, budesonide has a more rapid onset of action and shorter half-life, thus facilitating its proportional dose-response profile and enabling superior capacity to counter inflammatory fluctuations at both the blood and bone marrow levels without a sufficiently long bioavailability to initiate systemic adverse effectsCitation44,Citation45. Although beyond the scope of the current study, future analyses of subgroups defined by lung function, rescue medication use, treatment adherence, cardiovascular comorbidities, or exacerbation patterns may provide additional insight into the observed treatment differences.
Differences in airway penetration and greater airway deposition of BGF compared with FF/UMEC/VI may also contribute to the current findings. In ETHOS, treatment with a higher ICS dose (budesonide 320 µg) in BGF was shown to confer a mortality reduction versus LAMA/LABA dual therapy, but a lower ICS dose (budesonide 160 µg) did notCitation23, even though both doses similarly reduced moderate/severe exacerbation ratesCitation14. This suggests the absolute ICS dose delivered to the lung may have differential effects on mortality reduction versus on exacerbation rate reduction. Moreover, in silico modelling has demonstrated BGF has greater total lung deposition (47.0–49.2% of the delivered dose across different inhalation profiles) for each active component compared with FF/UMEC/VI (21.4–23.7%), with the largest magnitude differences observed for the ICS components, particularly in the small airways, where there was approximately 4-fold greater deposition as measured by a percentage of the delivered doseCitation46. Finally, the co-suspension delivery technology utilized to deliver BGF facilitates a consistent and high level of drug deliveryCitation20,Citation47,Citation48. Taken together, this suggests greater delivery of the ICS component of BGF, and possibly also the LAMA and LABA components, compared with that of FF/UMEC/VI throughout both small and large airways could contribute to the current findings.
There are limitations of this ITC that should be considered. First, as with any comparison of non-randomized treatment groups, ITCs can be biased by both observed and unobserved cross-trial differences. Despite balancing the observed patient characteristics through MAIC in our analyses, some unobservable differences may still exist between ETHOS and IMPACT. For example, there are differences in run-in periods and site locations (ETHOS was conducted in 26 countriesCitation14 and IMPACT was conducted in 37 countriesCitation16) between studies, although adjustments for race were conducted in the MAIC analysis in an attempt to overcome potential geographic differences. In addition, the studies were also conducted in different years, which could introduce bias. Moreover, the CV conditions sensitivity analysis may be limited by a lack of clarity regarding the severity level and potential therapies used for CV conditions in either study.
Second, this analysis includes assumptions inherent to all unanchored ITCs. Anchored and unanchored comparisons are distinguished according to whether a common comparator arm is used, with unanchored comparisons making the strong assumption that absolute outcomes can be predicted from the covariatesCitation30. To overcome this, as explained above, we further explored an anchored MAIC using the ETHOS and IMPACT studies. However, in this analysis, the anchored comparison had to accommodate the uncertainty in estimated quantities related to the common comparator assumption due to lack of the same control arm between ETHOS and IMPACT. Comparison of the Kaplan–Meier curves of the unanchored and the anchored analyses indicated that the anchored MAIC BGF curve deviated from the original BGF curve (unadjusted from ETHOS) and from the adjusted BGF curves used in the unanchored analyses (Supplementary Figure 3), showing that assumed proportional hazards in the anchored analysis increases the risk of bias.
The primary MAIC analysis utilized on- and off-treatment data from the ITT population for both ETHOS and IMPACT as prespecified primary analyses, whereas on-treatment analysis was assessed as exploratory. In this regard, it should be noted that in analyses comparing BGF to FF/UMEC/VI using only on-treatment data from the final retrieved BGF dataset and the original FF/UMEC/VI dataset, statistical significance was not observed. On-treatment datasets exclude information from data collected post treatment and thus exclude a number of deaths and reduce the number of events and power. Whereas, survival analyses primarily consider both on- and off-treatment data, which contain more information and is unbiased from the perspective of assuming that the risk for the event of death is independent of continued treatment.
