Abstract
Background
Advancing age is a risk factor for developing non-valvular atrial fibrillation (NVAF) or acute venous thromboembolism (VTE). We assessed the comparative effectiveness, safety, costs, and healthcare utilization associated with rivaroxaban versus warfarin in patients of advanced age managed in the United States (US).
Methods
We conducted a systematic review of Medline and Embase through April 2023 to identify real-world evidence (RWE) studies of older adults (at least 65+ years of age) with either NVAF or VTE who received either rivaroxaban or warfarin in the US and reported an outcome of stroke or systemic embolism (SSE), ischemic stroke (IS), recurrent VTE, major bleeding, intracranial hemorrhage, costs, or healthcare resource utilization. We classified each outcome of interest per study as “positive” (lower risk), “negative” (higher risk), or “neutral” based upon the summary effect size of rivaroxaban versus warfarin.
Results
Twenty-nine RWE studies met inclusion criteria, mostly (83%) in NVAF populations. For SSE with rivaroxaban versus warfarin, 68.8% of studies showed positive effects and 31.2% showed neutral outcome. For major bleeding, 57.7% showed neutral effects, 38.5% showed negative effects, and 3.8% of studies showed positive effects with rivaroxaban versus warfarin. Of the two studies reporting cost data, both were positive, showing lower costs for SSE for rivaroxaban versus warfarin and neutral cost for major bleeding costs.
Conclusions
This systematic review supports findings from subgroup analyses of randomized controlled trials that, compared with warfarin, rivaroxaban is associated with generally neutral or positive effects on thrombosis and a mixed picture on bleeding outcomes in older adults with either NVAF or VTE treated in the United States.
Introduction
Advancing age is a known risk factor for development of venous thromboembolismCitation1 (VTE) and stroke in non-valvular atrial fibrillation (NVAF)Citation2,Citation3. While clinical trials in NVAF and VTE have shown that stroke and major bleeding rates increase in older adults, the relative effectiveness of oral anticoagulants such as rivaroxaban compared with warfarin remain consistentCitation4,Citation5. While encouraging, these subgroup analyses may have had reduced power to detect meaningful differences. Information gained from real-world evidence (RWE) studies can augment randomized trial findings and may be beneficial in the context of oral anticoagulant use in older adultsCitation6,Citation7. We therefore conducted a systematic review of existing observational RWE studies comparing the effectiveness, safety, costs, and healthcare utilization associated with rivaroxaban versus warfarin in NVAF or acute VTE patients of advanced age managed in the United States. We limited the scope of our review to studies evaluating cohorts in the United States as anticoagulation practices may differ geographically and wanted, therefore, to limit heterogeneity related to this effect in the included studies. The findings of this systematic review may better inform shared decision making related to anticoagulation for older patients with NVAF and VTE treated within the United States.
Methods
This report was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statementCitation8.
Information sources and search strategy
Searches for potentially eligible studies and meta-analyses were performed in Medline and Embase from 1 January 2010 through 31 April 2023 and limited to full-text papers published in English. Our Medline (OVID) search strategy is depicted in eTable 1. Both searches were augmented by hand searches of the reference sections of identified relevant studies (backwards citation tracking).
Selection process
Observational studies published in English comparing rivaroxaban versus warfarin in United States treated patients of advanced age (greater than or equal to 65 years of age) with either NVAF or acute VTE and reporting an outcome of interest were sought. We excluded publications of case studies, editorials, and review articles. Studies using rivaroxaban for short-term anticoagulation (i.e. cardioversion, catheter ablation, hip/knee arthroplasty) were excluded. Two investigators performed comprehensive literature searches, removed duplicates, and selected potential articles based on the title and abstract. Two independent reviewers then screened these selections for inclusion eligibility based on the full-text and extract study data using a standardized form. Any disagreement during the study process was resolved by consensus and/or consultation with a third reviewer.
Data collection and risk of bias assessment
Information was collected regarding study details (i.e. first author’s last name, year of publication, data set/source used), patient characteristics (e.g. demographic characteristics, mean/median age, primary population [NVAF and/or VTE], secondary population, if any [≥65, 75, 80 years, etc.], whether the study used a new (incidence) or prevalent user design, secondary stroke, diabetes, CHA2DS2VASc score, bleeding risk score), intervention details, outcomes, and confounder adjustment strategy utilized (propensity score matching, weighting, multivariable regression, etc.). It was anticipated that the above-mentioned study details would be missing for subgroup analyses stratified by age (making the study eligible for inclusion but prohibiting the reporting of many of the patients/study characteristics). In these cases, we reported demographics/characteristics for the advanced age population only (or not available otherwise).
