Abstract
Objective
To provide a systematic overview of bioethical debate on somatic gene therapy as documented in the scientific literature.
Methods
We performed a systematic review of reasons, following Strech and Sofaer approach, which is a method to systematically identify and classify arguments (reasons) used in the scientific literature. We identified 217 eligible publications retrieved from PubMed, Lilacs, PhilPapers, and Google Scholar. A meta-synthesis was performed to analyze the data.
Results
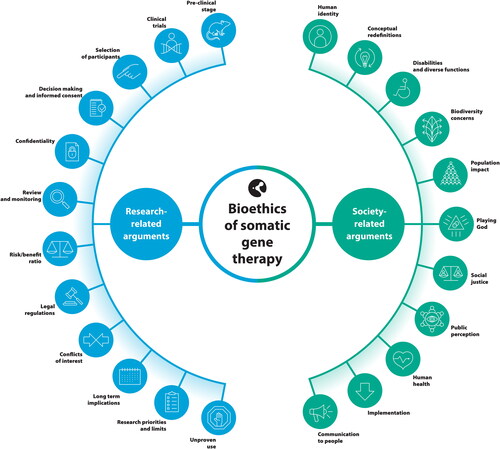
We extracted 189 arguments. Arguments were grouped into 23 categories. Twelve categories were classified as research-related, including the risk/benefit ratio, priorities and limits, informed consent, review, and monitoring. Eleven were classified as society-related, including population impact, human identity, public perception, human health.
Conclusion
Our study provides a database of existing challenges and arguments of somatic gene therapy and may serve as the basis of normative analysis. By presenting collected arguments, we contribute to the discussion about the ethics and social dimensions of somatic gene therapy.
Introduction
Gene therapy is defined as a technique that modifies a person’s genes for therapeutic purposesCitation1,Citation2. Introducing a new copy of an exogenous geneCitation3, replacing or inactivating a geneCitation1,Citation2 or editing genesCitation4 are some techniques used in the gene therapy field. According to the cellular target, gene therapy can also be classified in somatic and germline gene therapy. Somatic gene therapy is oriented to treat only the person receiving the therapy, whereas germline gene therapy treats the person, and the results of this procedure can be inherited by his/her descendantsCitation5,Citation6. Most current research focuses mainly on somatic gene therapyCitation7.
Somatic gene therapy is a promising approach that could provide treatment options for many diseasesCitation8. There are many preclinical and clinical studies that evaluate the therapeutic potential of interventions in human genesCitation5,Citation7. For example, in relation to curing various types of cancer (meningiomas and spinal cord, gastrointestinal, breast, etc.), genetic disorders (such as thalassemia or severe combined immunodeficiency), infectious diseases (such as HIV or hepatitis), cardiovascular diseases (such as coronary artery disease or ischemia), among othersCitation7.
The latest and more innovative techniques used for gene therapy are cutting-edge molecular tools that correct errors within genes, like CRISPR-CAS9, which is a simple, precise and rapid genome editing technology. Replacing, silencing or inserting an entire gene is now a kind of conventional somatic gene therapy after the emergence of CRISPRCitation4.
Conventional somatic gene therapy (i.e. non-editing somatic gene therapy) currently gets less attention in the discussion about ethics implications since the debate of CRISPR technologiesCitation9,Citation10. However, complex techniques or more invasive ones – such as CRISPR – should not distract us from the important ethical debate and unresolved questions. Somatic gene therapy will soon transform into a massive scale medical procedure: thus the unresolved ethical challenges need to be re-examinedCitation5,Citation6,Citation11–13.
In the following, we identify, categorize and analyze arguments on bioethical challenges of conventional somatic gene therapy. The aim of our study is to provide a systematic overview of the arguments used in the discussion about human gene therapy in somatic cells using conventional techniques that are documented in scientific literature.
Methods
We performed a systematic review of reasonsCitation14, following Strech and Sofaer approach, which is a method to systematically identify and classify arguments (reasons) used in the scientific literature. We described the methods in detail below. A meta-synthesisCitation15,Citation16 was performed to analyze all data. The study protocol was prospectively registered on Open Science Framework (see https://osf.io/fxuwj) (S1 Appendix).
Eligibility criteria
Publications were eligible if they had focused on somatic gene therapy with clear therapeutic goals and discussed reasons or premises about acceptability, importance, value, morality, ethics, or bioethics. We included articles in English or Spanish and the following types: (i) normative articles focusing on somatic gene transfer/therapy and its ethical/bioethical aspects; (ii) articles focusing on public perception or use of somatic gene transfer/therapy; (iii) articles focusing on professionals and researchers about ethical aspects of somatic gene transfer/therapy; (iv) narrative reviews, editorials, commentaries, opinions, letters, guidelines and policy recommendations. To make the search feasible, we excluded articles focused entirely on the ethics/bioethics of germline gene transfer or genome editing because it was out of the scope of our study; articles that focused on intrauterine, fetal, or prenatal gene transfer because we understand that this particular context could raise other ethical issues apart from those of the gene therapy itself; reports of interventional studies of gene transfer/therapy, as our objective is the ethical approach of the technique; articles from press and books, book chapters, comments on books, and congress abstracts/posters.
