Abstract
Introduction
The post-acute (long) COVID-19 Quality of Life instrument is the only specific instrument designed to assess the quality of life in long COVID patients. The present study aims to make a transcultural adaptation and validation into Spanish of the disease-specific (long COVID) quality of life instrument, post-acute (long) COVID-19 Quality of Life, to have a tool for objective measurement of quality of life in this population.
Methods
A descriptive cross-sectional study was divided into two phases. In phase one, the translation and cultural adaptation of the questionnaire was performed, while in phase two, the questionnaire was validated. The Spanish version of the questionnaire was used with a sample of 206 people, 40 males (19.4%) and 166 females (80.6%), with an age range between 21 and 70 years old. Participants completed the questionnaire through an online platform. Internal consistency, construct validity, convergent validity, test-retest reliability, and ceiling and floor effects of the Spanish version were analyzed.
Results
The Spanish version of the post-acute (long) COVID-19 Quality of Life instrument showed high internal consistency, with Cronbach's alpha= 0.922 and an intraclass correlation coefficient of 0.936. Mean scores obtained in the PAC-19QoL and SF-12 questionnaires showed that those who had a worse quality of life in the SF-12 tool also a had worse quality of life in the PAC-19QoL tool.
Conclusions
This study shows that the Spanish version of the post-acute (long) COVID-19 Quality of Life instrument is an appropriate and valid tool for assessing the quality of life of long COVID patients.
Introduction
A significant problem faced by people who have suffered from COVID-19 is “long-COVID”. The World Health Organisation (WHO) has defined this concept as the persistence of signs, symptoms, or abnormal clinical parameters persisting three months after the beginning of COVID-19 (with or without a confirmed diagnosis) and with a duration of at least two months that an alternative diagnosis cannot explainCitation1. This disease is estimated to affect up to 10% of those infected in SpainCitation2. In addition, recent studies suggest that the global prevalence of long COVID reaches 43%Citation3.
The persistence of symptomatology has led to the development of theories about long COVID etiopathogenesis. These are related to the persistence of the virus in the body, the inflammatory cytokine storm, and the existence of autoantibodies as immune function disruptorsCitation2. However, this area has a significant knowledge gapCitation2,Citation4.
The symptoms suffered by those with long COVID can be of different typesCitation2, but the latest research points to fatigue or asthenia as the most common. These symptoms may fluctuate or persist over time, worsen with physical and mental exertion, and limit functional capacityCitation2,Citation4–6. Furthermore, the long COVID profile seems to point to 40-year-old people as the most prevalent age and women as the majority group. Approximately 70%-80% of those with long COVID are womenCitation2.
It is known that long COVID decreases the quality of life of those who suffer from this illness, and it can be tremendously disabling in some casesCitation2,Citation7. Thus, long COVID directly impacts other areas, such as the economic system. It is estimated that 100,000 working-aged people in Spain are on sick leave 12 weeks after contracting the virusCitation2. All the above points show the importance of assessing the quality of life in people who suffer long COVID-19.
Currently, there are validated instruments that assess quality of life, including EQ-5D, SF-36, and its short version, SF-12. Health-related quality of life (HRQoL) is a generic tool and is therefore limited by the indicators included in the construct and variation in interpretation by different researchersCitation8. However, disease- or condition-specific HRQoL tools have advantages such as being clinically relevant to the health problem and responsive to clinically important changes in the stateCitation8,Citation9. In terms of long COVID-19, there is only one specific validated HRQoL, the post-acute (long) COVID-19 Quality of Life (PAC-19QoL)Citation9. This tool specifically assesses the quality of life in people with long COVID. This instrument has four domains (psychological, physical, social, and work), 19 subdomains, and 44 items. As far as it is known, it is only validated in the English version, thus resulting in the need to use this patient-centred quality-of-life instrument specific to long COVID for the Spanish population.
In this study, we aim to trans-culturally adapt and validate cross-culturally in Spanish the disease-specific instrument, post-acute COVID-19 Quality of Life (PAC-19QoL), to have a tool to objectively measure the quality of life of patients with long COVID within the Spanish population.
