Abstract
Objective
This Delphi method of consensus was designed to develop scientific statements for β-blockers in the continuum of cardiovascular diseases with a special focus on the role of bisoprolol.
Methods
Eleven experienced cardiologists from across the Asia-Pacific countries participated in two rounds of the survey. In the first round, experts were asked to rate agreement/disagreement with 35 statements across seven domains regarding the use of β-blockers for treating hypertension, heart failure, coronary artery diseases, co-morbidities, as well as their safety profile, usage pattern, and pharmacokinetic variability. A consensus for a statement could be reached with >70% agreement.
Results
Except for seven statements, all attained consensus in the first round. In the second round that was conducted virtually, the experts re-appraised their ratings for the seven statements along with a critical appraisal of two additional statements that were suggested by experts in the preceding round. At the end of the second round, the final version included 36 statements (34 original statements, two statements suggested by experts, and the omission of one statement that did not attain consensus). The final version of statements in the second round was disseminated among experts for their approval followed by manuscript development.
Conclusion
Attainment of consensus for almost all statements reconfirms the clinical benefits of β-blockers, particularly β1-selective blockers for the entire spectrum of cardiovascular diseases.
Introduction
β-blockers have been used for treating various cardiovascular diseases (CVD). They act by inhibiting the effects of catecholamines via multiple pathways that affect myocardial chronotropy, inotropy, and renin release with anti-ischemic and anti-arrhythmic effects. However, there is a possibility of various net effects on an individual’s clinical outcome due to heterogeneity of effects, which could depend on the patient’s characteristics, disease substrate, and the type of β-blocker usedCitation1. As patients who develop chronic heart disease commonly require lifelong treatment, using a β-blocker that provides a balance between risks and benefits is a crucial part of an optimal personalized treatment regimen for every patient. Here, it is important to note that all β-blockers decrease heart rate (HR) and blood pressure (BP); thus, reduced net myocardial oxygen consumption indicates that myocardial anti-ischemic action could be a class effect; but as all-cause mortality and sudden death are not similar for all β-blockers they might not be class effectsCitation2.
β-blockers faced a major setback when new hypertension guidelines recommended them as the second-line treatment for essential hypertension after angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs) in the absence of compelling indications. Indeed, these recommendations were based on meta-analyses that reported β-blockers to provide less favorable effects for total mortality, cardiovascular (CV) events, and stroke outcomes. Nevertheless, it is important to note that the majority of the analyzed data are derived from studies that used atenolol and propranolol, and not the new cardioselective β-blockersCitation3. Thus, compared to traditional β-blockers, newer agents with β1 selectivity or vasodilating properties reduce central pulse pressure and aortic stiffness more effectively than atenolol or metoprolol, and tend to have fewer metabolic side effects. Therefore, it is logical and practical for clinicians to reconsider the role of β-blockers in managing CVD in real-world clinical practice. It is noteworthy that, in the recent guidelines of the European Society of Cardiology in 2018, β-blockers are mentioned as the first-line therapy for hypertension in the same bracket as ACEIs, ARBs, CCBs, and diureticsCitation4.
In this Delphi review, we aimed to develop scientific statements with a special focus on bisoprolol, based on the outcomes of an expert discussion based on the current evidence for β-blockers in the CVD continuum.
Methods
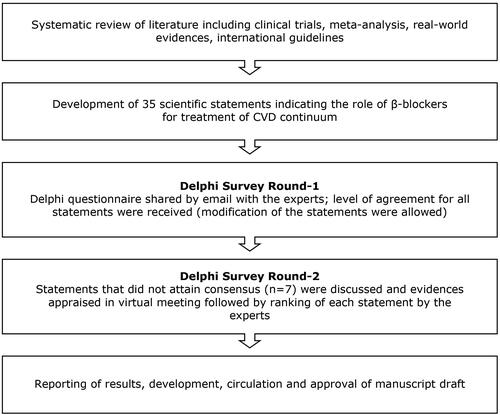
For developing this Delphi consensus, two iterations were planned for the expert panel as it is considered optimal to reach the consensus (). The Delphi method is a widely-used technique that uses a series of structured questionnaires and collects real-world knowledge from a small panel of experts (10–20) on a specific topic to arrive at a consensus. In the first round of this survey, 35 statements were shared with experts (N = 11) via email. They were allowed to modify the statements based on their experience and understanding. Agreement or disagreement with each statement was calculated based on the rating on a 9-point Likert scale. Scores of 1–4 indicated degrees of disagreement and scores of 6–9 indicated increasing degrees of agreement. A score of 5 was considered a neutral opinion. A consensus was said to have been reached when a statement was agreed upon by >70% of the experts. A “majority opinion” was reached when 51–69% of experts agreed, and a “minority opinion” was reached when ≤50% agreement was attained. In the second round, the statements (along with modifications, if any) which attained a consensus were communicated to the experts in the virtual meeting. The statements which did not attain consensus were presented for reconsideration along with the evidence supporting those statements. Evidence was critically appraised and was followed by a ranking of the statements. The final stage of this survey included drafting a manuscript and its dissemination among experts for their review, suggestion, and approval of the content.
Survey development
Statements provided in the first round of the Delphi survey were developed based on a comprehensive literature review (randomized controlled trials, meta-analysis, systematic review, and real-world evidence) and international guidelines for treating CVD. The first version of the statements was developed and divided into seven domains: (1) pattern of β-blocker used (n = 1); (2) β-blockers for hypertension (n = 9); (3) β-blockers for heart failure (HF) (n = 7); (4) β-blockers in treating coronary artery disease (CAD) (n = 6); (5) role of β-blockers in co-morbidities (n = 6); (6) safety profile (n = 3); and (7) pharmacokinetic variability and β-selectivity (n = 3).
To accomplish our study objective, we contacted experienced cardiologists from across the Asia-Pacific countries. Each cardiologist was fully informed regarding the survey, the Delphi technique, and the timing of their expected involvement. The final panel consisted of 11 experienced cardiologists from India, Indonesia, Malaysia, Philippines, Thailand, and Vietnam.
Results
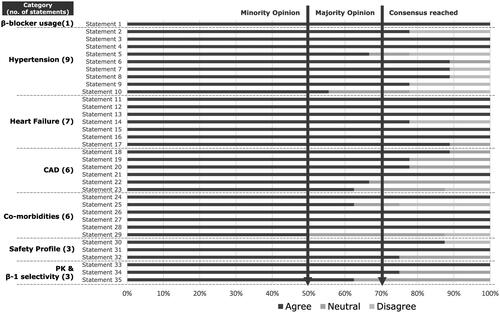
Of the 35 statements presented in the first iteration, 28 reached consensus, and seven statements attained majority opinion ().
Figure 2. Summary of results from the first round of the Delphi Survey (consensus reached: agreed by ≥70% of experts; majority opinion: agreed by 51–69% of experts; Minority opinion: agreed by ≤50% of the experts).
A summary of the collated scores of these seven statements was presented in a virtual meeting and was considered again by the experts. Evidence that supported those statements was critically appraised and was then ranked by the experts. Except for one, all statements achieved consensus. The statement (#23) which was not agreed upon was omitted in the final version. The experts suggested two additional statements (#24 and #34) which were approved by all experts and were thereby added to the final version of the statements (). There were 36 statements in the final version of the questionnaires ().