Search strategy
We performed the search in PubMed, Lilacs, PhilPapers, and Google Scholar on 26 July 2021. We chose these databases because they cover a wide range of biomedical and philosophical publications from all over the world. Choosing Lilacs, which is the most important Latin American database, allowed us to be sensitive to cultural or otherwise region-dependent differences. We performed the search without time restrictions. The only restriction that we used was in Google Scholar database because of the large number of articles that the search retrieved. We decided to use the first 100 hitsCitation17. The search strategy for each database is presented in the Supplementary Material section (S2 Appendix).
Selection process
Based on the pre-specified eligibility criteria, PB, AB, and KK independently screened the search results in two stages: first, titles and abstracts and second, the full texts. At each stage, we independently double screened all references. In case of any disagreements, a discursive consensus was reached.
Data extraction
The selected articles were analyzed using three prospectively designed data extraction documents (S3 Appendix). Contextual data from the included articles i.e. year, journal and language of publication, article type, field (according to Journal Citation Reports (JCR); if the journal were not indexed, we classified the journal field according to the journal scope based on its website), number, affiliation, and country of authors, were obtained using the first data extraction document. Subsequently, all arguments related to the bioethics of human gene therapy were extracted and organized in another data extraction document, including the argument extracted and the number of references. At this stage, we used the constant comparative method (CCM)Citation15. Before starting the extraction, researchers were trained in CCM.
Identification of codes and themes
We grouped the extracted arguments into categories related to a certain topicCitation18. The formulation of the categories was an iterative process. Categories are not supposed to be exhaustive or exclusive. There may be some arguments that correspond to two or more categories. However, we decided to include each argument only in one category to make our results more comprehensive. We discussed the categories several times among all researchers to find the best match for each argument. The categories were also grouped into two broad themes.
Quality appraisal
As described in the “Selection process” section, the article screening and extraction process was carried out independently by three researchers, who have different professional backgrounds (pharmacist, medical doctor and philosopher, with post-graduate studies in bioethics). The multidisciplinary approach was important to consider different points of view and ways of thinking. Screening, extraction, and category formulation were supervised by a bioethics expert (MW).
Data reporting
The data report follows the PRISMA Ethics – Reporting guideline for systematic reviews on ethics literature: development, explanations and examplesCitation19. The PRISMA-Ethics Reporting Guideline of this review can be found in the Supplementary Material section (S4 Appendix).
Results
Publication selection process
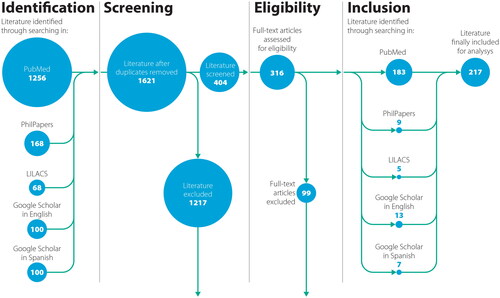
The systematic search yielded 1701 results. Removal of duplications left 1621 references. Title/Abstract screening resulted in 404 potentially eligible documents. After full text screening, we included 217 articles that met the eligibility criteria (). The cohort of included articles is listed in , and with full details in the Supplementary Material section (S5 Appendix).
Table 1. ID, authors and year of articles included in the cohort.
Characteristics of publications
Of the 217 articles included, 206 (94,9%) were published in English and 11 (5,1%) in Spanish. The earliest came from 1972, the last from 2020. The most prominent types of publications were reviews and theoretical/conceptual papers. Almost half of the authors (46,7%) of all selected publications were from the US, followed by Canada (12,4%) and the UK (9,8%). The journal that published the highest number of articles was Human Gene Therapy (n = 36; 16,6%). The largest number of articles were from the academic field of bioethics and genetics. More details can be found in the Supplementary Material section (S6 Appendix).
Results of syntheses
In total, 189 arguments were extracted from the included articles. These arguments were classified into 23 categories. All categories were grouped into two broad themes: research-related and society-related (). We present all research-related and society-related arguments by category and with references in the Supplementary Material section (S7 Appendix). Below we describe some relevant features of the categories, with the reference number of the article listed in where they are mentioned.
Research-related categories
Pre-clinical stage
As many articles agreed on the need for animal testing to evaluate safety, efficacy, and long-term effects (31, 35, 52, 56, 57, 59, 71, 97, 99, 100, 117, 123, 124, 155, 189, 191, 214), others argued that even when this is important, it is not always possible to extrapolate directly from animal experiments to human studies (7, 10, 17, 18, 22, 64, 88, 154, 161, 189, 209). Not only is it necessary to test the gene therapy technology itself, but also basic pathophysiology studies are required because there is difficulty in establishing causality in the occurrence of the disease (10, 45, 110, 161, 184, 189).
Clinical trials
Although some arguments are around the idea that clinical trials on somatic gene therapy are new and could have high/uncertain risks (10, 18, 22, 28, 32, 40, 41, 68, 90, 102, 104, 114, 117, 174, 175, 121), an article stated that these trials are not fundamentally different from those associated with other experimental therapies (173). Regarding adverse events in trials, some articles argued that even if they are present, they should not invalidate the therapy itself, as it is experimental and many patients are seriously ill (38, 45, 124, 154, 174, 175). This could be related to the idea that there should not be a delay in starting clinical trials, because this could also be a harm to people suffering from diseases that somatic gene therapy could prevent or treat (31, 81, 97, 104, 113, 143, 211). However, one concern was that many clinical trials lack adequate statistical power to draw valid conclusions about possible racial or ethnic differences in response to or toxicities of new treatments (141). The need for public input in the research process is emphasized (5, 10, 16, 53, 62, 66, 81, 141, 148, 149, 150, 151, 152, 184, 210, 213).