Methods
Study design
Transcultural adaptation and validation of the PAC-19QoL were conducted through a cross-sectional study. In phase one, the translation and cultural adaptation of the questionnaire was performed, while in phase two, the questionnaire was validated.
Sample size
An inappropriate sample size calculation can lead to erroneous results in the validation and adaptation of questionnaires. Currently, there is no consensus to calculate the sample size in this type of design, unlike research studies based on clinical or biological samples. Comrey and LeeCitation10 indicate that a sample size of 100 is poor, 200 is acceptable, 300 is good, and 500 or more is very good. The sample of the current study was 206. The sampling technique used for this design is consecutive.
Participants
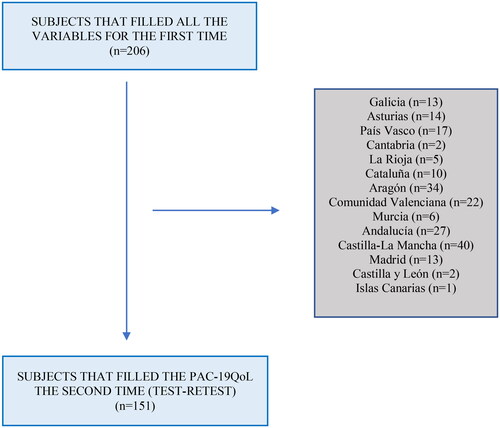
Adult members of Spain's different long COVID associations were invited to participate. The research team contacted them via social networks. The consent and the questionnaire were sent to them for dissemination after being informed about the objectives and procedures of the study. Finally, 206 people participated in the study.
The inclusion criteria were as follows: to be 18 years or older, have had COVID-19 or suspicions due to compatible symptomatology, have or have had symptomatology over three or more months, and be able to speak, read, and/or understand Spanish.
Transcultural adaptation
The transcultural adaptation of the PAC-19QoL questionnaire was carried out following the 5 phases described by Beaton et al.Citation11. In the first stage, the questionnaire was translated from English into Spanish by two translators. In the second stage, the discrepancies between these two initial versions were synthesised and resolved by a third translator. The version obtained at this point was back translated from Spanish into English by a native in the third stage. After this, a predefined version was obtained. In the fourth stage, an expert committee revised the predefined version by Delphi's method, in which we invited 3 primary care doctors, 3 primary care nurses, 2 psychologists, and 2 social workers to participate. The expert committee was asked about clarity, objectivity, relevance, organisation, sufficiency, intentionality, consistency, methodology, and applicability. All these heads were evaluated positively. Nevertheless, they proposed some changes to improve the understanding of the questionnaire, such as adding the option "not applicable" in items 7, 24, 31, 27, 28, and 37. In the fifth stage, a pre-test was conducted with 22 volunteer members of the Castilla-La Mancha Long COVID Association to obtain the definitive and final version of the questionnaire. The pre-test allowed us to check the correct understanding. Finally, the PAC-19QoL questionnaire was straightforward for the participants. However, one question related to the medical diagnosis of long COVID and another related to basal pathologies had to be reworded to clarify it. Both questions belonged to the clinic information section, not PAC-19QoL.
Validation of the Spanish version
Finally, 206 people participated in the study. The questionnaire was in an online format, and its completion time was 10 minutes. The SurveyMonkey online platform account of the University of Castilla-La Mancha was used, and the security protocols and protection of personal data were adhered to. Frequent problems in online surveys are poor response rate, representativeness, and item responseCitation12. To prevent these issues and improve the online survey quality, the use of grids or matrices to represent the Likert scale answer was avoided and the instruments used in the survey were pilot-tested. The questionnaire was opened from June 20 to 20 July 2022. After 10–15 days, participants received an email from the research team with the new link to access and complete the test–retest.
Variables and measurement instruments
The variables obtained and the measurement instruments used in the questionnaire for each participant were as follows:
Sociodemographic information: sex, age, weight, height, marital status, level of education, and employment status.
Clinical information: COVID-19 and long COVID questions related to symptomatology and its intensity, basal pathologies, and lifestyle habits such as drinking alcohol and smoking.