Figure 3. Summary of results from the combined first and second round of the Delphi survey (consensus reached: agreed by ≥70% of experts; majority opinion: agreed by 51–69% of experts; Minority opinion: agreed by ≤50% of the experts; *Statement 23 was omitted in the final version as consensus was not achieved in both rounds; *Statement 24 suggested by the experts in round-1 which was incorporated in the final version after achieving consensus; #Statement 34 was suggested by the experts in round-1 that was incorporated in the final version after achieving consensus).
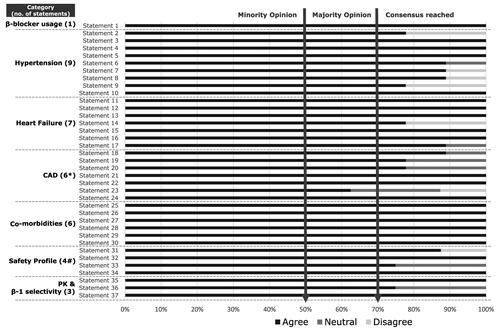
Table 1. Scientific statements related to the role of β-blocker therapy in the cardiovascular disease continuum.
Discussion
Hypertension
The current hypertension management guidelines recommended β-blockers in patients with compelling indications such as HF, angina, post myocardial infarction (MI), or CAD, and in younger hypertensive pregnant women or hypertensive women who are planning a pregnancyCitation4–6. However, recommendations regarding β-blockers as first-line antihypertensive agents for uncomplicated hypertension vary across guidelines as some recommend other classes over β-blockers, and others, especially from the Asia-Pacific countries, suggest β-blockers as first-line antihypertensives. Several studies show that hypertension occurs at a relatively younger age (20–44 years) in Asia and this subjects many young patients to an increased risk for premature mortality due to CV causesCitation7,Citation8. In young patients, hypertension is characterized by a marked adrenergic overdrive that could be effectively treated with β-1 selective β-blockersCitation9. Indeed, in a meta-analysis, Khan and McAlister studied CV events (stroke, MI, and death) in 145,811 patients from 21 hypertension trials. Among patients aged <60 years old, β-blockers reduced CV outcomes compared to placebo (relative risk [RR] = 0.86; 95% CI = 0.74–0.99) and were equivalent to other antihypertensive drugs (RR = 0.97; 95% CI = 0.88–1.07). But in patients who were ≥ 60 years old, β-blockers were equivalent to placebo (RR = 0.89; 95% CI = 0.75–1.09) and were less effective in reducing CV outcomes than other antihypertensive drugs (RR = 1.06; 95% CI = 1.01–1.10). These results showed improved clinical outcomes with β-blockers in younger hypertensive patientsCitation10.
This meta-analysis found that clinical events were largely determined by BP control rather than a therapeutic agent with two exceptions: the CCBs reduced stroke more and β-blockers provided a survival advantage for 2 years after a heart attack.
Guidelines recommend selecting evidence-based β-blockers. It should be noted that trials using β-blockers (particularly atenolol) were not performed to demonstrate effects on CVD outcomes. None of the clinical trials supports atenolol after a MI or in HF. Although atenolol was reportedly inferior to the comparator in LIFE and ASCOT-BPLA studies, interpretation of these results needs careful consideration of once-daily dosing of atenolol that creates a drug-free window period of almost 6 hCitation11.
There is no obvious evidence except for atenolol that β-blockers are worse than other antihypertensive classes. Moreover, β-blockers are also recommended for patients with resistant hypertension in addition to other medications, particularly when patients are not able to tolerate mineralocorticoid receptor antagonists (MRA)Citation4.
A Bayesian network meta-analysis showed that regression of left ventricular hypertrophy (LVH) produced by β-1 selective blockers is similar to that by ACE inhibitors and ARBs but more than that produced by diuretics or CCBs. Selective β-1 blockers showed a greater probability of being the most effective treatment for LVH regression in this analysisCitation12. Considering their clinical benefits, β-1 selective blockers may be re-evaluated in patients with LVHCitation13. In two randomized studies, bisoprolol showed greater reductions in systolic and diastolic BP compared to atenolol, particularly during the early morning “vulnerable” periodCitation14,Citation15. In another randomized study, bisoprolol produced a greater reduction in central aortic BP compared to atenololCitation16. Certain β-blockers have vasodilation properties; however, despite that, BP reduction by bisoprolol and nebivolol was found to be similar in a randomized studyCitation17. The scientific statements are shown in and their consensus in for the role of β-blockers in hypertension.
Heart failure
Heart failure with reduced ejection fraction (HFrEF)
Pivotal trials establish the safety and efficacy of β-blocker therapy in patients across the spectrum of chronic heart failure (CHF)Citation18–21. The Cardiac Insufficiency Bisoprolol Study-II (CIBIS-II) was designed to derive clinically relevant conclusions with bisoprolol for HFrEF. The patients (n = 2,647) were randomly assigned to receive bisoprolol and placebo. The estimated annual mortality rate was 8.8% and 13.2% (hazard ratio = 0.66; 95% CI = 0.54–0.81) in the bisoprolol group and placebo group, respectively. Patients receiving bisoprolol were less likely to be admitted to the hospital (p < 0.0001) or die due to a cardiac cause (p = 0.0049) compared to those receiving placeboCitation18. The MERIT-HF trial, another large randomized placebo-controlled trial, reiterated similar clinical benefits of metoprolol controlled-release/extended-release in patients with HFrEF (n = 3,991)Citation19. Adding carvedilol to standard therapy of HF reduced mortality by 35% (95% CI = 19–48%) in the COPERNICUS trial. The study also showed consistent benefits of β-blocker therapy across all strata of HFCitation21. Hence, based on unequivocal evidence of mortality- and morbidity-benefits of β-blocker therapy in HFrEF, international guidelines recommend evidence-based β-blockers, including bisoprolol, metoprolol succinate, and carvedilolCitation22,Citation23. Considering dose-dependent survival benefits of β-blockers, the clinicians are advised to titrate the dose to its targeted dose or maximally tolerated dose (whichever is feasible)Citation22,Citation24. Recently, Toyoda et al.Citation25 observed greater efficacy of bisoprolol over carvedilol regarding protection from myocardial injury and preservation of pulmonary function in patients with HFrEF. Despite the comparison being conducted in a small cohort, the findings may assist in selecting an appropriate agent in the setting of HFrEF.
Role in combination with other HF medications
As the comparison studies proved survival benefits with comprehensive pharmacological therapy (angiotensin receptor–neprilysin inhibitors [ARNIs], MRAs, and SGLT2 inhibitors) compared to established conventional pharmacological therapy (ACE-inhibitor or ARB plus β-blocker), patients are increasingly managed with comprehensive therapyCitation26. However, patients often demonstrate intolerance to therapy due to weight gain, hypotension, and orthostatic symptomsCitation27. In this scenario, switching from carvedilol to bisoprolol often avoids dizziness or hypotension and thereby facilitates the continuation of therapyCitation28.