Selection of participants
It is reported that there is a pressure to enroll record numbers of human subjects in record numbers of trials (207), but it is difficult to ensure fairness in the selection of subjects (7, 22, 31, 33, 40, 55, 64, 83, 97, 117, 129, 153, 176, 192, 200). Some argue that it could be justified in life-threatening diseases without any therapeutic alternative (55, 56, 57, 72, 74, 89, 100, 101, 110, 123, 158, 162, 183). On the contrary, end-stage disease should not be used to justify exposing participants to greater risks (196). But there is also a risk of exploitation related to what we call collateral affective benefits (hope and altruism) for research participants (196). It is reminded that the good of society should not come at the expense of individual persons (193, 200), and that society’s ethical commitments to people living today should be prioritized over those who may benefit in the future from gene therapy (176). It is claimed that it is unethical to recruit subjects from economically disadvantaged countries because they may not have access to gene therapy in the future, but, on the other hand, people from both developing and developed countries have something to gain by participating in gene therapy trials (214).
Decision making and informed consent
Regarding the decision-making process of potential participants in the trials, many arguments focus on informed consent itself. One of them highlights that consent form is an influential component of the consent process (209), another that informed consent seems to protect institutions and not participants (151) and some that there could be problems with understanding the nature of the intervention and risks for participants (1, 14, 42, 51, 104, 114, 149, 152, 165, 184, 201, 213). Several argued that participants may decide based on the hope that they will benefit themselves (28, 31, 32, 35, 104, 117, 130, 213) or that they will stop struggling with life-threatening diseases (51, 60, 66, 69). In summary, there are concerns based on evidence that the research subjects could overestimate the benefits and provide invalid informed consent (174, 176, 200, 204, 205, 209). It is important to provide very detailed information to patients participating in gene therapy trials to prevent unrealistic hopes (170, 196). The risks should be communicated even if they are unlikely to happen (8, 12, 18, 46, 50, 51, 159, 160, 214). It is also emphasized that receiving insufficient information about treatment is a main concern (144). On the other hand, some people prefer to wait for strong evidence before considering enrolling in a clinical trial (8, 73, 86). So far, we have mentioned empirical problems. In relation to conceptual problems that affect practice, many authors argue that the term gene therapy referring to research brings confusion and intensifies existing problems of informed consent (26, 31, 36, 40, 78, 174, 196, 201, 204, 205, 209). It should be clear that personal benefit does not overlap with the scientific purpose of the study (9, 13, 89, 95, 117, 122, 209) and that the benefits for the participants are not the same as the benefits for society (19, 174).
It is said that we should not only rely on the consent process to determine an acceptable level of harm, burden, or risk of harm (196) but also that informed consent could require a different strategy than usual to guarantee genuine decisions (51, 70, 81, 125, 138, 142, 148, 149, 189). Another thing to consider is that gene therapy could be irreversible, so the right to revoke one’s consent is not applicable here compared to continuing medical treatment and should be carefully explained (50).
Confidentiality
Many articles point out the difficulties in protecting privacy and confidentiality (4, 12, 36, 47, 64, 97, 162, 171, 187, 197, 198, 217), and that the information obtained during trials could be prejudicial to the individuals treated or to their families (50, 171, 187, 197, 198, 217).
Review and monitoring
As some articles discuss, there is no need for a special evaluation of the somatic gene therapy protocol (100, 107, 216) because somatic gene therapy arises ethical issues similar to other medical technologies/treatments (4, 6, 12, 18, 19, 22, 28, 32, 37, 38, 41, 44, 50, 63, 65, 66, 69, 70, 71, 72, 73, 76, 77, 78, 85, 93, 94, 96, 100, 102, 105, 111, 114, 122, 124, 126, 128, 143, 158, 162, 168, 171, 175, 178, 179, 181, 185, 191, 216). Others focus on the idea that there is a need for special evaluation and audit of somatic gene therapy protocols (11, 35, 40, 45, 57, 62, 81, 100, 113, 118, 121, 124, 131, 150, 154, 159, 160, 188, 190, 192, 200, 202, 210, 213), because gene therapy has very specific and unique ethical complexities compared to other medical practices (2, 39, 46, 71, 90, 119, 190, 208). Therefore, the bioethical implications of these experiments must be carefully considered (5, 16, 20) and security issues should not be confused with ethical issues (32). The protocol should be strictly followed, and any changes to the protocol must be documented (110, 115, 62, 89, 115, 137, 145, 187, 188) and should be an effective means of control and discipline after the protocol is approved (162). There is an obligation to avoid harm (19, 40, 87) and any adverse event must be reported (46, 62, 89, 115, 145).
The ethical complexity of gene therapy should not be approached only with an ethics committee (2, 147, 151, 154, 158, 159, 160, 162) and the public should be involved in the review and monitoring protocols as necessary (127).