Risk of bias assessment was performed using the validated Good ReseArch for Comparative Effectiveness (GRACE) checklist for evaluating the quality of observational studies for decision-making support (eTable 2)Citation9. A total of 11 items within two domains (data and methods) were evaluated. Studies were graded as “yes” meeting the criterion, “no”, or “not enough information in the article”. Each study was assessed by two investigators independently, with disagreements resolved through discussion or a third investigator.
Effect measures
The outcomes of interest for NVAF studies included stroke and/or systemic embolism (including ischemic stroke and intracranial hemorrhage [ICH]), ischemic stroke, major bleeding, ICH, extracranial bleeding, costs, and healthcare resource utilization. For VTE studies, the outcomes of interest included recurrent VTE, major bleeding, ICH, extracranial bleeding, costs, and healthcare resource utilization. For each outcome, definitions provided in the included studies were utilized. The point estimate (n, %; %/year, mean/median cost, or utilizations) in each treatment arm (rivaroxaban and warfarin) as well as the summary effect size (e.g. hazard ratio (HR) and 95% confidence interval (CI), absolute difference, etc.) were collected and reported.
Synthesis Methods
The direction of the association between rivaroxaban versus warfarin use (warfarin as referent) and each individual outcome identified in included studies was classified as “positive”, “negative”, or “neutral” based upon the summary effect sizeCitation10. “Positive” associations were defined as those in which the outcome improved in a statistically significantly manner with rivaroxaban versus warfarin use. An association was deemed “negative” if rivaroxaban was associated with a worsening of outcome that was statistically significant. If no statistically significant relationship is observed, the direction of association was deemed “neutral”. In all cases, the p-value used to conclude statistical significance was based on each study’s defined p-value. The frequencies (n, %) of each outcome were stratified by the direction of association with rivaroxaban. Subgroup analyses for each outcome were performed, separately in studies that evaluated an NVAF population and VTE population, as well as those that conducted their primary analysis using an intention-to-treat or on-treatment approach.
Results
Study characteristics
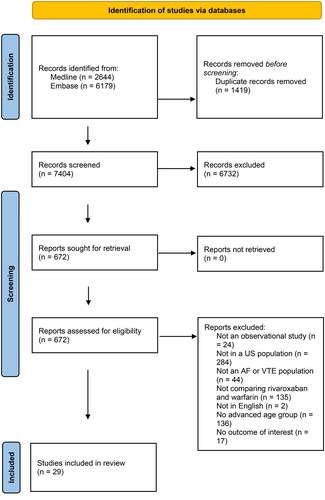
A total of 29 RWE studies met inclusion criteria ()Citation11–39. These studies represented a subset of the complete search of 7,404 abstracts for the full systematic review (). Twenty four (82.8%) studiesCitation11–16,Citation18–20,Citation22–28,Citation30–33,Citation35–38, were in an NVAF population with the remaining five (17.2%) studies in VTE populationsCitation17,Citation22,Citation29,Citation34,Citation39. Twelve (41.4%) studiesCitation19–22,Citation27,Citation29,Citation31,Citation33,Citation34,Citation36,Citation37,Citation39, reported findings on subgroups of advanced age. The sample sizes of the included studies ranged from 521Citation22 to 183,318Citation24 for the warfarin groups and 521Citation22 to 137,972Citation26 for the rivaroxaban groups. Nineteen (65.5%) studiesCitation12–19,Citation23–28,Citation31,Citation32,Citation34,Citation35,Citation38, conducted their primary analysis using an on-treatment approach with the remaining 10 (34.5%) studies using an intention-to-treat approachCitation11,Citation20–22,Citation29,Citation30,Citation33,Citation36,Citation37,Citation39. All but one studyCitation38 used a new (incident) anticoagulation user design.
Table 1. Characteristics of included studies.