However, to the best of our knowledge, to date, the extent of mortality benefit in HFrEF by β-blockers is unsurpassed by any other agent, including ACE-inhibitor, ARB, ARNI, MRA, and SGLT2 inhibitors.
Physicians are still in a dilemma regarding the use of β-blockers in treating acute HF (AHF) as its negative inotropic effects may transiently worsen the hemodynamic status and worsen the symptoms of HF. Heterogeneous results are reported by studies evaluating the effects of β-blockers in settings of AHCitation29–31.
Coronary artery disease
β-blockers have been implicated as the standard of care for the treatment of CAD because of their substantial clinical benefits. Several studies demonstrated decreased risk for adverse consequences of acute MI (including myocardial ischemia, reinfarction, and mortality) with β-blockers even in patients who opted for mechanical reperfusionCitation32. Early administration of intravenous β-blocker therapy in conjunction with percutaneous coronary intervention improves LV function, reduces the incidence of malignant arrhythmias, and decreases long-term HF readmissionCitation33–35. However, as intravenous administration of β-blockers is associated with side effects such as hypotension, shock, and bradycardia (observed in nearly 30% of the patients), especially in hemodynamically unstable patients, careful monitoring of the patients is advisableCitation36. While the efficacy of early oral β-blocker therapy was evaluated in a large cohort, it was significantly associated with reduced in-hospital mortality (odds ratio (OR) = 0.41; 95% CI = 0.21–0.80) and reduced incidence of severe LV dysfunction (OR = 0.57; 95% CI = 0.42–0.78) compared to delayed oral β-blocker therapyCitation37. Clinical outcomes of other studies are consistent with the aforementioned findingsCitation38,Citation39. Hence, considering mortality-reducing intervention in post-MI settings, the European Society of Cardiology (ESC) guidelines recommend the administration of oral β-blockers (as class I recommendation) within the first 24 h in all the patients with ST-elevation MI (STEMI) except those with signs of HF, evidence of a low output state, increased risk for cardiogenic shock, or other contraindications to using oral β-blockersCitation40. Similarly, oral β-blocker therapy has been recommended for all NSTEMI patients without pre-existing contraindicationCitation41. Although the prognostic implication of β-blockers for treating stable angina remains unanswered, they are extensively prescribed pharmacological therapyCitation42,Citation43. There is limited data related to the use of β-blockers in stable CAD without MICitation44,Citation45.
The intervention evidence review developed by the National guideline center based at the Royal College of Physicians noted bisoprolol as the most commonly prescribed β-blocker in routine clinical practice after ACSCitation46. Maclean et al.Citation39 assessed the efficacy of early administration of oral bisoprolol in patients with NSTEMI. They observed that patients receiving low-dose bisoprolol within 4 h of presentation were less likely to experience major adverse CV events (MACE: a composite of ventricular arrhythmia, cardiac death, or repeat infarction) during hospitalization compared to those receiving delayed bisoprolol (1 vs 27, p = 0.005). The comparison of bisoprolol with other β-blockers demonstrated a lower risk of mortality, angina, and MI with a HR of 0.45 (95% CI = 0.34–0.61), 0.58 (95% CI = 0.50–0.68), and 0.45 (95% CI = 0.27–0.75), respectively. Likewise, the HR of bisoprolol versus other than β-blocker therapy for mortality was 0.50 (95% CI = 0.38–0.66), for angina was 0.77 (95% CI = 0.68–0.88), and for MI was 0.34 (95% CI = 0.23–0.52), which favors bisoprolol in patients with angina. In addition, bisoprolol also reduced the risk for arrhythmiaCitation47.
Non-selective β-blockers are always preferred over β1-selective blockers for treatment or secondary prevention of variceal bleeding in cirrhotic patients. Likewise, the mortality benefits of β1-selective blockers have been well-documented in patients with acute MI. However, the coexistence of both diseases presents clinical challenges for the selection of appropriate agents from these two β-blocker subtypes to provide effective treatment for cirrhosis and at the same time offer mortality benefits from CVD. Wu et al.Citation48 compared clinical outcomes with β1-selective blockers and non-selective β-blockers in cirrhotic patients with coexistence acute MI in a 13-year population-based study. Propensity score matching identified 218 patients in each group. Kaplan-Meier survival analysis demonstrated significantly fewer MACCE with β1-selective blockers (hazard ratio = 0.62; 95% CI = 0.42–0.91; p = 0.014). There was no difference in all-cause mortality or non-worsening liver outcome. Hence, a β1-selective blocker can be considered a treatment approach in patients with cirrhosis and acute MI.
In chronic coronary syndrome, the β-blockers are the unchallenged drug of choice in most clinical scenarios, as per the ESC 2019 guidelinesCitation49.
High resting heart rate (> 80 bpm) in hypertension indicates increased sympathetic activity. As the heart rate rises, there’s a higher risk of AF, HF, and mortality in the general population and hypertensive patients. The ESH guidelines suggest that beta-blockers should be considered as the first drug treatment of choice for hypertensive patients with resting heart rates > 80 beats per minute50.
Sympathetic dysregulation has been observed in various stages of hypertension, including mild, moderate, and severe cases, across different age groups (young, middle-aged, and elderly patients) and in conditions such as white-coat hypertension, masked hypertension, and pregnancy-induced high blood pressure. Sympathetic overactivity has been linked to various clinical conditions, such as dipping or non-dipping hypertension, hypertension complicated by sleep apnea, metabolic syndrome, renal failure, and true resistant hypertension. This heightened sympathetic activation plays a significant role in developing and progressing vascular remodeling, endothelial dysfunction, and increased arterial stiffening observed in individuals with hypertensionCitation51.
Hence, based on an extensive literature review, we endorse the utilization of the β-blockers for the treatment of CAD.
Comorbidities
HF and renal function
Patients with CKD are prone to develop CVD, particularly ischemic heart disease, CHF, arrhythmias, and LV hypertrophy. According to an estimate, nearly 35–40% of mortality in CKD patients was attributed to cardiac causes. Considering the established benefits of β-blocker therapy in patients following MI or patients with CHF, it is expected that the therapy should have a similar degree of efficacy and safety profiles in patients with CKD and MI or CHF. These hypothetical benefits of β-blocker therapy are demonstrated in several observational and randomized controlled trials (RCTs).
Shaman et al.Citation52 performed a frequentist random-effects network meta-analysis of RCTs (n = 40) to assess the efficacy and safety of antihypertensive agents in dialysis-dependent patients. While all antihypertensive agents proved efficacious compared to placebo at lowering systolic BP, aldosterone antagonists (‒10.8 mm Hg; 95% CI = −14.8 to −6.7 mm Hg) and β-blockers (‒8.7 mm Hg; 95% CI = −10.9 to −6.4 mm Hg) were superior to others (ACE-I, angiotensin receptor blockers, calcium-channel blockers, and renin inhibitors). The results also demonstrated the increased risk of discontinuing aldosterone antagonist therapy due to adverse events. However, β-blockers were not associated with a higher risk for adverse events. Furthermore, only β-blockers were identified to have a marked effect on diastolic BP and HR. Several studies identified the clinical benefits of β-blocker therapy across all strata of CKD. A pile of evidence demonstrates a significant reduction in all-cause mortality and CV endpoints with β-blocker therapy for treating CHF in patients with CKDCitation53–57. Clinical benefits of β-blocker therapy were also reported in a nationwide study involving end-stage renal disease patients (also those who required dialysis) with co-existent AMICitation58.