Risk/benefit ratio
There is a claim that gene therapy should be treated as a conventional medical therapy when determining risk/benefit ratios (192) because the risks do not appear to be different from those encountered by any standard medical therapy (85). But other articles reveal that gene therapy has novel properties that can affect humans in unpredictable ways (7, 16, 61, 63, 64, 70, 90). Probabilities and outcomes for adverse events related to gene transfer are difficult to define (7, 10, 18, 22, 40, 42, 51, 63, 67, 104, 114, 117, 165, 184, 190). Gene therapy raises concerns about long-term safety and efficacy (12, 16, 17, 31, 40, 41, 45, 59, 60, 61, 63, 64, 67, 69, 76, 77, 89, 90, 105, 123, 166, 175, 182) and about serious and/or irreversible side effects (10, 17, 18, 23, 43, 50, 54, 60, 64, 69, 71, 85, 86, 88, 90, 100, 101, 114, 126, 167, 176, 183, 192).
Principal risks include technical issues in terms of the quality and stability of transgene expression (17, 31, 41, 59, 70, 85, 90, 110, 161, 168, 183, 184, 192, 196, 200, 202, 213), transfer of an unwanted gene, administration of replication-competent virus or bacterial contamination of vector preparation (177, 196, 202), immune response against both the vector and the transgene (54, 62, 118, 161, 164, 165, 168, 169, 175, 176, 177, 194, 196, 202, 213), activation of oncogene or inactivate a tumor suppressor gene caused by gene vector (164) and unintentional modification of germinal cells (31, 54, 64, 67, 85, 88, 107, 114, 117, 125, 126, 164, 175, 177, 180, 202).
On the other hand, viral vectors seem effective but are still not quite safe (17, 39, 61, 62, 64, 70, 71, 73, 90, 99, 100, 110, 118, 131, 142, 161, 165, 167, 183, 187, 196, 202, 213), non-viral vectors could be safer but still not efficient (17, 39, 62, 67, 70, 73, 131) and transgene expression worked but in long term is limited (142).
There are difficulties in balancing benefits and risks in relation to the burden and prognosis of the disease (18, 34, 40, 41, 48, 63, 95, 100, 104, 114, 121, 125, 190), but also because the risks are uncertain and cannot be reduced to a single utility (176, 193).
Furthermore, difficulties in the balance of risk/benefit relate to how potential social benefits should be balanced against individual risks (196, 201). There could be subtle social benefits of gene therapy (88, 100, 125). The problem with social benefit is that it can be as broad or narrow as one chooses (201). Beneficence is based on the potential for net benefit in the entire population while doing minimal harm to the individual (32, 81), and the distinction between medical benefits and collateral benefits is highlighted (196).
Conflicts of interest
The difficulties in managing conflicts of interest were highlighted in several articles (33, 39, 40, 53, 77, 85, 93, 100, 102, 121, 124, 115, 145, 146, 188, 207, 213), showing that important stakeholders have deep interests in gene therapy as a product (127, 155). Therefore, due to the great investments, scientists face a high pressure for success to develop gene therapy (4, 53, 117, 121). It is clear that clinical investigators should not have a personal financial relationship with companies that may benefit from the results (46). It is also shared that conflicts of interest do not need to be financial. They can be personal. For example, most Institutional Review Boards members in medical schools are employees of those institutions and have personal relationships with researchers (207). The overlapping roles could lead to potential conflicts in subject recruitment (104).
Regulations
Some articles expressed that the regulatory system is likely to be challenged by gene therapy (6, 21, 22, 31, 45, 67, 66, 68, 69, 121, 159, 160, 190). Regulations cannot be a general “blanket,” but each type of gene therapy must be evaluated on its own merits and risk analysis (149). However, others showed that gene therapy research is, without any scientific or medical basis, the most highly regulated procedure in medicine (135). Gene therapy is subject to too strict rules and is affected by overregulation (65, 68). No other form of therapy has ever been subjected to such strict control in its development and clinical trials as somatic gene therapy (179). Therefore, overregulation of gene therapy can lead to increased bureaucracy (207) and can profoundly slow its testing and ultimate adoption (135). An article suggests a worldwide accepted and controlled bioethics convention for somatic gene therapy (126).
Research priorities and limits
Some articles proposed that gene therapy per se is no longer being debated, but its application to particular diseases or particular patients is (179, 193, 216). In this sense, some authors mention that gene therapy used in diseases should be evaluated in advance (71, 85, 101, 125) or that the goal of the therapy has yet to be determined (175). There is also a back and forth about when to apply gene therapy. One position is that there should be more efforts to prevent rather than treat (4). The other is that gene therapy should not be a “first line” of defense therapy as long as an alternative is available (18). About priorities, there is a concern about who should decide what to investigate: companies, scientists or other? Pharmaceutical companies and other corporate interests often determine research priorities, which may not be aligned with public health needs (4, 191). Furthermore, scientists should decide about gene therapy research priorities on the basis of enlightened and broad-based public opinion (156).
The need to redefine the rights and responsibilities of all involved actors is noted (14, 17, 109, 117, 150, 152, 155, 184, 210, 213), as well as the need for public participation in genetic research policy (200). Lay people and stakeholders should be involved in the ethics discussion about gene therapy (4, 53, 58) as human gene pools are viewed as collective property. Public debate is necessary (50). But, with so many stakeholders, it could be difficult to design a regulation considering both political and cultural differences (17, 62, 60, 63, 64, 68, 76, 83, 85, 120, 127, 152, 201).
