Abstract
Objective
To update on and describe the role of Disease Specific Programmes (DSPs), a multi-perspective real-world data (RWD) source, in the context of the evolution of the value and acceptance of real-world evidence (RWE) in clinical, regulatory and guideline decision-making.
Methods
DSPs are multi-national, multi-subscriber, multi-therapy cross-sectional surveys incorporating retrospective data collection from patient, caregiver and physician perspectives. Information collected covers the patient journey, including treatment/prescribing patterns and rationale, patient-reported outcomes, impact on work and everyday activities, attitudes towards and perceptions of the condition, adherence to treatment and burden of illness. Published peer-reviewed DSP papers were aligned with current key RWE themes identified in the literature, alongside their contribution to RWE.
Results
RWE themes examined were: using RWE to inform clinical practice, patient and caregiver engagement, RWE role in supporting health technology assessments and regulatory submissions, informing value-driven healthcare decisions, real-world patient subgroup differences and therapeutic inertia/unmet needs; highlighting patients’ and caregivers’ experience of living with a disease, disconnect from their physicians, unmet needs and educational gaps.
Conclusions
DSPs provide a wealth of RWD in addition to evidence generated by registries, clinical trials and observational research, with wide use for the pharmaceutical industry, government, funding/regulatory bodies, clinical practice guideline insights and, most importantly, informing improvements in people’s lives. The depth, breadth and heritage of information collected via DSPs since 1995 is unparalleled, extending understanding of how diseases are managed by physicians in routine clinical practice and why treatment choices are made, patients’ perceptions of their disease management, and caregiver burden.
Introduction
Real-world data (RWD) are data about the health of patients and the routine delivery of healthcare, collected using a variety of methods other than traditional clinical trials (CTs).Citation1 Sources of RWD are generally observational and/or non-interventional, i.e. interventions are not determined by a study protocol but rather decided by healthcare professionals (HCPs) and patients according to clinical need. Examples of non-interventional sources of RWD include pre-existing data such as electronic medical records (EMRs), claims and administrative data. RWD can also be generated as primary data. An example of experimental and/or interventional study that can generate RWD is a pragmatic CT. RWD can be either quantitative or qualitative.Citation2
RWD help to improve understanding of health and social care delivery, patient health and experiences, and any effects of interventions on the patient and their outcomes in routine clinical practice settings.Citation2–4 RWD can also provide more representative and faster access to information compared with findings from more traditional research sources such as randomized controlled trials (RCTs) and observational clinical studies.Citation5 In particular, real-world studies, unlike RCTs, have wider eligibility criteria which often include patients with comorbidities or populations from diverse cultural and ethnic backgrounds.Citation6 Similarly, RWD may provide a more appropriate split of data across the sexes, making them more likely to be generalizable to the wider patient population than RCTs.Citation7 In addition, the restrictive nature of RCTs can mean that real-world studies can be a valuable way to gather data on smaller populations, such as those with rare diseases, as, with their less restrictive methodologies, researchers can maximize the number of patients eligible for inclusion and increase samples to improve statistical power.
Real-world evidence (RWE), which is derived from RWD, can provide clinical evidence regarding the use and possible benefits/risks of medical products, how drugs are being used and by whom, patient-reported outcomes (PROs) and impact on caregivers. It can also offer information regarding the effectiveness of healthcare delivery and services, and identify unmet needs.Citation4 RWD and resulting RWE are now accepted by decision-makers and stakeholders including governments, regulators, payors and clinicians to inform healthcare policy, guidance and decisions. As global interest in RWE has grown, there has been increasing scrutiny of RWD collection methods and the appropriateness of the analyses that can be performed using specific data sources.Citation4,Citation8
Questions that can only be properly addressed using RWD and RWE include: ‘Are the best standards of care followed in routine clinical practice and if not, why?’ ‘Are patients adhering to prescribed treatment and if not, why?’ ‘What is the burden – both humanistic and economic – on professional and non-professional caregivers?’ ‘Are key players in the patient journey aligned on their understanding of the disease burden, what is most important to the patient and, consequently, what is the best management approach for that patient and in general?’