The dialyzability of β-blockers was assumed to have a noticeable impact on its efficacy in dialysis-dependent patients. The results of a large retrospective cohort study favored poorly dialyzable β-blocker (bisoprolol) compared to highly dialyzable β-blockers (atenolol or metoprolol)Citation59. However, contradictory results of recent studies underscore the importance of larger randomized controlled trialsCitation60,Citation61.
The comparison of cardioselective and non-selective β-blockers favors the use of cardioselective β-blockers in dialysis-dependent patients with a statistically significant 17% reduction in mortality and 17% in CV morbidity and mortalityCitation62. Similarly, Wu et al.Citation63 reported a lower risk of MACEs (HR = 0.85; 95% CI = 0.80–0.91), HF (HR = 0.83; 95% CI = 0.77–0.91), and ischemic stroke (HR = 0.84; 95% CI = 0.72–0.97) with the use of bisoprolol, a cardioselective β-blocker. The results were consistent in propensity score matching analyses, stratified analyses, and analyses that considered prescribed dosages or censored patients discontinuing or switching β-blockers. Furthermore, non-selective β-blockers (particularly carvedilol) are likely to cause intradialytic hypotension owing to their significant α1-blocking effectCitation64,Citation65.
Chronic obstructive pulmonary disease (COPD)/asthma and CVD
Patients with COPD are at an increased risk for developing ischemic heart disease, CHF, and cardiac arrhythmia. The higher mortality rate and adverse consequences of CVD further highlight the significance of an effective therapeutic approach for the prevention and treatment of CVD in patients with COPDCitation66. Conventionally, β-blockers are contraindicated in COPD patients because of several theoretical safety concernsCitation67. However, current evidence supports the role of β-blockers as a pharmacological approach for treating CVD in this patient population. Clinical benefits of improved survival and decreased risk of re-hospitalization are documented in a meta-analysis evaluating β-blockers in COPD patients with concomitant CVDCitation68,Citation69.
Evidence suggests that cardioselective β1-blockers are well tolerated in patients with COPD, even during hospitalization for COPD exacerbationCitation70. While the effects of cardioselective β1-blocker were assessed in patients with HF, and moderate or severe COPD, a significant reduction of FEV1 in patients receiving a therapeutic dose of bisoprolol as compared to those receiving placebo was seen. However, treatment with bisoprolol did not affect reversibility following inhaled β2-agonist and static lung volumesCitation71. The use of β-blockers is also supported by significant improvement in quality of life and non-significant impact on COPD exacerbationCitation71,Citation72. In several studies, cardioselective β1-blocker performed better than other pharmacological agents as evident by reduced exacerbation of COPD or improved lung functionsCitation73–76. The aforementioned findings are translated clinically in several studies with reduced risk for all-cause mortality and re-hospitalization due to CHF and/or COPD exacerbation with the use of cardioselective β1-blockers in COPD patients diagnosed with cardiac illnessCitation77–83. Su et al.Citation84 evaluated the survival benefits of different β-blockers (carvedilol, bisoprolol, and metoprolol) in a large patient cohort with coexistent HF and COPD (N = 11,558). At a mean follow-up period of 4.07 years, bisoprolol use was associated with a dose–response survival benefit (low dose: adjusted hazard ratio = 0.76; 95% CI = 0.59–0.97, p = 0.030; high dose: adjusted hazard ratio = 0.40; 95% CI = 0.26–0.63, p < 0.001) compared to those who were not prescribed bisoprolol. The study did not report any survival difference for carvedilol or metoprolol. Improved clinical outcomes with the use of bisoprolol compared to carvedilol for treating HF in patients with COPD were also reported in other studiesCitation85,Citation86. With the evolution of β-blockers, newer evidence enumerates the beneficial effects of β-blocker therapy even in patients with concomitant diabetes and COPDCitation87.
Although several guidelines also recommend using β-blockers in this cohortCitation88, a considerable number of eligible patients are not prescribed β-blockers in a real-world scenarioCitation89–91. Cardioselective β1-blockers have been used in asthmatic patients with promising resultsCitation92,Citation93. However, it needs to be thoroughly evaluated in controlled trials. Herein, we recommend the use of cardioselective β1-blockers in patients with COPD.
The safety profile of β-blockers
A substantial proportion of potentially eligible patients are not treated with β-blockers as a result of physicians’ misperception regarding the safety profile of therapy. These patients who may have otherwise gained benefits from β-blocker therapy include those with concomitant disorders such as diabetes, COPD, and men with erectile dysfunction.
Earlier trials reported increased incidences of new onset of diabetes and increased insulin resistance among patients who were prescribed β-blockers. In contrast, laboratory investigations of patients treated with cardioselective β-blocker questioned this safety concern. The studies did not show any change in carbohydrate/lipid metabolism following β-blocker therapyCitation94,Citation95. A recent study also demonstrated a negative association (OR = 0.31; 95% CI = 0.11–0.83; p = 0.02) of prior β-blocker use with the incidence of severe hypertension during severe hypoglycemia, a potentially lethal combination that may contribute to the increased risk for CVD or mortalityCitation96. Likewise, a prospective cohort study conducted by Witte et al.Citation97 delineated the significance of a higher dose of β-blocker therapy when prescribed in diabetic patients for treating CHF, especially in those with severely impaired left ventricular function.
As erectile dysfunction is one of the well-recognized side-effects of β-blockers, disqualifying the patients from this effective therapy needs careful consideration of the following facts: (1) β-blockers are a heterogeneous class of drugs; (2) CVD for which β-blockers are prescribed itself is associated with the erectile dysfunction; (3) CVD and erectile dysfunction share similar risk factors; and (4) psychological effects influence patient’s perception, especially when patients know erectile dysfunction is one of the side-effects of the therapyCitation98,Citation99. Furthermore, many studies did not find any association between decreased sexual activity with β1-selective blockers (bisoprolol and nebivolol) Citation100,Citation101.
β-blockers can be categorized based on their distribution coefficient into either lipophilic or hydrophilic drugs. Hydrophilic β-blockers include Atenolol and sotalolCitation102, while lipophilic ones comprise propranolol and metoprololCitation103. Bisoprolol and betaxolol fall into an intermediate positionCitation104 Notably, highly lipophilic β-blockers like metoprolol and propranolol can cross the blood–brain barrier, potentially leading to central nervous system side-effects during treatment, such as sleep disturbances, depression, and hallucinationsCitation103.
Hence, the available evidence allows us to conclude that the benefits of β-blockers outweigh their risk (as mentioned in ).