Unproven use
Unproven use refers to pre-approval, non-trial access to potentially beneficial therapies (3). For some rare diseases, experimental therapies such as gene therapy may be the only way to provide a treatment option (3). Patients who have exhausted other therapeutic options may not meet the restrictive criteria for inclusion in the trial (3). However, a failed use attempt with gene therapy may make a patient unable to try similar intervention again (215). In this sense, companies that might produce gene therapies want to “preserve the pool of future customers” and the reputation image, so they restrict unproven use (215). Moreover, since some gene therapies are one-dose treatments and the rare diseases patients are a small number of customers, there could be a commercial disincentive for unproven use (215).
Long term implications
The need to consider long-term implications was raised in some articles (4, 154, 162, 164) along with the need for adequate follow-up and ongoing care for the participants (10, 22, 54). However, this is not easy, as several factors seem to complicate the achievement of follow-up of patients participating in gene therapy trials (187).
Society-related categories
Human identity
Those who do not believe that somatic gene therapy could change human identity state that the essence of the human person is not something that we can change at will, regardless of our technological capabilities (216). Human identity is more than a pool of genes (127) and is constantly redefined in biomedicine (76, 91, 105). Furthermore, an article states that gene therapy objectifies the disease in the person rather than the person (217).
However, others declare that somatic gene therapy could modify human identity, humanness or personal perception (11, 19, 27, 47, 69, 79, 101, 103, 109, 123, 131, 133, 191, 199, 212, 216) and could threaten human dignity (208). The body could be perceived as an enemy or as a source of weakness that is perfectible by technology (133), and eventually, the use of gene therapy could make certain human individuals cease to exist (4, 103). Gene therapy could reshape the ideas on how to live better (2), that effort is part of what makes us appreciate our life, so we do not have to eliminate all the pain or suffering (47). If we do so, we could lose our caring characteristics (47). Finally, gene therapy is said to not be used to change human traits (162).
Conceptual redefinitions
Gene therapy could open up some conceptual redefinitions. Some authors announce that could create a need for new disease/illness prevention and treatment concepts (11, 14, 49, 81, 110, 113, 122, 126, 133, 208). Additionally, it could be difficult to distinguish enhancement from treatment (11, 14, 29, 44, 47, 64, 66, 72, 74, 80, 81, 85, 94, 96, 97, 101, 102, 109, 110, 113, 114, 120, 122, 126, 132, 179, 185), and enhancement or eugenic therapy could be captured as human genetic therapy (167). In this sense, experiments in somatic gene therapy cannot be tainted by past associations with eugenics (172). Biotechnology is said to highlight moral problems, but not create them (44). Another conceptual issue that appears in some articles is that there are no ethical differences between germline and somatic gene therapy (25, 29) and that we are not conceptually forced to allow all types of gene therapy once we allow one (96).
Disability and diverse functions
Gene therapy could have an impact on social attitudes toward disability (133). On the one hand, gene therapy could not increase discrimination, but could make us aware of it (6, 81). On the other hand, the possibility of treatments could lead to more discrimination for disabled people (47). This is because diverse functions or bodies do not imply disabilities to prevent or treat, for example, deafness, but that community may argue that the only reason that deafness confers any disadvantages in society is because of societal discrimination (47). Also, in some cases, disability could be an integrated aspect of a person’s identity (133). Some articles mention that it is not necessary to overcome every human “limitation” (4, 47, 79, 81, 83, 91, 103, 105), and instead of working on solutions based on social bias, we need to think again about our social values (47).
Biodiversity concerns
There seems to be little concern about the impact of gene therapy on biodiversity (4). Gene therapy could replace the use of animal tissue culture used in current treatments (164), but the manufacture of gene therapy could be hazardous to the environment (1). In another sense, this field seems to avoid the issue that we are part of the environment because we put an anthropocentric distance ourselves from nature as if it were something different from human beings (4), and so gene therapy needs to consider the environmental effects on genes (4, 47, 49, 50).
Population impact
Gene therapy could have an impact on the population in different ways. To start, gene therapy research is a significant step in science evolution and therefore for well-being of humanity (40, 65, 67, 69, 70, 72, 74, 76, 78, 83, 105, 106, 107, 118, 124, 126). However new approaches have novel properties that may affect humans in unpredictable ways (142). There is a need to consider broad and long-range research consequences: public health, environmental and evolutionary concerns (200, 201).
Gene therapy for one person could have adverse repercussions on others (16, 27, 37, 44, 70, 77, 82, 85, 90, 93, 97, 114, 121, 126, 157, 200), for example, by making genetic diseases more prevalent in each generation after somatic gene therapy (37, 43, 202). In this sense, it is said that it could modify human evolution (37, 43, 76, 77, 81, 82, 91, 93, 94, 96, 101, 109, 122, 123, 126, 157, 167, 183, 184, 212, 217) because “bad” genes are needed from the viewpoint of the species (106). In opposition, other article advised that gene therapy will not affect human evolution (165).