PAC-19QoL as an index test. This questionnaire specifically assesses the quality of life in people with long COVID. This instrument has four domains (psychological, physical, social, and work), 19 subdomains, and 44 items. This allows us to estimate the impact of long-term COVID on the quality of life of affected patients. Scores range from 0 to 220, with higher scores indicating a worse quality of lifeCitation9.
SF-12 as a reference test: is a self-report questionnaire that assesses quality of life in general terms. It was constructed using the questions of the eight dimensions of the SF-36. In this validated instrument, the 12 items referred to different health aspects that allow us to know to what extent the person can carry out habitual activitiesCitation13. To score the SF-12, two summary techniques have been reported: the mental component score (MCS-12) and the physical component score (PCS-12)Citation14. Scores range from 0 to 100, with higher scores indicating better physical and health functioningCitation15.
Data analysis
The data analysis was performed using version 28.0 of SPSS statistical software. A descriptive analysis was carried out to provide a profile of the participants in the study. The scores of the SF-12 questionnaire and the PAC-19QoL were obtained.
The statistical analysis to test the psychometric properties was performed by:
Internal consistency was measured by Cronbach's alpha. Values below 0.7 indicate low consistency, from 0.7 to 0.8 moderate, from 0.8 to 0.9 good, and more than 0.9 indicate excellent consistencyCitation16.
Ceiling and floor effects were calculated considering the scores of the questionnaire. These effects are present if more than 15% of the participants have the highest or lowest total score possibleCitation17.
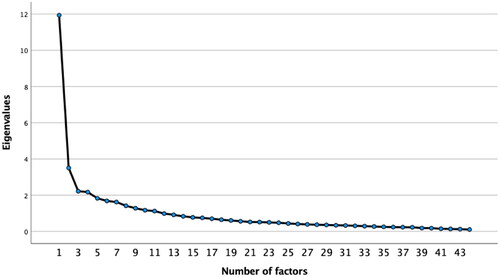
Construct validity was carried out using principal component analysis (PCA) to analyze the number of factors underlying the scale. The Kaiser–Meyer–Olkin (KMO) test and the Bartlett sphericity test were used to determine the suitability to carry out the exploratory factor analysis. An eigenvalue of 1 was obtained as a criterion for factor extraction. A screen plot was also carried out to analyze the adequacy of the number of factors extracted.
Convergent validity was performed. The total PAC-19QoL score was categorized as follows: low quality of life (first quartile), moderate quality of life (second and third quartiles), and high quality of life (fourth quartile). The highest levels of quality of life are associated with better health conditionsCitation17. The convergent validity of the scale was analyzed by gender and by ANCOVA models using SF-12 as the dependent variable, PAC-19QoL categories as fixed factors, and age as a covariate. Effect sizes 'd' were calculated employing the estimated marginal means and were interpreted as small (0.20-0.50), moderate (0.51-0.80) or large (>0.8)Citation9,Citation16.
Test-retest reliability was analyzed by the intraclass correlation coefficient (ICC). An ICC value above 0.8 is considered a good resultCitation16.
Ethical considerations
The study protocol was registered and approved with number 2022/001 by The Ethics Committee for Research on Medicines of the Albacete Integrated Health Care Management System. All research procedures used in this study were established per the Declaration of Helsinki. All participants gave their consent to participate in the study after they were duly informed about its purposes and procedures.
Results
In total, 206 people participated in the study, 40 males (19.4%) and 166 females (80.6%). Mean age of the participants was 46.81 years (SD 8.53). In the test-retest, 151 people participated, and the response rate was 73.30% (). The sociodemographic data obtained are shown in . The clinical characteristics related to COVID-19 and the long COVID of the sample population are shown in .
Table 1. Socio-demographic characteristics of the sample population.
Table 2. Clinical data and covid-19 and long covid characteristics of the sample population.
To obtain the PAC-19QoL score, 45 questionnaires with unanswered items were considered missing dates. Therefore, 161 questionnaires were scored: 35 (21.7%) were from males, and 126 (78.3%) were from females. The medium score in the test was 113.8 (SD 25.8) and segmented by gender, 115.3 (SD 29.3) for males and 113.4 (SD 24.8) for females.