Pharmacokinetic variability and β-1 selectivity
Despite the enormous clinical benefits of β-blockers for the almost entire spectrum of CVD, considerable inter-individual pharmacokinetic variability warrants a note of caution to cardiologists for their widespread prescription. Ågesen et al.Citation105 reviewed pharmacokinetic studies to assess the pharmacokinetic variability of 14 different β‐blockers in healthy individuals who received a single or multiple doses; none of the studies of bisoprolol showed coefficients of variance for the area under the plasma concentration–time curve <40% indicating its lowest pharmacokinetic variability. Ågesen et al.Citation105 conducted a review of pharmacokinetic studies on 14 β-blockers in healthy individuals. The studies included reported maximum plasma concentration (Cmax) and/or area under the concentration curve (AUC). Among them, bisoprolol had the lowest variabilityCitation105, with no AUC coefficients of variance exceeding 40% in those studies. In contrast, metoprolol showed the highest pharmacokinetic variability, with steady-state ratios reaching as high as 30Citation105. This ultimately results in a reduced incidence of angina attacks as a persistent reduction in exercise BP and HR could be achieved with a single daily dose. The once-daily dose of bisoprolol further facilitates persistent control of lower BP through continual blockage of β1-receptors which may not be the case with multiple doses of other β1-blockersCitation106.
The literature review demonstrated conflicting results for β1- and β2-adrenoceptor selectivity of carvediol, bisoprolol, and nebivololCitation107–109. Maack et al.Citation107 evaluated the interaction between nebivolol and bisoprolol with β-adrenoceptor-G-protein by radioligand binding experiments with [125I]-lodocyanopindolol and compared the intrinsic activity of these β1-selective blockers. Although animal studies demonstrated higher β1-selectivity of nebivolol over bisoprolol, the latter exhibited higher affinity when the binding characteristics were assessed to human β1- and β2-adrenoreceptors. Bisoprolol shows higher selectivity for β1-adrenoreceptors over β3-adrenoreceptors in the heart and thereby contributes toward greater clinical advantages (higher effects in reducing HR)Citation110. Bisoprolol reduces mean dynamic HR greater than that of metoprolol in patients with mild-to-moderate hypertensionCitation111. A randomized placebo-controlled trial evaluating carvedilol, bisoprolol, and nebivolol also demonstrated the largest acute HR reduction with bisoprololCitation112.
Scientific statements about pharmacokinetic variability and β1-adrenoreceptors selectivity of β-blockers are summarized in .
Conclusion
Evidence suggests that β-blockers should be a mainstay in the treatment of several entities of the CVD continuum. While considering recent contradictory results for the safety and tolerability of β-blockers, “β-blockers” include an array of molecules with largely different pharmacokinetic and pharmacodynamic characteristics culminating in their different effects.
Transparency
Declaration of funding
The consensus meeting of the advisory board members was funded by Merck Pte Ltd, Singapore, an affiliate of Merck KGaA, Darmstadt, Germany under Good Publication Practice (GPP3) guidelines. The sponsor also funded the medical writing support and article processing charges of this manuscript.
Declaration of financial/other relationships
The authors received honoraria as consultants for the advisory board meeting from Merck Pte Ltd, Singapore, an affiliate of Merck KGaA, Darmstadt, Germany. There are no other competing interests for the authors. Dr. Harshal and Dr. Harshveer are employees of Merck Specialities Pvt. Ltd., India, an affiliate of Merck KGaA, Darmstadt, Germany.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All the authors attended meetings conducted for the development of consensus statements. All authors were involved during the draft development and approved the final version of the manuscript.
Acknowledgements
Writing assistance was provided by CBCC Global Research.
References
- Ziff OJ, Samra M, Howard JP, et al. Beta-blocker efficacy across different cardiovascular indications: an umbrella review and meta-analytic assessment. BMC Med. 2020;18(1):103. doi: 10.1186/s12916-020-01564-3.
- Koracevic G, Micic S, Stojanovic M, et al. Compelling indications should be listed for individual beta-blockers (due to diversity), not for the whole class. Curr Vasc Pharmacol. 2021;19(4):343–346. doi: 10.2174/1570161118666200518113833.
- Wiysonge CS, Bradley HA, Volmink J, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2012;11: Cd002003.
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339.
- Whelton P, Carey R, Aronow W, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood ppressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065.
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
- Kaul U, Bhagwat A, Omboni S, et al. Blood pressure and heart rate related to sex in untreated subjects: the India ABPM study. J Clin Hypertens (Greenwich). 2020;22(7):1154–1162. doi: 10.1111/jch.13894.
- Ramakrishnan S, Zachariah G, Gupta K, et al. Prevalence of hypertension among indian adults: results from the great India blood pressure survey. Indian Heart J. 2019;71(4):309–313. doi: 10.1016/j.ihj.2019.09.012.
- Floras JS, Hara K. Sympathoneural and haemodynamic characteristics of young subjects with mild essential hypertension. J Hypertens. 1993;11(6):647–655. doi: 10.1097/00004872-199306000-00009.
- Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ. 2006;174(12):1737–1742. doi: 10.1503/cmaj.060110.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338(may19 1):b1665–b1665. doi: 10.1136/bmj.b1665.
- Xing F, Chen J, Zhao B, et al. Real role of β-blockers in regression of left ventricular mass in hypertension patients: Bayesian network meta-analysis. Medicine (Baltimore). 2017;96(10):e6290. doi: 10.1097/MD.0000000000006290.
- Muresan L, Cismaru G, Muresan C, et al. Beta-blockers for the treatment of arrhythmias: bisoprolol – a systematic review. Ann Pharm Fr. 2022;80(5):617–634. doi: 10.1016/j.pharma.2022.01.007.
- Bühler FR, Berglund G, Anderson OK, et al. Double-blind comparison of the cardioselective beta-blockers bisoprolol and atenolol in hypertension: the bisoprolol international multicenter study (BIMS). J Cardiovasc Pharmacol. 1986;8(Suppl 11): s122–7. doi: 10.1097/00005344-198511001-00022.
- Neutel JM, Smith DH, Ram CV, et al. Application of ambulatory blood pressure monitoring in differentiating between antihypertensive agents. Am J Med. 1993;94(2):181–187. doi: 10.1016/0002-9343(93)90181-n.
- Zhou WJ, Wang RY, Li Y, et al. A randomized controlled study on the effects of bisoprolol and atenolol on sympathetic nervous activity and Central aortic pressure in patients with essential hypertension. PLoS One. 2013;8(9):e72102. doi: 10.1371/journal.pone.0072102.
- Czuriga I, Riecansky I, Bodnar J, et al. Comparison of the new cardioselective beta-blocker nebivolol with bisoprolol in hypertension: the Nebivolol, Bisoprolol Multicenter Study (NEBIS). Cardiovasc Drugs Ther. 2003;17(3):257–263. doi: 10.1023/a:1026180325278.
- Lechat P, Brunhuber K, Hofmann R, et al. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet. 1999;353(9146):9–13.
- Group M-HS. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in-congestive heart failure (MERIT-HF). The Lancet. 1999;353(9169):2001–2007.
- Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26(3):215–225. doi: 10.1093/eurheartj/ehi115.
- Eichhorn EJ, Bristow MR. The carvedilol prospective randomized cumulative survival (COPERNICUS) trial. Curr Control Trials Cardiovasc Med. 2001;2(1):20–23. doi: 10.1186/cvm-2-1-020.