Risk of bias
The results of the GRACE checklist are shown in . Only three (10.3%) studiesCitation22,Citation36,Citation37, reported information on both dose of rivaroxaban and time in therapeutic range of warfarin. All included studies adequately reported their primary outcomes, objectively measured the clinical outcomes, used outcome definitions that were previously validated or adjudicated, identified outcome measures equivalently between groups, and adjusted for known confounders or effect modifiers. A majority (28 of 29, 96.6%) included new anticoagulation users and used concurrent comparators. All studies also took important covariates, confounding, and effect modifying variables into account in the design/analysis and were free of immortal time bias. Ten (34.5%) studiesCitation16,Citation17,Citation20,Citation21,Citation25,Citation28,Citation30,Citation32,Citation35,Citation38,Citation39, did not conduct analyses to test a-priori identified key assumptions. Of the studies that did (65.5%), none resulted in substantive conclusions changes.
Table 2. GRACE Checklist scoring of individual studies.
Evidence Synthesis
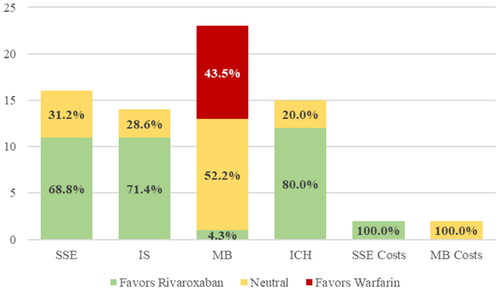
Of the 29 included studies, 16 (55.2%)Citation12–15,Citation18,Citation20,Citation22–25,Citation27,Citation28,Citation30,Citation31,Citation36,Citation37, reported comparisons for the outcome of stroke and systemic embolism, 14 (48.3%)Citation11–16,Citation18,Citation23,Citation26,Citation28,Citation30,Citation32,Citation33,Citation35, reported on ischemic stroke, and 5 (17.2%)Citation18,Citation21,Citation29,Citation34,Citation39, reported on VTE (). For each outcome, all studies showed either a positive or neutral outcome with 68.8% being positive for stroke and systemic embolism and 71.4% for ischemic stroke favoring rivaroxaban ().
Figure 2. Effectiveness and safety of rivaroxaban and warfarin in observational studies of Elderly US patients. ICH, intracranial hemorrhage; IS, ischemic stroke; MB, major bleed; SSE, stroke or systemic embolism; VTE, venous thromboembolism.
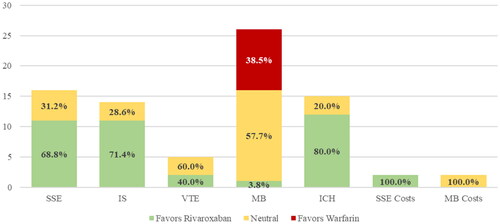
Table 3. Proportion of positive, neutral and negative studies by outcome of interest.
Twenty six of the 29 included studies (89.7%)Citation11–23,Citation25–28,Citation30–38, reported comparisons for major bleeding and 15 (51.7%)Citation12–15,Citation18,Citation23–26,Citation28,Citation30,Citation32,Citation38,Citation40, reported on ICH (). For major bleeding, 57.7% showed neutral effects, 38.5% showed negative effects, and 3.8% of studies showed positive effects with rivaroxaban versus warfarin while for ICH, all studies were positive or neutral ().
Two of the 29 (6.9%)Citation12,Citation15, studies reported data on cost of stroke and systemic embolism and major bleeding. Both studies were positive for rivaroxaban versus warfarin showing lower cost for stroke and systemic embolism and neutral cost for major bleeding (, ). None of the included studies reported findings on the outcome of healthcare resource utilization.
Subgroup analyses
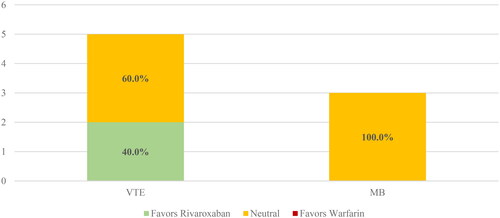
When looking at subgroup analyses, studies using either an intention-to-treat (eFigure 1) or on-treatment (eFigure 2) analysis had generally similar results to the overall cohort. One notable difference is that a majority (88.9%) of studies using an intention-to-treat analytic approach showed positive or neutral effects for rivaroxaban versus warfarin for major bleeding. The subgroup of studies limited to just NVAF populations () also showed similar findings to the overall cohort. Studies limited to just VTE populations () reported only VTE and major bleeding outcomes, with all studies showing positive or neutral effects.