The Adelphi Real World Disease Specific Programmes (DSPs) have been a valuable and referenced source of RWD since their inception in 1995.Citation9 They are well-positioned to answer many of these questions and needs, across multiple geographies and a multitude of disease areas, with a consistent approach. The 2008 publication described the DSP methodology and utility in the context of data sources including CTs and RWD.Citation9 Since then, the number of data sources and variety of methodologies, coupled with an increased understanding, acceptance, use of and need for RWD, have resulted in an exponential growth in both the sources and accepted value of RWD.
The aim of this paper was to give an update on the DSP methodology and describe the role that DSPs can play as a multi-perspective source of RWD, demonstrating how DSPs have contributed to RWE generation, in the context of the evolution of the value and acceptance of RWD, and the RWE derived from RWD, in clinical, regulatory and guideline decision-making.
Methods
Description of data source
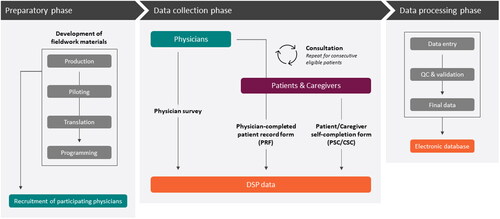
DSPs are multi-national, multi-subscriber, multi-therapy cross-sectional surveys with elements of retrospective data collection from patients, their caregivers and physicians. Each DSP is assembled through primary data collection conducted by Adelphi Real World. Once this data set has been assembled, it is available for use on subscription as a secondary data source. Each DSP is an independent source of data, owned by Adelphi Real World. The methodology has been previously described,Citation9 validated,Citation10 and demonstrated to be representative and consistent over time.Citation11 DSPs are designed without a prior hypothesis to holistically and impartially understand patients, the impact of their disease and associated treatment/management approaches in real-world settings, reflecting current real-world clinical practice irrespective of national or international clinical guidelines. The results of DSP studies are not intended to provide direct advice for physicians about how to best care for individual patients, but rather to influence decision-making on a population level through the publication of their findings in clinical journals.
Each DSP comprises data from physicians and their consulting patients and, where possible and relevant, patients’ caregivers. Eligible physicians are identified from public lists of HCPs by independent fieldwork partners familiar with local healthcare systems, according to predefined selection and eligibility criteria. Physicians are eligible to participate in a DSP subject to minimal inclusion criteria ensuring they have the relevant clinical specialty, see a required minimum number of eligible patients, make treatment and management decisions for these patients, and can provide insights around why clinical decisions are made.
Once selected, physicians complete an online survey including their disease management approach, prescribing of current and awareness of future drugs, and their patient caseload. Each physician then completes a detailed online patient record form for eligible patients who present to them consecutively with a physician-identified diagnosis of the relevant disease. These physician-completed patient records include, among other things, demographic and clinical characteristics, tests and investigations conducted (including results where known), disease and treatment history, comorbidities and healthcare resource utilization (HCRU), supplemented by questions exploring the reasons behind the decision-making for that individual patient. DSPs collect only information available to the physician/HCP at the time of consultation. This includes findings garnered from the conversation between physician and patient during the consultation, as well as retrospective data from the patient’s medical record.
The same patients for whom a physician-completed patient record form has been provided are themselves then invited to fill out a questionnaire independently and unaided. These patient self-completion forms include validated disease-specific and general PRO measures (PROMs) covering, for example, health-related quality of life (HRQoL), symptom assessment, impact on activities of daily living and adherence to treatment. Both patients and physicians provide subjective assessment on disease severity at various timepoints, symptoms and satisfaction with disease control. If a patient decides not to complete a self-completion form, this does not disqualify data recorded by the physician for that patient from being included in the database.