- Maddox TM, Januzzi JL, Jr., Allen LA, et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2021;77(6):772–810. doi: 10.1016/j.jacc.2020.11.022.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (ESC). developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592.
- Simon T, Mary-Krause M, Funck-Brentano C, et al. Bisoprolol dose–response relationship in patients with congestive heart failure: a subgroup analysis in the cardiac insufficiency bisoprolol study (CIBIS II). Eur Heart J. 2003;24(6):552–559. doi: 10.1016/s0195-668x(02)00743-1.
- Toyoda S, Haruyama A, Inami S, et al. Protective effects of bisoprolol against myocardial injury and pulmonary dysfunction in patients with chronic heart failure. Int J Cardiol. 2017;226:71–76. doi: 10.1016/j.ijcard.2016.10.046.
- Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121–128. doi: 10.1016/S0140-6736(20)30748-0.
- Liu RC. Focused treatment of heart failure with reduced ejection fraction using sacubitril/valsartan. Am J Cardiovasc Drugs. 2018;18(6):473–482. doi: 10.1007/s40256-018-0280-5.
- Taniguchi T, Ohtani T, Mizote I, et al. Switching from carvedilol to bisoprolol ameliorates adverse effects in heart failure patients with dizziness or hypotension. J Cardiol. 2013;61(6):417–422. doi: 10.1016/j.jjcc.2013.01.009.
- Orso F, Baldasseroni S, Fabbri G, et al. Role of beta-blockers in patients admitted for worsening heart failure in a real world setting: data from the Italian survey on acute heart failure. Eur J Heart Fail. 2009;11(1):77–84. doi: 10.1093/eurjhf/hfn008.
- Böhm M, Link A, Cai D, et al. Beneficial association of β-blocker therapy on recovery from severe acute heart failure treatment: data from the survival of patients with acute heart failure in need of intravenous inotropic support trial. Crit Care Med. 2011;39(5):940–944. doi: 10.1097/CCM.0b013e31820a91ed.
- Prins KW, Neill JM, Tyler JO, et al. Effects of beta-blocker withdrawal in acute decompensated heart failure: a systematic review and meta-analysis. JACC Heart Fail. 2015;3(8):647–653. doi: 10.1016/j.jchf.2015.03.008.
- Chatterjee S, Chaudhuri D, Vedanthan R, et al. Early intravenous beta-blockers in patients with acute coronary syndrome–a meta-analysis of randomized trials. Int J Cardiol. 2013;168(2):915–921. doi: 10.1016/j.ijcard.2012.10.050.
- Sterling LH, Filion KB, Atallah R, et al. Intravenous beta-blockers in ST-segment elevation myocardial infarction: a systematic review and meta-analysis. Int J Cardiol. 2017;228:295–302. doi: 10.1016/j.ijcard.2016.11.133.
- García-Ruiz Jose M, Fernández-Jiménez R, García-Alvarez A, et al. Impact of the timing of metoprolol administration during STEMI on infarct size and ventricular function. J Am Coll Cardiol. 2016;67(18):2093–2104. doi: 10.1016/j.jacc.2016.02.050.
- Podlesnikar T, Pizarro G, Fernández-Jiménez R, et al. Left ventricular functional recovery of infarcted and remote myocardium after ST-segment elevation myocardial infarction (METOCARD-CNIC randomized clinical trial substudy). J Cardiovasc Magn Reson. 2020;22(1):44. doi: 10.1186/s12968-020-00638-8.
- Boudonas GE. β-Blockers in coronary artery disease management. Hippokratia. 2010;14(4):231–235.
- Bugiardini R, Cenko E, Ricci B, et al. Comparison of early versus delayed oral β blockers in acute coronary syndromes and effect on outcomes. Am J Cardiol. 2016;117(5):760–767. doi: 10.1016/j.amjcard.2015.11.059.
- de Matos Soeiro A, de Barros ESPG, Roque EA, et al. Mortality reduction with use of oral beta-blockers in patients with acute coronary syndrome. Clinics (Sao Paulo). 2016;71(11):635–638. doi: 10.6061/clinics/2016(11)03.
- Maclean E, Zheng S, Nabeebaccus A, et al. Effect of early bisoprolol administration on ventricular arrhythmia and cardiac death in patients with non-ST elevation myocardial infarction. Heart Asia. 2015;7(2):46–51.
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393.
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Heart Journal. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575.
- Huang HL, Fox KA. The impact of beta-blockers on mortality in stable angina: a meta-analysis. Scott Med J. 2012;57(2):69–75. doi: 10.1258/smj.2011.011274.
- Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391(10115):31–40. doi: 10.1016/S0140-6736(17)32714-9.
- Bangalore S, Steg G, Deedwania P, et al. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. Jama. 2012;308(13):1340–1349. doi: 10.1001/jama.2012.12559.
- Bangalore S, Bhatt DL, Steg PG, et al. β-blockers and cardiovascular events in patients with and without myocardial infarction: post hoc analysis from the CHARISMA trial. Circ Cardiovasc Qual Outcomes. 2014;7(6):872–881. doi: 10.1161/CIRCOUTCOMES.114.001073.
- National Guideline Centre (UK). Evidence review for beta-blockers: acute coronary syndromes: evidence review H. London: National Institute for Health and Care Excellence (NICE); 2020 Nov. (NICE Guideline, No. 185.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK565360/
- Sabidó M, Thilo H, Guido G. Long-term effectiveness of bisoprolol in patients with angina: a real-world evidence study. Pharmacol Res. 2019;139:106–112. doi: 10.1016/j.phrs.2018.10.031.
- Wu VC, Chen SW, Ting PC, et al. Selection of β-blocker in patients with cirrhosis and acute myocardial infarction: a 13-Year nationwide population-based study in Asia. J Am Heart Assoc. 2018;7(19):e008982.
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425.
- Mancia G, Kreutz R, Brunström M, et al. 2023 ESH guidelines for the management of arterial hypertension the Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023 Jun 21 [cited 2023; Jul 21]; Publish Ahead of Print. Available from: doi: 10.1097/HJH.0000000000003480.
- Grassi G, et al. Sympathetic overdrive in hypertension: clinical and therapeutic relevance. ESC Council Cardiol Pract. 2015;13:36–24.
- Shaman AM, Smyth B, Arnott C, et al. Comparative efficacy and safety of BP-Lowering pharmacotherapy in patients undergoing maintenance dialysis: a network Meta-Analysis of randomized, controlled trials. Clin J Am Soc Nephrol. 2020;15(8):1129–1138. doi: 10.2215/CJN.12201019.
- Badve SV, Roberts MA, Hawley CM, et al. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(11):1152–1161. doi: 10.1016/j.jacc.2011.04.041.
- Kotecha D, Gill SK, Flather MD, et al. Impact of renal impairment on Beta-Blocker efficacy in patients with heart failure. J Am Coll Cardiol. 2019;74(23):2893–2904. doi: 10.1016/j.jacc.2019.09.059.