Discussion
Our systematic review of a broad range of well-conducted large RWE studies has shown that use of rivaroxaban is associated with similar or better effects on stroke or systemic embolism, ischemic stroke, VTE, and ICH compared with warfarin and similar (57.7%), worse (38.5%), or improved (3.8%) effects on major bleeding in individuals 65 years of age or older treated within the United States. These effects are consistent across populations with both NVAF and VTE, regardless the analytic approach taken. Only two studies reported cost outcomes, both in NVAF populations, which were favorable to rivaroxaban for SSE-related costs and neutral regarding bleeding-related costs. No included studies evaluated healthcare utilization.
The results of the included RWE studies are generally consistent with secondary analyses of randomized controlled trials. A subgroup analysis of the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) showed a neutral effect of rivaroxaban versus warfarin on the endpoints of stroke and systemic embolism (HR 0.80, 95% CI 0.63–1.02), ischemic stroke (HR 0.88, 95% CI 0.67–1.16), and major bleeding (HR 1.11, 95% CI 0.92–1.34) in participants 75+ years of ageCitation4. A pooled analysis of the EINSTEIN-DVT and EINSTEIN-PE trials showed similar findings in participants 75+ years of age with neutral effects of rivaroxaban on symptomatic recurrent VTE and positive effects on major bleeding versus the control group of enoxaparin-vitamin K antagonistCitation5. A recently published systematic review of randomized controlled trials similarly showed efficacy and safety of direct oral anticoagulants in older individuals, including those 85+ years of ageCitation41.
Our findings are also generally in alignment with systematic reviews of observational studies comparing rivaroxaban versus warfarin in broader populationsCitation42,Citation43. Almutairi et al.Citation42 showed that rivaroxaban use, as compared with warfarin in NVAF patients, was associated with a 20% reduction in SSE in RCTs (HR 0.80, 95% CI 0.67–0.95) and 22% reduction in observational studies (HR 0.78, 95% CI 0.59–1.04). The outcome of recurrent VTE or fatal PE was not different in RCTs (HR 0.88, 95% CI 0.54–1.43) nor observational studies (HR 0.91, 95% CI 0.54–1.54). Major bleeding in both AF and VTE was also not different in RCTs (HR 0.99, 95% CI 0.97–1.01) nor observational studies (HR 1.03, 95% CI .81–1.32). The meta-analysis by Briere et al.Citation43 showed similar results. Our findings are in general agreement with these meta-analyses for the effectiveness of rivaroxaban versus warfarin for treating NVAF and VTE in older patients. We did see some studies suggesting a higher risk of major bleeding with rivaroxaban; although, 61.5% of included studies suggested either improved or neutral effects of rivaroxaban on major bleeding risk versus warfarin.
While our review found many studies in patients aged 65+ years with either NVAF or VTE, some were excluded due to including younger individuals in their populations. Mittal et al.Citation44 included individuals with AF and a mean age around 77 years within a skilled nursing facility (SNF) who started either rivaroxaban (n = 519) or warfarin (n = 1129) within the first 3 days of their stay using Optum Clinformatics Extended Data Mart from January 2013 through December 2017. After adjusting for confounders using inverse probability of treatment weighting, they showed that rivaroxaban use was associated with a lower odds of hospitalization (p < 0.001) and lower SNF-related medical costs (p < 0.0001) but higher pharmacy costs (p < 0.0001) versus warfarin use. The significantly lower healthcare costs with rivaroxaban use also extended 100 days beyond the index SNF stay (p < 0.0001). The author group then extended their investigation (over the same time) to include experienced users who had been on either rivaroxaban (n = 4,423) or warfarin (n = 22,796) within 6 months preceding their SNF stayCitation45. This analysis of an expanded population similarly showed significantly lower costs with rivaroxaban versus warfarin use during the index SNF stay (p < 0.0001), mostly related to longer SNF stay durations with warfarin use (p < 0.0001). This lower healthcare utilization and cost with rivaroxaban also extended beyond the SNF stay. These findings extend the results of the studies included in our review in similar populations.