Where appropriate, caregivers accompanying the patient provide information regarding the amount and type of care they provide, the impact on their own ability to perform usual activities, including work, and their own quality of life (QoL). They may also complete proxy measures of disease impact on behalf of the patient. Caregiver forms are often used where the patients are too young or too impaired by their disease to complete a form themselves.
Data collection forms for each disease area are designed by a dedicated DSP project team with review by internal therapy experts plus specialist statistics, health outcomes, scientific and compliance teams at Adelphi Real World. Qualitative development interviews with patients and physicians are undertaken to ensure questions are clear, appropriate for the disease area and clinically meaningful. For some disease areas, partnerships with academic collaborators and patient advocacy groups (PAGs) are developed. To ensure continued relevance, each time a DSP is run in a specific condition for a second or subsequent time, the fieldwork materials are updated to incorporate changes in healthcare delivery, clinical guidelines, regulatory policy and payment mechanisms; to include new/different treatment alternatives; and to ensure novel topics important to patients, caregivers and physicians are included.
DSP forms are developed in British English and, once programmed, the online text is then translated into the language of the study country by an accredited translation agency. The DSP fieldwork agency proofreads the online translated text ensuring it reflects country-specific differences in healthcare provision and, once satisfied, it approves the link for use in the target market. This ensures fieldwork materials are consistent across countries.
A proportion of data completed via online links is quality-checked by the DSP project team during the data collection period. Checking includes an assessment of data completeness, questionnaire logic and the speed at which participants complete forms. If issues are uncovered, then local fieldwork partners may recontact participating physicians to clarify answers and, where appropriate, make corrections. However, patients cannot be recontacted after they have provided data. In cases where the integrity of data provided by a physician is questioned, for example, if a physician was seen to be providing the same/similar data for a number of patients, completing forms in an unusually short time, providing minimal responses, etc., data from that physician would be removed as necessary.
Each DSP is submitted to an institutional review board/ethics committee for review. Data are collected in line and full accordance with relevant data protection guidelines and legislation.Citation12–14 All participants provide informed consent to take part in the survey. Data are collected in such a way that participants cannot be identified. Physicians are compensated for participation according to fair market rates.
Sample size and data analysis
DSPs do not require any sample size calculations as data are collected without any predetermined hypotheses in mind. The sample size is chosen simply to give a reasonable level of precision within some potentially interesting subgroups (e.g. individual countries). As a secondary data source, subscribers develop their own analysis plans/protocols in partnership with Adelphi Real World and, where relevant, thought leaders. An overview of how DSPs are designed, implemented and analyzed is shown in .
Figure 1. Overview of how DSPs are designed, implemented and analyzed.
CSC, caregiver self-completion form; DSP, Disease Specific Programme; PRF, patient record form; PSC, patient self-completion form; QC, quality control
Source: Adapted with permission from the DSP methodology previously published in 2008.Citation9 To access the original source, please, visit https://www.tandfonline.com/
Identifying key RWE themes
In order to assess DSP contribution to the RWE landscape, we reviewed all peer-reviewed papers based on DSP data published between December 2001 and September 2023. DSP publications were broadly aligned with current key RWE themes based on trends identified by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).Citation15
Results
DSPs have been conducted in both common and rare conditions across the majority of healthcare specialties, including autoimmune (covering dermatology, gastroenterology and rheumatology), rare (covering hematology, immunology, nephrology, neurology, pulmonology and multi-organ/other diseases), cardiovascular, metabolic, renal, oncology, psychiatry, neurology, respiratory, ophthalmology, urology, infectious and other diseases. Peer-reviewed papers, using DSP data (n = 301), were categorized by topic (n = 13) which were then grouped into current key RWE themes (n = 6), as shown in .
Table 1. Current key RWE themes covered by DSPs with example findings supporting clinical, regulatory and guideline decisions.
Assessment of insights gathered from a representative subset (n = 45) of published peer-reviewed DSP papers is presented in , demonstrating the utility and application of this methodology in supporting clinical, regulatory and guideline decisions.