There were no significant differences by sex (p = 0.148) or age (p = 0.71). Regarding the test-retest, 121 complete questionnaires were considered 25 (20.7%) were male, and 96 (79.3%) were female. The medium score in the test-retest was 115 (SD 26.8) and segmented by gender, 119.6 (SD 29) for males and 113.8 (SD26.2) for females. There were no significant differences by sex (p = 0.634) or age (p = 0.776). A ceiling or floor effect was absent, as any participants scored the minimum (0) or the maximum score (220).
Internal consistency
The Cronbach's alpha coefficient for the complete questionnaire was 0.922. Cronbach's alpha coefficient for each domain was 0.850 for the first (items 1–18), 0.856 for the second (items 19–34), 0.779 for the third (items 35–41), and 0.723 for the fourth (items 42–44).
Construct validity
An exploratory analysis identified 11 factors with an eigenvalue greater than one and with 68.1% of the variance. After analyzing the screen plot, it was observed that a solution with four factors would be appropriate (). This four-factor solution showed good sampling adequacy indexes (Kaiser–Meyer–Olkin 0.853; Bartlett sphericity p<.000), explained 45.1% of the variance, and was more comparable with the original version. shows the items of the scale with their respective factorial weights. The items corresponding to the first factor are framed in the "Social" dimension, those corresponding to the second factor in the "Psychological" dimension, those of the third factor in the "Self-recognition" dimension, and those of the fourth factor in the "Physical" dimension.
Table 3. Exploratory factor analyses: factor loadings after varimax rotations of PAC-19qol questionnaire.
Convergent validity
The differences in the mean score of the SF-12 by quality-of-life category, controlling for age, by gender, are shown in . There were no differences by age. The quality of life in males and females was significantly worse in participants with higher quality of life scores.
Table 4. Mean score of the sf-12 index by quality-of-life category, by ancova models controlling for age, by sex.
Test-retest reliability
It was calculated with a subsample of 121 participants who completed all the items of the PAC-19QoL questionnaire ten days later. The reliability of the instrument assessed by the ICC showed very high levels of consistency (ICC = 0.936).
Discussion
This is the first study that validates the original English version of the PAC-19QoL in Spanish. This study aimed to perform the transcultural adaptation and validation of the Spanish-language version of the PAC-19QoL. The results of our study confirm that the Spanish version of the PAC-19QoL has acceptable psychometric properties and a good level of validity and reliability in adults with long COVID. Additionally, this new version showed adequate convergent validity. In addition, no floor or ceiling effects are present in our study, which indicates that our questionnaire seems able to distinguish patients with the lowest or highest possible scoreCitation18.
As, in the original PAC-19QoL design, validation, and implementation, a novel method named “The Jandhyala Method” was used, other statistical analyses, such as exploratory factor analysis and confirmatory factorial analysis, have not been included in this study. “The Jandhyala Method” is a unique method for observing consensus and measuring subject matter across expert knowledge, and improves upon traditional Delphi-style methodologiesCitation9,Citation19. Additionally, this method generated the best neutral list of quality-of-life indicatorsCitation20,Citation21. Other studies have previously used this new method, and the results obtained were goodCitation22. This makes our validation benefit from the positive results of this new and innovative method.
Our adaptation seems logical for the study population, as the number of items and dimensions that make up the original scale are the same. The method to score both questionnaires is the same too. Nonetheless, discrepancies are found in item placement. It is considered appropriate a Spanish version of the PAC-19QoL with the 44 items restructured. It has been considered to change some items to other dimensions in which they fit better. Item 27 was restructured to dimension 1 (Social). Items 4 and 40 were restructured to dimension 2 (Psychological). Item 25 was restructured to dimension 3 (Self-recognition). Items 22, 28, 34, 30 and 29 were restructured to dimension 4 (Physical).