Gene therapy could increase the possibility of the development of other new genetic technologies that have undesirable consequences (4, 35, 71, 72, 80, 93, 94, 96, 97, 101, 106, 122, 123, 128, 165, 183, 191, 199). For example, this could lead us to accept eugenic medical goals (4, 49, 52, 74, 81, 85, 94, 96, 157, 172, 208, 217), to a willingness to modify the color of the skin or change personality (167, 171) or that we are logically committed to accepting germline therapy (44, 72, 122, 208).
Despite the fact that gene therapy is offered with a focus on individual patient choice (70, 72, 79), it could motivate/deepen conflicts between values (17, 35, 101, 107, 121, 152, 163) and turn social problems into genetic problems (4, 29, 85, 93). In addition, gene therapy could raise issues of fairness, justice, or equity in access to therapy (69, 67, 75, 81). Gene therapy could cause population aging (180) and longevity could cause loneliness and overpopulation, despite improving quality of life (1).
Social justice
Across social justice and similarly to what happened to other biomedical innovations, gene therapy could only be available in countries or for people with high income (1, 14, 17, 21, 33, 34, 36, 76, 77, 79, 90, 96, 101, 102, 183, 189, 197). It could be discriminatory to people who do not have access to gene therapy (11, 28, 36, 63, 81, 84, 101, 123, 185, 198, 212). An article argued that these economic inequities could affect human biology (112). Some propose that justice debates should take seriously the fact of scarcity in the field of gene therapy (195, 197), because it may also relegate funding from other areas of healthcare (4, 21, 32, 34, 36, 38, 61, 64, 69, 83, 75, 77, 79, 85, 112, 119, 125, 197, 202). The fact that gene therapy could be cost-effective compared to current therapies (50, 53, 55, 69, 143, 162, 164, 189, 202, 215) opens the possibility that gene therapy can be available for universal access to health care (86, 197).
Public perception
There is an ambivalence about the perception of gene therapy (208). Some authors show that people are unaware of the term “gene therapy” and its availability (69, 86, 97, 126). Others reported that there is no public trust in gene therapy (4, 8, 127) and that gene therapy has a long way to go before gaining widespread acceptance (180). The frequent reasons for not accepting gene therapy are fears of adverse effects, high cost, and a belief that it went against nature (180, 216). There are concerns about the political uses of gene technology, genetic discrimination, and misuse of power (180, 208). The possible consequences of manipulating genes or designing humans arise fear (9, 15, 60, 86, 93, 97, 98, 101, 105, 106, 126, 212). People think it is a risky procedure (127). It still provokes negative emotional reactions due to the stories of deaths (23, 62, 121, 131, 150, 163, 165, 210). On the other hand, many articles describe that there is high public support for the use of gene therapy to cure serious diseases but not for human enhancement (9, 19, 45, 50, 61, 63, 66, 67, 73, 74, 81, 85, 90, 97, 101, 106, 107, 113, 144, 167, 180, 212). Gene therapy is seen by most as a desirable extension to the range of available medical options (179) and people are interested in learning about gene therapy (212). The guarantee of sound research in general and the safety of patients is crucial for public support and recruitment (146).
With regard to religions, if it is for therapeutic purposes, gene therapy is accepted and encouraged, as long as proper precautions are taken (186, 198) considering that genetic manipulation leads to a delicate issue about soul alteration (186).
Human health
A common argument with respect to human health is that gene therapy could prevent and/or treat serious diseases that cause humanity to suffer and improve quality of life (60, 64, 68, 69, 73, 74, 80, 83, 84, 133, 140, 143, 169, 182, 185, 192, 199, 211). Furthermore, it could be the only possibility of treatment in particular diseases (11, 23, 31, 43, 50, 60, 62, 68, 70, 110, 111, 123, 128, 175, 179, 181, 182, 183, 185, 192). Therefore, there is a moral obligation to develop gene therapy if we consider it to be the only treatment for particular diseases (12, 19, 33, 36, 76, 125, 129, 194). It is also underlined that gene therapy has many potential applications, in addition to its application in monogenetic diseases (59, 62, 64, 69, 70, 73, 145, 161, 175, 181). Not only what gene therapy could do, but how: gene therapy may provide a curative rather than a symptomatic approach to diseases (143), holds the promise of preventing diseases (155) and restoring functions (175). An article presents that the progress in gene therapy is clearly relevant to women’s health for understanding and treating common diseases (197). Two important points were that gene therapy could avoid anxiety associated with the life-threatening nature of the underlying disease (53) and that therapeutic abortion could be rare if genetic diseases could be treated (53, 129).
Implementation
Gene therapy could create problems in its implementation in medicine (38, 59, 66, 67, 68, 131, 159, 165, 193, 194, 213). Specific standard operational procedures and cooperation between healthcare workers may be needed (64). Furthermore, some authors said that a genetic diagnosis is needed prior to therapy, so it should already be available (56, 81, 84, 123, 189). Therefore, if alternative treatment exists, the use of gene therapy will depend on its efficiency, costs, and level of discomfort for patients (59).
Communication with society
Many articles support the need for public trust on the basis of proper knowledge and transparency in the research process (14, 15, 17, 62, 68, 66, 81, 84, 90, 100, 108, 150, 152, 161, 163, 165, 184, 213). Hence, public opinion should be adequately informed about gene therapy (81), and scientists must spend adequate time communicating science to the media (8, 137, 149, 212).