- Molnar AO, Petrcich W, Weir MA, et al. The association of beta-blocker use with mortality in elderly patients with congestive heart failure and advanced chronic kidney disease. Nephrol Dial Transplant. 2020;35(5):782–789. doi: 10.1093/ndt/gfz167.
- Fu EL, Uijl A, Dekker FW, et al. Association between β-Blocker use and mortality/morbidity in patients with heart failure with reduced, midrange, and preserved ejection fraction and advanced chronic kidney disease. Circ Heart Fail. 2020;13(11):e007180.
- Jin J, Guo X, Yu Q. Effects of beta-blockers on cardiovascular events and mortality in dialysis patients: a systematic review and meta-analysis. Blood Purif. 2019;48(1):51–59. doi: 10.1159/000496083.
- Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42(2):201–208. doi: 10.1016/s0735-1097(03)00572-2.
- Weir MA, Dixon SN, Fleet JL, et al. β-Blocker dialyzability and mortality in older patients receiving hemodialysis. J Am Soc Nephrol. 2015;26(4):987–996. doi: 10.1681/ASN.2014040324.
- Wu PH, Lin YT, Kuo MC, et al. β-blocker dialyzability and the risk of mortality and cardiovascular events in patients undergoing hemodialysis. Nephrol Dial Transplant. 2020;35(11):1959–1965. doi: 10.1093/ndt/gfaa058.
- Assimon MM, Brookhart MA, Fine JP, et al. A comparative study of carvedilol versus metoprolol initiation and 1-year mortality among individuals receiving maintenance hemodialysis. Am J Kidney Dis. 2018;72(3):337–348. doi: 10.1053/j.ajkd.2018.02.350.
- Tao S, Huang J, Xiao J, et al. Cardio-selective versus non-selective β-blockers for cardiovascular events and mortality in long-term dialysis patients: a systematic review and meta-analysis. PLoS One. 2022;17(12):e0279171. doi: 10.1371/journal.pone.0279171.
- Wu P-H, Lin Y-T, Liu J-S, et al. Comparative effectiveness of bisoprolol and carvedilol among patients receiving maintenance hemodialysis. Clin Kidney J. 2021;14(3):983–990. doi: 10.1093/ckj/sfaa248.
- Giannattasio C, Cattaneo BM, Seravalle G, et al. Alpha 1-blocking properties of carvedilol during acute and chronic administration. J Cardiovasc Pharmacol. 1992;19(Suppl 1): s18–22. doi: 10.1097/00005344-199219001-00005.
- Tamargo J, Delpón E. Optimization of beta-blockers’ pharmacology. J Cardiovasc Pharmacol. 1990;16(Suppl 5): s10–S18. doi: 10.1097/00005344-199006165-00003.
- Leitao Filho FS, Alotaibi NM, Yamasaki K, et al. The role of beta-blockers in the management of chronic obstructive pulmonary disease. Expert Rev Respir Med. 2018;12(2):125–135. doi: 10.1080/17476348.2018.1419869.
- Baker JG, Wilcox RG. β-blockers, heart disease and COPD: current controversies and uncertainties. Thorax. 2017;72(3):271–276. doi: 10.1136/thoraxjnl-2016-208412.
- Gulea C, Zakeri R, Alderman V, et al. Beta-blocker therapy in patients with COPD: a systematic literature review and meta-analysis with multiple treatment comparison. Respir Res. 2021;22(1):64. doi: 10.1186/s12931-021-01661-8.
- Yang Y-L, Xiang Z-J, Yang J-H, et al. Association of β-blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2020;41(46):4415–4422. doi: 10.1093/eurheartj/ehaa793.
- Neef P, Burrell L, McDonald C, et al. Commencement of cardioselective bet-blockers during hospitalisation for acute exacerbations of chronic obstructive pulmonary disease. Intern Med J. 2017;47(9):1043–1050. doi: 10.1111/imj.13518.
- Hawkins NM, MacDonald MR, Petrie MC, et al. Bisoprolol in patients with heart failure and moderate to severe chronic obstructive pulmonary disease: a randomized controlled trial. European J of Heart Fail. 2009;11(7):684–690. doi: 10.1093/eurjhf/hfp066.
- Foresi A, Cavigioli G, Signorelli G, et al. Is the use of β-blockers in COPD still an unresolved dilemma? Respiration. 2010;80(3):177–187. doi: 10.1159/000318583.
- Zvizdic F, Begic E, Mujakovic A, et al. Beta-blocker use in moderate and severe chronic obstructive pulmonary disease. Med Arch. 2019;73(2):72–75. doi: 10.5455/medarh.2019.73.72-75.
- Bhatt SP, Wells JM, Kinney GL, et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax. 2016;71(1):8–14. doi: 10.1136/thoraxjnl-2015-207251.
- Agostoni P, Contini M, Cattadori G, et al. Lung function with carvedilol and bisoprolol in chronic heart failure: is beta selectivity relevant? Eur J Heart Fail. 2007;9(8):827–833. doi: 10.1016/j.ejheart.2007.04.006.
- Contini M, Apostolo A, Cattadori G, et al. Multiparametric comparison of CARvedilol, vs. NEbivolol, vs. BIsoprolol in moderate heart failure: the CARNEBI trial. Int J Cardiol. 2013;168(3):2134–2140. doi: 10.1016/j.ijcard.2013.01.277.
- Puente-Maestu L, Álvarez-Sala LA, de Miguel-Díez J. Beta-blockers in patients with chronic obstructive disease and coexistent cardiac illnesses. COPD Res Pract. 2015;1(1):1–10. doi: 10.1186/s40749-015-0013-y.
- Salpeter SR, Ormiston TM, Salpeter EE, et al. Cardioselective beta‐blockers for reversible airway disease. Cochrane Database Syst Rev. 2002;(4):CD002992. doi: 10.1002/14651858.CD002992.
- Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD003566. doi: 10.1002/14651858.CD003566.pub2.
- Lainscak M, Podbregar M, Kovacic D, et al. Differences between bisoprolol and carvedilol in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized trial. Respir Med. 2011;105(Suppl 1):S44–S49. doi: 10.1016/S0954-6111(11)70010-5.
- Jabbour A, Macdonald PS, Keogh AM, et al. Differences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trial. J Am Coll Cardiol. 2010;55(17):1780–1787. doi: 10.1016/j.jacc.2010.01.024.
- Statsenko M, Derevianchenko M, Chernikov M, et al. Efficacy and safety of bisoprololal in hypertensive patients with cardiovascular disease and chronic obstructive pulmonary disease. Kardiologiia. 2014;54(1):48–54. doi: 10.18565/cardio.2014.1.48-54.
- Sessa M, Mascolo A, Mortensen RN, et al. Relationship between heart failure, concurrent chronic obstructive pulmonary disease and beta‐blocker use: a Danish nationwide cohort study. Eur J Heart Fail. 2018;20(3):548–556. doi: 10.1002/ejhf.1045.
- Su VY-F, Chang Y-S, Hu Y-W, et al. Carvedilol, bisoprolol, and metoprolol use in patients with coexistent heart failure and chronic obstructive pulmonary disease. Medicine (Baltimore). 2016;95(5):e2427. doi: 10.1097/MD.0000000000002427.