This systematic review has limitations of note. First, we limited our inclusion to observational studies. While they all conducted risk-adjusted analysis, most using important variables known to impact outcomes in NVAF or VTE, unmeasured confounding is still a concern and causality cannot be established. As our intention was to evaluate outcomes specifically related to effectiveness and safety in NVAF and VTE, general outcomes such as all-cause mortality were not included. Related, many of the studies were missing key information that may increase their risk of bias including the absence of rivaroxaban dosing and quality of warfarin treatment (e.g. time in therapeutic range). Many studies used an on-treatment approach to their study design, resulting in some having relatively short durations of follow-up. Some studies only had information on outcomes of interest through subgroup analyses, potentially leading to type 2 error. Most studies included individuals with AF resulting in few data on individuals with VTE. Because of the heterogeneity of the patient populations, missing information on sample size in some, and overlap in timeframe from analyses of the same database, we were unable to conduct meta-analyses of the included outcomes. To the last point, as some studies used the same database with timeframes that overlap in years of inclusion, the precision of point estimates may vary and should be interpreted with caution.
Conclusions
This systematic review supports findings from subgroup analyses of randomized controlled trials that, compared with warfarin, rivaroxaban is associated with generally neutral or positive effects on thrombosis outcomes in older adults with either NVAF or VTE treated in the United States. For major bleeding, 57.7% showed neutral effects, 38.5% showed negative effects, and 3.8% of studies showed positive effects with rivaroxaban versus warfarin.
Transparency
Author contributions
All authors had a role in study design; data collection, analysis, and interpretation of data; writing the report; and approved the manuscript for submission.
Acknowledgements
None declared.
Supplemental Material
Download MS Word (79.2 KB)Declaration of funding
This study was supported by Janssen Scientific Affairs, LLC, Titusville, NJ, USA. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of financial/other relationships
WLB has received consulting fees from Bayer AG and Astra Zeneca. CIC has received research funding and honoraria from Janssen Scientific Affairs, LLC; Bayer AG; and Alexion Pharmaceuticals. VA and BB are employees of Janssen Scientific Affairs, LLC. Other authors have no conflicts to declare. Janssen Pharmaceuticals markets rivaroxaban in the Unites States of America and Bayer AG markets rivaroxaban globally. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Gregson J, Kaptoge S, Bolton T, et al. Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol. 2019;4(2):163–173. doi: 10.1001/jamacardio.2018.4537.
- Kim TH, Yang PS, Yu HT, et al. Age threshold for ischemic stroke risk in atrial fibrillation. Stroke. 2018;49(8):1872–1879. doi: 10.1161/STROKEAHA.118.021047.
- Mitrousi K, Lip GYH, Apostolakis S. Age as a risk factor for stroke in atrial fibrillation patients: implications in thromboprophylaxis in the era of novel oral anticoagulants. J Atr Fibrillation. 2013;6(1):783. doi: 10.4022/jafib.783.
- Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;130(2):138–146. doi: 10.1161/CIRCULATIONAHA.113.005008.
- Prins MH, Lensing AW, Bauersachs R, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11(1):21. doi: 10.1186/1477-9560-11-21.
- Franklin JM, Patorno E, Desai RJ, et al. Emulating randomized clinical trials with nonrandomized Real-World evidence studies. Circulation. 2021;143(10):1002–1013. doi: 10.1161/CIRCULATIONAHA.120.051718.
- Wang SV, Schneeweiss S, Franklin JM, RCT-DUPLICATE Initiative., et al. Emulation of randomized clinical trials with nonrandomized database analyses: results of 32 clinical trials. JAMA. 2023;329(16):1376–1385. doi: 10.1001/jama.2023.4221.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Dreyer NA, Schneeweiss S, McNeil BJ, et al. GRACE principles: recognizing high-quality observational studies of comparative effectiveness. Am J Manag Care. 2010;16(6):467–471.
- Weeda ER, Nguyen E, Martin S, et al. The impact of non-medical switching among ambulatory patients: an updated systematic literature review. J Mark Access Health Policy. 2019;7(1):1678563. doi: 10.1080/20016689.2019.1678563.
- Alcusky M, Tjia J, McManus DD, et al. Comparative safety and effectiveness of direct-acting oral anticoagulants versus warfarin: a national cohort study of nursing home residents. J Gen Intern Med. 2020;35(8):2329–2337. doi: 10.1007/s11606-020-05777-3.
- Amin A, Keshishian A, Trocio J, et al. Risk of stroke/systemic embolism, major bleeding and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran or rivaroxaban compared with warfarin in the United States Medicare population. Curr Med Res Opin. 2017;33(9):1595–1604. doi: 10.1080/03007995.2017.1345729.