Neither ETHOS nor IMPACT were designed to assess mortality as the primary endpoint; however, both studies included planned follow-ups to determine vital status regardless of treatment or study discontinuation. ETHOS included all-cause mortality as a prespecified secondary endpoint, while mortality was a prespecified “other” efficacy endpoint in IMPACTCitation24,Citation49. Therefore, the sample size was smaller than required to detect a clinically meaningful impact of treatment (statistical power <0.80). However, not being powered for the analysis did not diminish from the observed signal, and it should be noted that it is very common for ITCs to include secondary or other endpoints from RCTs.
The current research provides an important contribution to the published literature by using a MAIC approach, which leads to more balanced and homogeneous populations across different studies and increases the robustness of ITCs. The MAIC can be considered a strong tool to adjust for the impact of covariates compared with meta-regression techniques. In addition, the current research used a large effective sample size for BGF after adjustment and a large number of matching covariates, included several sensitivity analyses and additional analysis with a different methodology to determine the consistency and robustness of the primary results, and assessed data from the two large one-year RCTs with prespecified all-cause mortality efficacy endpoints. Taken together, these findings provide new evidence on mortality risk reduction with fixed-dose triple combinations, as recognized by GOLD 2023 reportCitation1.
Conclusion
In conclusion, this MAIC demonstrated a statistically significantly greater all-cause mortality risk reduction with BGF compared with FF/UMEC/VI in patients with moderate-to-very-severe COPD. Given that COPD elevates risk of cardiopulmonary events and continues to exert a considerable mortality burdenCitation3,Citation9,Citation12,Citation13,Citation50, these findings provide important evidence on the comparative magnitude of all-cause mortality reduction for two of the three approved triple therapies for COPD and may help inform clinical decision making.
Transparency
Author contributions
Daiana Stolz was involved in the Conceptualization, Methodology, Supervision, Data visualization, and Writing – review and editing of the analysis and manuscript. Erik Hermansson was involved in the Data curation, Formal analysis, Investigation, Methodology, Data visualization, and Writing – review and editing of the analysis and manuscript. Mario Ouwens was involved in the Investigation, Methodology, Data visualization, and Writing – review and editing of the analysis and manuscript. Barinder Singh was involved in the Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Data visualization, and Writing – review and editing of the analysis and manuscript. Akanksha Sharma was involved in the Conceptualization, Data curation, Formal analysis, Methodology, Data visualization, and Writing – review and editing of the analysis and manuscript. Dan Jackson was involved in the Investigation, Methodology, and Writing – review and editing of the analysis and manuscript. Patrick Darken was involved in the Conceptualization, Methodology, Supervision, and Writing – review and editing of the analysis and manuscript. Jonathan Marshall was involved in the Conceptualization, Investigation, Supervision, and Writing – review and editing of the analysis and manuscript. Karin Bowen was involved in the Supervision and Writing – review and editing of the analysis and manuscript. Hana Müllerová was involved in the Conceptualization, Methodology, and Writing – review and editing of the analysis and manuscript. Bernardino Alcázar Navarrete was involved in the Supervision, and Writing – review and editing of the analysis and manuscript. Richard Russell was involved in the Conceptualization, and Writing – review and editing of the analysis. MeiLan K. Han was involved in the Conceptualization and Writing – review and editing of the analysis and manuscript. Deniz Tansey-Dwyer was involved in the Conceptualization, Methodology, Project administration, Supervision, and Writing – review and editing of the analysis and manuscript.
Acknowledgements
The authors thank Andrey Pavlov from the Everest Clinical Research team for his valuable contributions to the validation of the analyses and Michel Pszczol, Sr and James Cranfield, both of whom were compensated by AstraZeneca for their time, for their review of the plain language summary as people living with COPD. Medical writing support, under the guidance of the authors, was provided by Daniel Spindlow, MSc, CMC Connect, a division of IPG Health Medical Communications, funded by AstraZeneca, in accordance with Good Publication Practice (GPP 2022) guidelinesCitation51.