Discussion
We aimed to provide an update on the DSP methodology since the original publication in 2008,Citation9 including DSPs’ contribution to the evolution of the value and acceptance of RWD and RWE in clinical, regulatory and guideline decision-making by aligning more than 300 published peer-reviewed papers using DSP data with current key themes in RWE.
The majority of papers using DSP data have been published in clinical journals because, as well as influencing decision-making on a macro level, it is also important that individual physicians have access to the findings and can choose to incorporate any learnings into their own clinical practice as they see appropriate. Publications analyzed spanned a number of specialties (). Thirteen topics were grouped into six themes: using RWE to inform clinical practice, patient and caregiver engagement, role of RWE in supporting health technology assessments and regulatory submissions, informing value-driven healthcare decisions, real-world patient subgroup differences and therapeutic inertia/unmet needs. The consistent, validated methodology across DSPs has contributed to them becoming a valued source of RWD.Citation9–11 Building on such robust methodology, the range of publication examples supporting the identified themes underlined how DSPs have enabled gather of RWE for many types of analyses and provided insights into market understanding, treatment patterns, patient subpopulations, unmet needs, humanistic and economic disease burden, patient and caregiver engagement, and degree of alignment between patients, caregivers and healthcare providers.Citation16–28 They have also enabled evidence generation in support of payor and regulatory submissions, identified gaps between clinical practice guidelines (CPGs) and clinical practice, and, ultimately, influenced CPG recommendations.Citation18,Citation29–33
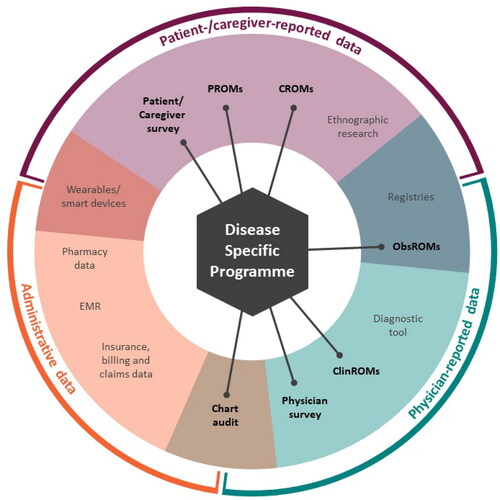
Figure 2. A map of the RWD source landscape and the placement of DSPs within it.
ClinROM, clinical-reported outcome measure; CROM, caregiver-reported outcome measure; DSP, Disease Specific Programme; EMR, electronic medical record; ObsROM, observer-reported outcome measure; PROM, patient-reported outcome measure; RWD, real-world data
RWD and RWE in general have become invaluable sources of information in the pharmaceutical, healthcare and regulatory space, increasingly demanded and accepted by key stakeholders, to inform healthcare policy, guidance and decision-making.Citation34 While historically stakeholder use of RWE was low, advances in technology to collect RWD, and its resulting ubiquity, have meant the availability, quality and use of RWD and RWE have grown enormously over the last ten years. Use of RWD and RWE has also increased following the 2016 21st Century Cures Act in the United States of America, a legislation which advanced medical technological developments and approvals by incorporating RWE and the patient perspective into assessments, enabling new treatments to reach patients faster.Citation35,Citation36 Subsequently, the formalization of the framework for the Food and Drug Administration Real-World Evidence Program in 2018 has provided stakeholders with fundamental guidance on how to produce and use RWD robustly.Citation3 ISPOR has identified that the most frequent uses of RWE in 2023 included informing value-driven and healthcare decision-making, addressing disparities, incorporating the patient voice and enabling cross-country comparisons,Citation15 some of which were covered by and discussed in DSP publications ().