According to some authors, terminology has been shown to influence risk and benefit perception (205, 209), and here the term “gene therapy” used in research does not reflect whether it is a therapy or research (50, 53, 54, 89, 93, 95, 104, 107, 113, 117, 124, 150, 161, 201, 204, 213). It has been shown that the potential of somatic cell gene therapy may have been exaggerated, especially in relation to the timeline of its successful implementation (202, 216) with a tendency to amplify potential benefits and minimize potential risks (68, 66, 78, 124, 134, 190). Reinforcing this, the oversell of gene therapy research could cause a slowdown in gene therapy if something bad happens (155). As an emotionally volatile topic, if no patient is helped, the negative reaction can halt the entire field of gene therapy (169). However, advances have been made during the last few years, and there are reasons to hope clinically important results will be presented (175).
Playing God
Some articles came with the topic of “playing God,” referring to actions that could be done without any limit and have serious effects on people’s lives, as someone could have unlimited power. Some stated that humankind should not play God (76, 81, 91, 106, 122, 157, 167, 208), others that we are not playing God with gene therapy, as science is a human activity (127), and that there may be both proper and improper ways of “playing God” (203).
Discussion
To our knowledge, this article constitutes the first systematic review of reasons in bioethics for somatic gene therapy. Systematic reviews of reasons are relatively new in descriptive ethics. Recent articles that have applied this method include bioethical debates about organoids modelsCitation20, permissibility in research with great apesCitation21, germline modificationsCitation22, genome editing in non-human animalsCitation23, among others. Systematic reviews of reasons provide broader perspective of the chosen topic.
Somatic gene therapy following conventional techniques has the potential to be a major step in science and humanity’s well-beingCitation5. After analyzing all the arguments provided in this review, we can agree that at the same time, this technology could have repercussions on a massive scale and we do not have clear answers how to deal with these challengesCitation6,Citation11,Citation24.
The impact that gene therapy could have -or already has had- on society is different from any other social impact of a non-genetic health-related biotechnologyCitation13,Citation24,Citation25. The role that we give to genes impacts in how we understand our health and the functionality of our bodyCitation26. We are targeting genes as mediators of human illness, which play a role in some kinds of disease; but they are not always the whole explanation and the social considerations surrounding them should be seriously consideredCitation13,Citation27. Somatic gene therapy on a massive scale could have repercussions on human identityCitation24. For example, many deaf people do not consider themselves as a person with disability, but rather identify deafness as personal feature that is part of their identityCitation25. If somatic gene therapy could play a role in “treating” this diverse function through genes, then diverse functions could be seen just as a genetics problem. In this context, people with deafness might be seen as people with a genetic abnormality that may have impact on the identity of those who do not consider themselves with an abnormality. And in this context, deciding not to “treat the abnormality” will be out of a personal decision, but on the social framework of an abnormal who actually needs to correct the abnormality. The deaf situation is one example of how somatic gene therapy is very close to the genetic determination idea, and this is one of the reasons it is not similar to other non-genetics biotechnologies. Another specific issue is that we cannot guarantee that all people could eventually access this kind of therapy. This should be considered in advance, because there is a great risk of transforming genetics modifications into a social disadvantage based on the economic situation of a personCitation25.
Although new techniques in the genetic field, like CRISPR, raise ethical challenges and attention, we want to highlight the problems of conventional somatic gene therapy that already existCitation26. Debates on certain topics should not be marginalized because other challenges appear, but rather that there is a minimum consensus on the discussionCitation24,Citation28, which has not yet been consolidated in the case of conventional somatic gene therapy. As we demonstrate in this review of arguments, procedural, conceptual, and social issues about somatic gene therapy remain issues that need to be addressed.
Moreover, society should be part of the debate, defining priorities and limits in gene therapy research, ethical permissibility and nuances regarding its acceptance by certain communities and for certain usesCitation9,Citation24. All of this could also have positive influences on the development of the somatic gene therapy fieldCitation9.
Our analysis should be interpreted in light of the following limitations. First, there were some terms that were not included in the search strategy that may be associated with ethics and bioethics, for example informed consent or risks/benefits. This was intentional to make the systematic review feasible. Second, we are aware that a different group of researchers could have selected or grouped the included reasons in a different way. Third, we did not assess the scientific validity of the articles included.
Conclusion
This article is a starting point in a systematic re-evaluation of the ethical arguments before somatic gene therapy will transform into a massive-scale procedure. Our study provides a database of existing challenges and arguments of somatic gene therapy and may serve as the basis of normative analysis.
Transparency
S5 Appendix.docx
Download MS Word (57.8 KB)S3 Appendix.docx
Download MS Word (14 KB)S7 Appendix.docx
Download MS Word (35.6 KB)S1 Appendix.pdf
Download PDF (82.9 KB)S4 Appendix.docx
Download MS Word (19 KB)S2 Appendix.docx
Download MS Word (13.5 KB)S6 Appendix.docx
Download MS Word (693.1 KB)Acknowledgements
The authors thank Phyllis Zych Budka for linguistic edits and Lech Mazurczyk for figures design.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- National Human Genome Research Institute. Gene therapy; [accessed 2022 Dec 14]. Available from: https://www.genome.gov/genetics-glossary/Gene-Therapy
- U.S. Food & Drug Administration. What is gene therapy?; [accessed 2022 Dec 14]. Available from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy
- Flomemberg P, Daniel R. Overview of gene therapy, gene editing, and gene silencing; [accessed 2022 Dec 14]. Available from: https://www.uptodate.com/contents/overview-of-gene-therapy-gene-editing-and-gene-silencing
- Landhuis E. The definition of gene therapy has changed. Nature. 2021. doi: 10.1038/d41586-021-02736-8.