- Amin A, Keshishian A, Dina O, et al. Comparative clinical outcomes between direct oral anticoagulants and warfarin among elderly patients with non-valvular atrial fibrillation in the CMS medicare population. J Thromb Thrombolysis. 2019;48(2):240–249. doi: 10.1007/s11239-019-01838-5.
- Amin A, Garcia Reeves AB, Li X, et al. Effectiveness and safety of oral anticoagulants in older adults with non-valvular atrial fibrillation and heart failure. PLoS One. 2019;14(3):e0213614. doi: 10.1371/journal.pone.0213614.
- Amin A, Keshishian A, Hines DM, et al. Risk of stroke/systemic embolism, major bleeding, and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran, or rivaroxaban compared with warfarin in the United States medicare population: updated analysis. Curr Med Res Opin. 2022;38(12):2131–2140. doi: 10.1080/03007995.2022.2115772.
- Briasoulis A, Inampudi C, Akintoye E, et al. Safety and efficacy of novel oral anticoagulants versus warfarin in Medicare beneficiaries with atrial fibrillation and valvular heart disease. J Am Heart Assoc. 2018;7(8):e008773. doi: 10.1161/JAHA.118.008773.
- Coleman CI, Turpie AGG, Bunz TJ, et al. Effectiveness and safety of rivaroxaban versus warfarin in frail patients with venous thromboembolism. Am J Med. 2018;131(8):933–938.e1. doi: 10.1016/j.amjmed.2018.02.015.
- Coleman CI, Weeda ER, Nguyen E, et al. Effectiveness and safety of rivaroxaban vs. warfarin in patients 80+ years of age with non-valvular atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. 2018;4(4):328–329. doi: 10.1093/ehjqcco/qcx044.
- Coleman CI, Baker WL, Meinecke AK, et al. Effectiveness and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and coronary or peripheral artery disease. Eur Heart J Cardiovasc Pharmacother. 2020;6(3):159–166. doi: 10.1093/ehjcvp/pvz047.
- Coleman CI, Costa OS, Brescia CW, et al. A RIVA-DM subanalysis investigating patients with nonvalvular atrial fibrillation and type 2 diabetes aged under versus over 80 years. Clin Appl Thromb Hemost. 2022;28:10760296221133083. doi: 10.1177/10760296221133083.
- Costa OS, Beyer-Westendorf J, Ashton V, et al. Effectiveness and safety of rivaroxaban versus warfarin in obese patients with acute venous thromboembolism: analysis of electronic health record data. J Thromb Thrombolysis. 2021;51(2):349–358. doi: 10.1007/s11239-020-02199-0.
- Costa OS, Beyer-Westendorf J, Ashton V, et al. Effectiveness and safety of rivaroxaban versus warfarin in obese nonvalvular atrial fibrillation patients: analysis of electronic health record data. Curr Med Res Opin. 2020;36(7):1081–1088. doi: 10.1080/03007995.2020.1762554.
- Deitelzweig S, Keshishian A, Li X, et al. Comparisons between oral anticoagulants among older nonvalvular atrial fibrillation patients. J Am Geriatr Soc. 2019;67(8):1662–1671. doi: 10.1111/jgs.15956.
- Graham DJ, Baro E, Zhang R, et al. Comparative stroke, bleeding, and mortality risks in older Medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596–604.e11. doi: 10.1016/j.amjmed.2018.12.023.
- Hernandez I, Zhang Y, Saba S. Comparison of the effectiveness and safety of apixaban, dabigatran, rivaroxaban, and warfarin in newly diagnosed atrial fibrillation. Am J Cardiol. 2017;120(10):1813–1819. doi: 10.1016/j.amjcard.2017.07.092.
- Kim DH, Pawar A, Gagne JJ, et al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation : a cohort study. Ann Intern Med. 2021;174(9):1214–1223. doi: 10.7326/M20-7141.
- Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49(12):2933–2944. doi: 10.1161/STROKEAHA.118.020232.
- Lip GYH, Keshishian AV, Kang AL, et al. Oral anticoagulants for nonvalvular atrial fibrillation in frail elderly patients: insights from the ARISTOPHANES study. J Intern Med. 2021;289(1):42–52. doi: 10.1111/joim.13140.
- Lutsey PL, Norby FL, Zakai NA, et al. Oral anticoagulation therapy and subsequent risk of vnous thromboembolism in atrial fibrillation patients. Curr Med Res Opin. 2019;35(5):837–845. doi: 10.1080/03007995.2018.1541445.