Ethics statement
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplemental Material
Download MS Word (342.1 KB)Declaration of funding
This analysis was sponsored by AstraZeneca. The sponsor was involved in the design, collection, analysis, and interpretation of data; the writing of the report; and in the decision to submit the article for publication. Medical writing support for the development of this manuscript was funded by AstraZeneca.
Declaration of financial/other relationships
Daiana Stolz reports participation in clinical research grants to the institution by AstraZeneca, Boston Scientifics, Curetis AG, and Swiss National Foundation; is an advisory board member for Almirall, AstraZeneca, Bayer, Berlin-Chemie AG, Boehringer Ingelheim, Chiesi, CSL Behring, Curetis, GlaxoSmithKline, MSD, Novartis, Sanofi, and Vifor; is a consultant and has been invited to lecture for Almirall, AstraZeneca, Bayer, Behring, Berlin-Chemie AG, Boehringer Ingelheim, Chiesi, Curetis AG, CSL, GlaxoSmithKline, MSD, Novartis, Sanofi, and Vifor; is a speaker and advisory panel member for Almirall, AstraZeneca, Bayer, Behring, Berlin-Chemie AG, Boehringer Ingelheim, CSL, Curetis AG, GlaxoSmithKline, MSD, Novartis, Sanofi, and Vifor; has received grants from AstraZeneca, Boston Scientific, and Curetis; and has received lecture fees from AstraZeneca, GSK, Novartis, Pfizer, Roche, Schwabe Pharma, Vifor, and Zambon. Erik Hermansson, Mario Ouwens, Dan Jackson, Patrick Darken, Jonathan Marshall, Karin Bowen, Hana Müllerová, and Deniz Tansey-Dwyer are employees of AstraZeneca and own stock/stock options in the company. Barinder Singh and Akanksha Sharma are employees of Pharmacoevidence Pvt. Ltd, which was funded by AstraZeneca to conduct these analyses. Bernardino Alcázar Navarrete reports grants from AstraZeneca and GlaxoSmithKline; is an advisory committee member for AstraZeneca, Boehringer Ingelheim, Gilead, and GlaxoSmithKline; and is a speaker for AstraZeneca, Boehringer Ingelheim, Chiesi, Gilead, and GlaxoSmithKline. Richard Russell is an advisory committee member, trial safety monitoring board member for AstraZeneca and is a speaker for AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. MeiLan K. Han reports personal consulting fees from Aerogen, Altesa Biopharma, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, DevPro, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Merck, Mylan, NACE, Novartis, Polarian, Pulmonx, Regeneron, Sanofi, Teva, UpToDate, and Verona; has received either in kind research support or funds paid to the institution from the American Lung Association, AstraZeneca, Biodesix, Boehringer Ingelheim, the COPD Foundation, the NIH, Novartis, Nuvaira, Sanofi, Sunovion, and Gala Therapeutics; has participated in data safety monitoring boards for Medtronic and Novartis with funds paid to the institution; and has received stock options from Altesa Biopharma and Meissa Vaccines.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.
References
- Global Initiative for Chronic Obstructive Lung Disease. 2023 GOLD Report. Global strategy for prevention, diagnosis and management of COPD [Internet]. 2023 [cited November 20, 2022]. Available from: https://goldcopd.org/2023-gold-report-2/.
- Safiri S, Carson-Chahhoud K, Noori M, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the global burden of disease study 2019. BMJ. 2022;378:e069679. doi: 10.1136/bmj-2021-069679.
- Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7.
- Rothnie KJ, Connell O, Müllerová H, et al. Myocardial infarction and ischemic stroke after exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2018;15(8):935–946. doi: 10.1513/AnnalsATS.201710-815OC.
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883.
- Feary JR, Rodrigues LC, Smith CJ, et al. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi: 10.1136/thx.2009.128082.
- Berry CE, Wise RA. Mortality in COPD: causes, risk factors, and prevention. COPD. 2010;7(5):375–382. doi: 10.3109/15412555.2010.510160.