Another interesting consideration is the approach of a fifth dimension in the Spanish PAC-19QoL version named “Work”. Items referring to the labour sphere (42, 43 and 44) are framed in this additional dimension. This approach gives due importance to the work or labour sphere and does not minimize it in the other dimension as was “physical”. This is an important fact, as other studies show how much COVID affects the work capacity of these patientsCitation2. Additionally, in our results, we show this trend. A total of 42.7% of study participants had three months or more of sick leave.
Regarding the subdomains established in the original version of the PAC-19QoL, it is considered appropriate to remove them in the Spanish version of the PAC-19QoL. This is because the subdomains are not considered to score the scale, a characteristic that differs from the SF-12 questionnaire in which a mental and a physical part is found to obtain the total scoreCitation14. Moreover, the restructured main domains or dimensions are descriptive enough on their own and do not require subdomains.
It is also worth remarking that our results show that the quality of life is moderate in people who suffer long COVID. In this line, other studies also confirm the reduction in quality of life that is associated with this emergent diseaseCitation2,Citation7. Our Spanish validation of the PAC-19QoL instrument contributes to its use as a disease-specific tool in another language and patient population. This reliable tool can effectively measure the impact of long COVID on the quality of life of affected Spanish patients. Furthermore, an important fact is that this tool can be used in the clinical context and can help to modify therapeutic interventions, improving the quality of the diary life of Spanish people with long COVID.
The limitations of the present study are as follows. First, the PAC-19QoL has only been validated in another language with our study, so more studies validating this questionnaire in other languages could be better to compare results. Due to this lack of other long COVID questionnaires for comparison, it was not possible to perform external validation analyses. Second, our study is a cross-sectional study, and therefore, it is not possible to test the predictive validity of the scale that we propose. A longitudinal study will allow us to understand this issue. Third, the low response rate could affect the results obtained. In addition, a sample with the same number of women as men would show a better inference of our results to the general population, although our study matches with the prevalence by sex that other studies showCitation2. Also, this survey may be too long for the population with this condition. Many people with long COVID have neural sequelae that make cognitive tasks such as reading or paying attention difficultCitation23. This could result in these patients not completing the survey due to mental fatigue. Finally, the fluctuating nature of the disease, as well as the existence of relapses or disease outbreaksCitation2, can have quite an impact on the scores of this questionnaire.
As strengths of our study, it should be noted that it is the first validation study of the PAC-19QoL in Spanish carried out in different autonomous communities of Spain. Additionally, PAC-19QoL is a simple tool that requires little time to complete, making its administration more efficient and suitable for clinical use and in community studies. In addition, comparing the PAC-19QoL with a validated tool in different languages, such as the SF-12, contributes to quality results.
Conclusion
The Spanish-language version of the PAC-19QoL is a valid and reliable instrument for the assessment of quality of life in Spanish patients with long COVID. It is an understandable and easy instrument that provides Spanish clinical practice with an assessment tool that has been unavailable until now. It facilitates clinical decisions and encourages future research with a common and specific tool. Finally, this study confirms the use of PAC-19QoL in the Spanish-speaking population.
Transparency
Declaration of funding
This study did not receive any public funding.
Declaration of financial/other relationships
R. Jandhyala is a visiting senior lecturer at the Centre for Pharmaceutical Medicine Research at King’s College London and is responsible for research into real-world evidence approaches. He is also the founder and CEO of Medialis Ltd, a medical affairs consultancy and contract research organization involved in the design and delivery of real-world evidence in the pharmaceutical industry. The Jandhyala method was developed by R Jandhyala but is free of commercial licensing restrictions and while used as part of proprietary methodology, is not a direct means of commercial gain for the author.
The rest of authors listed above certify that they have no affiliations or involvement in any organisation or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Authors thank all the participants involved in this study.
References
- World Health Organisation. A clinical case definition of post COVID-19 condition by a Delphi consensus Accessed December 15, 2021 https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
- Rodriguez Ledo, Pilar. "Guía clínica para la atención al paciente long covid/covid persistente." (2021).
- Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post COVID-19 condition or long COVID: A meta-analysis and systematic review. Journal Infect Dis 2022 doi: 10.1093/INFDIS/JIAC136.
- Akbarialiabad H, Taghrir MH, Abdollahi A, et al. Long COVID, a comprehensive systematic scoping review. Infection Published online 2021. doi: 10.1007/s15010-021-01666-x.
- Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis.; 2021 https://ssrn.com/abstract=3769978
- Amenta EM, Spallone A, Rodriguez-Barradas MC, Sahly HME, Atmar RL, Kulkarni PA. Postacute COVID-19: An overview and approach to classification. Open Forum Infect Dis. 2020;7(12). doi: 10.1093/OFID/OFAA509.
- Malik P, Patel K, Pinto C, et al Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J Med Virol. 2022;94(1):253–262. doi: 10.1002/JMV.27309.
- Jandhyala R. Neutral theory: applicability and neutrality of using generic health-related quality of life tools in diseases or conditions where specific tools are available. BMC Medical Research Methodology 2021;21(1):1–8. doi: 10.1186/S12874-021-01279-W/TABLES/2.
- Jandhyala R. Design, validation and implementation of the post-acute (long) COVID-19 quality of life (PAC-19QoL) instrument. Health and Quality of Life Outcomes 2021;19(1):1–11. doi: 10.1186/S12955-021-01862-1/FIGURES/1.
- Comrey AL, Lee HB. A First Course in Factor Analysis A First Course in Factor Analysis Published online November 12, 2013. doi: 10.4324/9781315827506.
- Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25(24):3186–3191. doi: 10.1097/00007632-200012150-00014.
- Polit DF, Beck CT. Nursing research: Generating and assessing evidence for nursing practice. Lippincott Williams & Wilkins; 2017.
- Vilagut G, Valderas JM, Ferrer M, Garin O, López-García E, Alonso J. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: componentes físico y mental. Medicina Clínica. 2008;130(19):726–735. doi: 10.1157/13121076.
- Ware JE, Kosinski M, Keller SD. How to score SF-12 items Estimation of medical care total expenditures View project Published online 2002 Accessed May 12, 2022 https://www.researchgate.net/publication/291994160
- Soh SE, Morello R, Ayton D, et al. Measurement properties of the 12-item Short Form Health Survey version 2 in Australians with lung cancer: a Rasch analysis. Health and Quality of Life Outcomes 2021;19(1):1–13. doi: 10.1186/S12955-021-01794-W/FIGURES/2.
- Plichta S. B, Kelvin E. Munro's Statistical Methods for Health Care Research 6th ed.; 2013.
- Jávorné-Erdei R, Takács P, Fábián G. Relation of health condition and quality of life: examination of the quality of life of the disadvantaged population in Nyíregyháza by the FT quality of life index Practice and Theory in Systems of Education 2015;10(2):157–164. doi: 10.1515/ptse-2015-0015.
- Terwee CB, Bot SDM, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology 2007;60(1):34–42. doi: 10.1016/J.JCLINEPI.2006.03.012.
- Jandhyala R. Delphi, non-RAND modified Delphi, RAND/UCLA appropriateness method and a novel group awareness and consensus methodology for consensus measurement: a systematic literature review Curr Med Res Opin. 2020;36(11). doi: 10.1080/03007995.2020.1816946.
- Jandhyala R. Development and validation of the medical affairs pharmaceutical physician value (MAPPval) instrument. Pharmaceut Med. 2022;36(1):47–57. doi: 10.1007/s40290-021-00413-9.
- Jandhyala R. Development, validation and implementation of the medical affairs pharmaceutical physician work-related quality of life instrument [published online ahead of print, 2023 Feb 8]. Curr Med Res Opin. 2023;1–8. doi: 10.1080/03007995.2023.2174747.
- Jandhyala R. Development of a definition for medical affairs using the jandhyala method for observing consensus opinion among medical affairs pharmaceutical physicians Frontiers in Pharmacology 2022;13:136. doi: 10.3389/FPHAR.2022.842431/BIBTEX.
- Calabria M, García-Sánchez C, Grunden N, Pons C, Arroyo JA, Gómez-Anson B, et al. Post-COVID-19 fatigue: the contribution of cognitive and neuropsychiatric symptoms. J Neurol [Internet]. 2022;269(8):3990–9. Disponible en: doi: 10.1007/s00415-022-11141-8.