- Martinez BK, Sood NA, Bunz TJ, et al. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in frail patients with nonvalvular atrial fibrillation. J Am Heart Assoc. 2018;7(8):e008643. doi: 10.1161/JAHA.118.008643.
- Mehta HB, An H, Ardeshirrouhanifard S, et al. Comparative effectiveness and safety of direct oral anticoagulants versus warfarin among adults with cancer and atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2022;15(12):e008951. doi: 10.1161/CIRCOUTCOMES.122.008951.
- Mentias A, Heller E, Vaughan Sarrazin M. Comparative effectiveness of rivaroxaban, apixaban, and warfarin in atrial fibrillation patients with polypharmacy. Stroke. 2020;51(7):2076–2086. doi: 10.1161/STROKEAHA.120.029541.
- Norby FL, Bengtson LGS, Lutsey PL, et al. Comparative effectiveness of rivaroxaban versus warfarin or dabigatran for the treatment of patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. 2017;17(1):238. doi: 10.1186/s12872-017-0672-5.
- Pawar A, Gagne JJ, Gopalakrishnan C, et al. Association of type of oral anticoagulant dispensed with adverse clinical outcomes in patients extending anticoagulation therapy beyond 90 days after hospitalization for venous thromboembolism. JAMA. 2022;327(11):1051–1060. doi: 10.1001/jama.2022.1920.
- Palamaner Subash Shantha G, Bhave PD, Girotra S, et al. Sex-Specific comparative effectiveness of oral anticoagulants in elderly patients with newly diagnosed atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2017;10(4):e003418. doi: 10.1161/CIRCOUTCOMES.116.003418.
- Sood N, Ashton V, Bessada Y, et al. Effectiveness and safety of rivaroxaban versus warfarin among nonvalvular atrial fibrillation patients with concomitant obstructive sleep apnea. TH Open. 2023;7(1):e82–e93. doi: 10.1055/a-2013-3346.
- Weir MR, Ashton V, Moore KT, et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and stage IV-V chronic kidney disease. Am Heart J. 2020;223:3–11. doi: 10.1016/j.ahj.2020.01.010.
- Wong JM, Maddox TM, Kennedy K, et al. Comparing major bleeding risk in outpatients with atrial fibrillation or flutter by oral anticoagulant type (from the national cardiovascular disease registry’s practice innovation and clinical excellence registry). Am J Cardiol. 2020;125(10):1500–1507. doi: 10.1016/j.amjcard.2020.02.028.
- Zakai NA, Walker RF, MacLehose RF, et al. Venous thrombosis recurrence risk according to warfarin versus direct oral anticoagulants for the secondary prevention of venous thrombosis. Res Pract Thromb Haemost. 2021;5(6):e12575. doi: 10.1002/rth2.12575.
- Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3(2):e000718. doi: 10.1161/JAHA.113.000718.
- Doni K, Bühn S, Weise A, et al. Safety outcomes of direct oral anticoagulants in older adults with atrial fibrillation: a systematic review and meta-analysis of (subgroup analyses from) randomized controlled trials. Geroscience. 2023; doi: 10.1007/s11357-023-00825-2.
- Almutairi AR, Zhou L, Gellad WF, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants for atrial fibrillation and venous thromboembolism: a systematic review and meta-analyses. Clin Ther. 2017;39(7):1456–1478.e36. doi: 10.1016/j.clinthera.2017.05.358.
- Briere JB, Bowrin K, Millier A, et al. Number needed to treat based on real-world evidence for non-vitamin K antagonist oral anticoagulants versus vitamin K antagonist oral anticoagulants in stroke prevention in patients with non-valvular atrial fibrillation. J Med Econ. 2019;22(8):760–765. doi: 10.1080/13696998.2019.1606001.
- Mittal VS, Wu B, Song J, et al. Healthcare resource utilization and costs among nonvalvular atrial fibrillation patients initiating rivaroxaban or warfarin in skilled nursing facilities: a retrospective cohort study. Curr Med Res Opin. 2020;36(4):529–536. doi: 10.1080/03007995.2019.1706464.
- Mahajan D, Wu B, Song J, et al. Comparison of healthcare resource utilization and costs between rivaroxaban and warfarin for nonvalvular atrial fibrillation in a skilled nursing facility setting. Drugs Aging. 2020;37(4):281–289. doi: 10.1007/s40266-019-00737-x.