- Liao K-M, Lin T-Y, Huang Y-B, et al. The evaluation of β-adrenoceptor blocking agents in patients with COPD and congestive heart failure: a nationwide study. Int J Chron Obstruct Pulmon Dis. 2017;12:2573–2581. doi: 10.2147/COPD.S141694.
- Kubota Y, Asai K, Furuse E, et al. Impact of β-blocker selectivity on long-term outcomes in congestive heart failure patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:515–523. doi: 10.2147/COPD.S79942.
- Kukes VG, Ostroumova OD, Mamaev VI[, et al. Efficacy and safety of different beta-blockers in patients with isolated systolic hypertension associated with diabetes mellitus and obstructive pulmonary diseases. Ter Arkh. 2003;75(8):43–47.
- Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):e1-194–194.
- Neef PA, McDonald CF, Burrell LM, et al. Beta-blockers are under-prescribed in patients with chronic obstructive pulmonary disease and co-morbid cardiac disease. Intern Med J. 2016;46(11):1336–1340. doi: 10.1111/imj.13240.
- Pinner N, Oliver W, Veasey T, et al. Frequency of β-Blocker use following exacerbations of COPD in patients with compelling indication for use. South Med J. 2019;112(11):586–590. doi: 10.14423/SMJ.0000000000001038.
- Lipworth B, Skinner D, Devereux G, et al. Underuse of β-blockers in heart failure and chronic obstructive pulmonary disease. Heart. 2016;102(23):1909–1914. doi: 10.1136/heartjnl-2016-309458.
- Chatterjee SS. The cardioselective and hypotensive effects of bisoprolol in hypertensive asthmatics. J Cardiovasc Pharmacol. 1986;8(Suppl 11):S74–S7. doi: 10.1097/00005344-198511001-00013.
- Huang K-Y, Tseng P-T, Wu Y-C, et al. Do beta-adrenergic blocking agents increase asthma exacerbation? A network meta-analysis of randomized controlled trials. Sci Rep. 2021;11(1):452. doi: 10.1038/s41598-020-79837-3.
- Janka HU, Ziegler AG, Disselhoff G, et al. Influence of bisoprolol on blood glucose, glucosuria, and haemoglobin A1 in noninsulin-dependent diabetics. J Cardiovasc Pharmacol. 1986;8(Suppl 11):S96–S9. doi: 10.1097/00005344-198511001-00018.
- Giesecke HG, Buchner-Möll D. Three years of experience with bisoprolol in the treatment of mild to moderate hypertension. J Cardiovasc Pharmacol. 1990;16(Suppl 5):S175–S178. doi: 10.1097/00005344-199000165-00031.
- Tsujimoto T, Yamamoto-Honda R, Kajio H, et al. Effectiveness of prior use of beta-blockers for preventing adverse influences of severe hypoglycemia in patients with diabetes: an observational study. Medicine (Baltimore). 2015;94(39):e1629. doi: 10.1097/MD.0000000000001629.
- Witte KK, Drozd M, Walker AMN, et al. Mortality reduction associated with β-Adrenoceptor inhibition in chronic heart failure is greater in patients with diabetes. Diabetes Care. 2018;41(1):136–142. doi: 10.2337/dc17-1406.
- Rerkpattanapipat P, Stanek MS, Kotler MN. Sex and the heart: what is the role of the cardiologist? Eur Heart J. 2001;22(3):201–208. doi: 10.1053/euhj.1999.2010.
- Erdmann E. Safety and tolerability of beta-blockers: prejudices and reality. Eur Heart J Suppl. 2009;11(Suppl A):A21–A25. doi: 10.1093/eurheartj/sup001.
- Nurmamedova GS, Mustafaev II. Analysis of variability of cardiac rhythm and sexual function in men with arterial hypertension during therapy with biosporolol and nebivolol. Klin Med (Mosk). 2012;90(12):56–59.
- Broekman CP, Haensel SM, Van de Ven LL, et al. Bisoprolol and hypertension: effects on sexual functioning in men. J Sex Marital Ther. 1992;18(4):325–331. doi: 10.1080/00926239208412857.
- Fumagalli C, Maurizi N, Marchionni N, et al. β-blockers: their new life from hypertension to cancer and migraine. Pharmacol Res. 2020;151:104587. doi: 10.1016/j.phrs.2019.104587.
- Lertvipapath P, Warunyuwong W. Beta-blocker and its neuropsychiatric effects. Thai J Pharm Sci. 2020;44(2):117–123.
- Cojocariu SA, Maștaleru A, Sascău RA, et al. Neuropsychiatric consequences of lipophilic beta-blockers. Medicina (Kaunas). 2021;57(2):155. doi: 10.3390/medicina57020155.
- Ågesen FN, Weeke PE, Tfelt-Hansen P, et al. Pharmacokinetic variability of beta-adrenergic blocking agents used in cardiology. Pharmacol Res Perspect. 2019;7(4):e00496-e. doi: 10.1002/prp2.496.
- Haasis R, Bethge H. Exercise blood pressure and heart rate reduction 24 and 3 hours after drug intake in hypertensive patients follwing 4 weeks of treatment with bisoprolol and metoprolol: a randomized multicentre double-blind study (BISOMET). Eur Heart J. 1987;8(suppl M):103–113. doi: 10.1093/eurheartj/8.suppl_m.103.
- Maack C, Tyroller S, Schnabel P, et al. Characterization of beta(1)-selectivity, adrenoceptor-G(s)-protein interaction and inverse agonism of nebivolol in human myocardium. Br J Pharmacol. 2001;132(8):1817–1826. doi: 10.1038/sj.bjp.0703992.
- Bundkirchen A, Brixius K, Bölck B, et al. Beta 1-adrenoceptor selectivity of nebivolol and bisoprolol. A comparison of [3H]CGP 12.177 and [125I]iodocyanopindolol binding studies. Eur J Pharmacol. 2003;460(1):19–26. doi: 10.1016/s0014-2999(02)02875-3.
- Brixius K, Bundkirchen A, Bölck B, et al. Nebivolol, bucindolol, metoprolol and carvedilol are devoid of intrinsic sympathomimetic activity in human myocardium. Br J Pharmacol. 2001;133(8):1330–1338. doi: 10.1038/sj.bjp.0704188.
- AlHabeeb W, Mrabeti S, Abdelsalam AAI. Therapeutic properties of highly selective β-blockers with or without additional vasodilator properties: focus on bisoprolol and nebivolol in patients with cardiovascular disease. Cardiovasc Drugs Ther. 2021;36(5):959–971. doi: 10.1007/s10557-021-07205-y.
- Yang T, Jiang Y, Hao Y, et al. Comparison of bisoprolol to a metoprolol CR/ZOK tablet for control of heart rate and blood pressure in mild-to-moderate hypertensive patients: the CREATIVE study. Hypertens Res. 2017;40(1):79–86. doi: 10.1038/hr.2016.101.
- Stoschitzky K, Stoschitzky G, Brussee H, et al. Comparing beta-blocking effects of bisoprolol, carvedilol and nebivolol. Cardiology. 2006;106(4):199–206. doi: 10.1159/000093060.