- McGarvey LP, John M, Anderson JA, et al. Ascertainment of cause-specific mortality in COPD: operations of the TORCH clinical endpoint committee. Thorax. 2007;62(5):411–415. doi: 10.1136/thx.2006.072348.
- Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):51–57. doi: 10.1164/rccm.201711-2239OC.
- Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi: 10.1136/thoraxjnl-2011-201518.
- Whittaker H, Rubino A, Müllerová H, et al. Frequency and severity of exacerbations of COPD associated with future risk of exacerbations and mortality: a UK routine health care data study. Int J Chron Obstruct Pulmon Dis. 2022;17:427–437. doi: 10.2147/COPD.S346591.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442.
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9.
- Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi: 10.1056/NEJMoa1916046.
- Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi: 10.1016/S2213-2600(18)30327-8.
- Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901.
- Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi: 10.1164/rccm.201703-0449OC.
- Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi: 10.1016/S0140-6736(18)30206-X.
- Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. doi: 10.1016/S0140-6736(16)31354-X.
- AstraZeneca Pharmaceuticals LP. Breztri Aerosphere™ Prescribing Information [Internet]. 2020 [cited June 13, 2023]. Available from: https://www.azpicentral.com/breztri/breztri.pdf.
- GlaxoSmithKline. Anoro® Ellipta® Prescribing Information [Internet]. 2019 [cited December 15, 2022]. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Anoro_Ellipta/pdf/ANORO-ELLIPTA-PI-MG-IFU.PDF.
- Chiesi Limited. Trimbow 87 µg/5 µg/9 µg pressurised inhalation solution SmPC [Internet]. 2022 [cited October 31, 2022]. Available from: https://www.medicines.org.uk/emc/product/761/smpc.
- Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021;203(5):553–564. doi: 10.1164/rccm.202006-2618OC.
- Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201(12):1508–1516. doi: 10.1164/rccm.201911-2207OC.
- Vestbo J, Fabbri L, Papi A, et al. Inhaled corticosteroid containing combinations and mortality in COPD. Eur Respir J. 2018;52(6):1801230. doi: 10.1183/13993003.01230-2018.
- Usmani OS, Roche N, Jenkins M, et al. Consistent pulmonary drug delivery with whole lung deposition using the aerosphere inhaler: a review of the evidence. Int J Chron Obstruct Pulmon Dis. 2021;16:113–124. doi: 10.2147/COPD.S274846.
- Lee HW, Kim HJ, Jang EJ, et al. Comparisons of efficacy and safety between triple (inhaled corticosteroid/long-acting muscarinic antagonist/long-acting beta-agonist) therapies in chronic obstructive pulmonary disease: systematic review and bayesian network meta-analysis. Respiration. 2021;100(7):631–643. doi: 10.1159/000515133.
- Rogliani P, Ora J, Cavalli F, et al. Comparing the efficacy and safety profile of triple fixed-dose combinations in COPD: a meta-analysis and IBiS score. J Clin Med. 2022;11(15):4491. doi: 10.3390/jcm11154491.
- Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–211. doi: 10.1177/0272989X17725740.
- Phillippo D, Ades T, Dias S, et al. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. Commissioned report. Decision Support Unit, ScHARR, University of Sheffield: NICE Decision Support Unit; 2016.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947. doi: 10.1016/j.jval.2012.05.004.
- Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28(10):935–945. doi: 10.2165/11538370-000000000-00000.
- Zhang Z, Kim HJ, Lonjon G, et al. Balance diagnostics after propensity score matching. Ann Transl Med. 2019;7(1):16. doi: 10.21037/atm.2018.12.10.
- Rohatgi A. WebPlotDigitizer. v4.6. 2022. Available from: https://automeris.io/WebPlotDigitizer/.
- Guyot P, Ades AE, Ouwens MJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Medical Res Methodol. 2012;12(1):1–13.
- André S, Conde B, Fragoso E, et al. COPD and cardiovascular disease. Pulmonology. 2019;25(3):168–176. doi: 10.1016/j.pulmoe.2018.09.006.