- Riva L, Petrini C. A few ethical issues in translational research for gene and cell therapy. J Transl Med. 2019;17(1):395. doi: 10.1186/s12967-019-02154-5.
- Freire J, Medeiros S, Lopes Neto A, et al. Bioethical conflicts of gene therapy: a brief critical review. Rev Assoc Med Bras (1992). 2014;60(6):520–524. doi: 10.1590/1806-9282.60.06.008.
- Alhakamy N, Curiel D, Berkland C. The era of gene therapy: from preclinical development to clinical application. Drug Discov Today. 2021;26(7):1602–1619. doi: 10.1016/j.drudis.2021.03.021.
- High K, Roncarolo M. Gene therapy. N Engl J Med. 2019;381(5):455–464. doi: 10.1056/NEJMra1706910.
- Delhove J, Osenk I, Prichard I, et al. Public acceptability of gene therapy and gene editing for human use: a systematic review. Hum Gene Ther. 2020;31(1-2):20–46. doi: 10.1089/hum.2019.197.
- Cwik B. Moving Beyond ‘therapy’ and ‘enhancement’ in the ethics of gene editing. Camb Q Healthc Ethics. 2019;28(4):695–707. doi: 10.1017/S0963180119000641.
- Aiyegbusi O, Macpherson K, Elston L, et al. Patient and public perspectives on cell and gene therapies: a systematic review. Nat Commun. 2020;11(1):6265. doi: 10.1038/s41467-020-20096-1.
- Kimmelman J. The ethics of human gene transfer. Nat Rev Genet. 2008;9(3):239–244. doi: 10.1038/nrg2317.
- McGuire A, Gabriel S, Tishkoff S, et al. The road ahead in genetics and genomics. Nat Rev Genet. 2020;21(10):581–596. doi: 10.1038/s41576-020-0272-6.
- Strech D, Sofaer N. How to write a systematic review of reasons. J Med Ethics. 2012;38(2):121–126. doi: 10.1136/medethics-2011-100096.
- Gibbs G. Analysing qualitative data, qualitative research kit. London: SAGE Publications; 2008.
- Sandelowski M, Barroso J. Handbook for synthesizing qualitative research. New York (NY): Springer Publishing Company; 2007.
- Piasecki J, Waligora M, Dranseika V. Google search as an additional source in systematic reviews. Sci Eng Ethics. 2018;24(2):809–810.
- Mezinska S, Kakuk P, Mijaljica G, et al. Research in disaster settings: a systematic qualitative review of ethical guidelines. BMC Med Ethics. 2016;17(1):62. doi: 10.1186/s12910-016-0148-7.
- Kahrass H, Borry P, Gastmans C, et al. PRISMA-ethics – reporting guideline for systematic reviews on ethics literature: development, explanations and examples. OSF Preprints. 2021. doi: 10.31219/osf.io/g5kfb.
- Barnhart A, Dierickx K. The many moral matters of organoid models: a systematic review of reasons. Med Health Care Philos. 2022;25(3):545–560. doi: 10.1007/s11019-022-10082-3.
- Aguilera B, Perez Gomez J, DeGrazia D. Should biomedical research with great apes be restricted? systematic review of reasons. BMC Med Ethics. 2021;22(1):15. doi: 10.1186/s12910-021-00580-z.
- van Dijke I, Bosch L, Bredenoord A, et al. The ethics of clinical applications of germline genome modification: a systematic review of reasons. Hum Reprod. 2018;33(9):1777–1796. doi: 10.1093/humrep/dey257.
- de Graeff N, Jongsma K, Johnston J, et al. The ethics of genome editing in non-human animals: a systematic review of reasons reported in the academic literature. Philos Trans R Soc Lond B Biol Sci. 2019;374(1772):20180106.
- Mills M, Tropf F. Sociology, genetics, and the coming of age of sociogenomics. Annu Rev Sociol. 2020;46(1):553–581. doi: 10.1146/annurev-soc-121919-054756.
- Harden K, Koellinger P. Using genetics for social science. Nat Hum Behav. 2020;4(6):567–576. doi: 10.1038/s41562-020-0862-5.
- Machirori M, Patch C, Metcalfe A. “It didn’t mean anything”–moving within a landscape of knowledge to interpret genetics and genetic test results within familial cancer concerns. New Genet Soc. 2021;40(4):570–598. doi: 10.1080/14636778.2021.1997575.
- Raz A, Pontarotti G, Weitzman J. Epigenetic metaphors: an interdisciplinary translation of encoding and decoding. New Genet Soc. 2019;38(3):264–288. doi: 10.1080/14636778.2019.1601009.
- Moor J. Why we need better ethics for emerging technologies. Ethics Inf Technol. 2005;7(3):111–119. doi: 10.1007/s10676-006-0008-0.