- Böhm M, Schumacher H, Teo KK, et al. Cardiovascular outcomes in patients at high cardiovascular risk with previous myocardial infarction or stroke. J Hypertens. 2021;39(8):1602–1610. doi: 10.1097/HJH.0000000000002822.
- Chen H, Deng ZX, Sun J, et al. Association of inhaled corticosteroids with all-cause mortality risk in patients with COPD: a meta-analysis of 60 randomized controlled trials. Chest. 2023;163(1):100–114. doi: 10.1016/j.chest.2022.07.015.
- Suissa S, Dell’Aniello S, Ernst P. Fluticasone-based versus budesonide-based triple therapies in COPD: real-world comparative effectiveness and safety. COPD. 2022;19(1):109–117. doi: 10.1080/15412555.2022.2035705.
- Chen H, Sun J, Huang Q, et al. Inhaled corticosteroids and the pneumonia risk in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12:691621. doi: 10.3389/fphar.2021.691621.
- Maltais F, Ferguson GT, Feldman GJ, et al. A randomized, double-blind, double-dummy study of glycopyrrolate/formoterol fumarate metered dose inhaler relative to umeclidinium/vilanterol dry powder inhaler in COPD. Adv Ther. 2019;36(9):2434–2449. doi: 10.1007/s12325-019-01015-3.
- Gong Y, Lv Y, Liu H, et al. Quantitative analysis of efficacy and safety of LABA/LAMA fixed-dose combinations in the treatment of stable COPD. Ther Adv Respir Dis. 2022;16. doi: 10.1177/17534666211066068.
- Lee HW, Park J, Jang EJ, et al. Comparisons of exacerbations and mortality among LAMA/LABA combinations in stable chronic obstructive pulmonary disease: systematic review and bayesian network meta-analysis. Respir Res. 2020;21(1):310. doi: 10.1186/s12931-020-01540-8.
- Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584–594. doi: 10.1111/joim.12067.
- Brattsand R, Selroos O. May a different kinetic mode explain the high efficacy/safety profile of inhaled budesonide? Pulm Pharmacol Ther. 2022;77:102167. doi: 10.1016/j.pupt.2022.102167.
- Marshall J, Li G, Backer JD, et al. Small airways deposition of two fixed-dose triple therapy combinations assessed with functional respiratory imaging (FRI). American Throracic Society International Conference 2023 (poster presentation P406). doi: 10.1164/ajrccm-conference.2023.207.1_MeetingAbstracts.A1556.
- Doty A, Schroeder J, Vang K, et al. Drug delivery from an innovative LAMA/LABA co-suspension delivery technology fixed-dose combination MDI: evidence of consistency, robustness, and reliability. AAPS PharmSciTech. 2018;19(2):837–844. doi: 10.1208/s12249-017-0891-1.
- Taylor G, Warren S, Dwivedi S, et al. Gamma scintigraphic pulmonary deposition study of glycopyrronium/formoterol metered dose inhaler formulated using co-suspension delivery technology. Eur J Pharm Sci. 2018;111:450–457. doi: 10.1016/j.ejps.2017.10.026.
- Rabe KF, Martinez FJ, Ferguson GT, et al. A phase III study of triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler 320/18/9.6 μg and 160/18/9.6 μg using co-suspension delivery technology in moderate-to-very severe COPD: the ETHOS study protocol. Respir Med. 2019;158:59–66. doi: 10.1016/j.rmed.2019.08.010.
- Mannino DM, Doherty DE, Sonia Buist A. Global initiative on obstructive lung disease (GOLD) classification of lung disease and mortality: findings from the atherosclerosis risk in communities (ARIC) study. Respir Med. 2006;100(1):115–122. doi: 10.1016/j.rmed.2005.03.035.
- DeTora LM, Toroser D, Sykes A, et al. Good publication practice (GPP) guidelines for company-sponsored biomedical research: 2022 update. Ann Intern Med. 2022;175(9):1298–1304. doi: 10.7326/M22-1460.