As well as DSPs, there are many alternative RWD sources that can generate RWE. These include both pre-existing data collected for other purposes and primary data to answer specific research questions. Each data source has its own strengths and weaknesses. A broad landscape of evidence generation, including RWD and DSPs, is shown in . Data sources such as the DSPs do not fit easily into existing hierarchies of evidence as they include various elements associated with a number of these data sources. For example, DSPs routinely capture evidence derived from patient/caregiver surveys, patient-, caregiver-, observer- and clinical-reported outcome measures, physician surveys and retrospective data with elements similar to chart audits, while also documenting thought processes involved in making clinical and prescribing decisions, as illustrated in . Although DSPs are an invaluable component of the RWE landscape and can answer many key questions, in some cases, multiple sources in combination may be required.
It could be argued that the DSPs mirror some of the elements of claims/medical records due to the inclusion of retrospective data. However, DSPs collect evidence that is not available from EMR databases. For instance, as well as providing RWE about which clinical decisions were made, DSPs also capture details around why decisions were made, for example, regarding diagnostic processes, treatment goals and associated reasons for choice of drugs/other treatments, together with information regarding satisfaction with treatment and overall disease control.Citation16,Citation17,Citation30,Citation37,Citation38 Another important distinction is that DSPs collect evidence directly from the patient and, where appropriate, the caregiver.Citation27,Citation38–40 As well as validated PROMs, patients provide insights from their own perspective including severity, satisfaction, adherence and activities of daily living.Citation22,Citation41,Citation42 Caregivers can also provide information as a proxy for the patient and regarding the impact of the patient’s condition on their own lives. The patient/caregiver perspective is increasingly being recognized as important to inform healthcare decisions, with ISPOR identifying previously unrecognized elements of value that should be incorporated into such assessments.Citation43,Citation44 Many of these new elements comprise patient/caregiver-centric measures including the value of hope, fear of contagion, value of knowing (that they have a disease or treatment they may receive) and family spillover (impact of patients’ disease on caregivers and other family members).Citation43,Citation44 Understanding the impact of these aspects on patient and caregiver HRQoL can help decision-makers to obtain a more well-rounded view of a health intervention’s value. For example, a diagnostic test may greatly improve patient HRQoL despite having no immediate treatment outcome as it can inform the patient of what future treatment plans may look like or involve, which could alleviate their worries or enable them to feel more knowledgeable and/or involved in decisions about their journey as a patient.Citation43
Prospective data sources such as registries capture data on specific diagnoses or treatments. They serve to evaluate the progression of disease, response to treatment and, ultimately, the outcomes of patients with/at risk of a specific disease or condition.Citation45 They can also be used to recruit patients to RCTs and develop treatments to target diseases,Citation46 and provide information that can complement RCT findings. A drawback of registries is that they often take many years to develop and run, and can be expensive. Registries are also usually developed with specific hypotheses in mind which can limit their use in answering more general questions.Citation45 In contrast, while DSPs do not currently provide prospective longitudinal information on disease progression and patient outcomes, they do provide historical information, as well as detailed information about why physicians make certain treatment decisions, how well patients feel their disease is being managed and the patient-physician relationship.Citation47–50
The key strengths of the DSPs are that, as a RWD source, they include routinely consulting patients with a given condition as opposed to the selective patient populations included in RCTs because of age restrictions, requirements to meet stringent eligibility criteria and the need for protocol-driven consultations.Citation5 Through the collection of routine clinical practice data, DSPs also include patients who may be less likely to be adherent to medication than those included in RCTs.Citation51 All patients included in DSPs have their diagnosis subjectively confirmed by the participating physicians without resorting to reliance on International Classification of Diseases codes. This can include specific patient subpopulations within a wider condition. DSPs provide RWE from patients, caregivers and physicians who are representative of the consulting population, and the matching of these data allows for patient, caregiver and physician alignment analyses. Collection of data from these multiple participant sources and representing their voices also enable the understanding of PRO results in the context of patient-, caregiver- and physician-reported data on disease severity, symptomatology and treatment. A unique element of the DSPs is the consistent methodology and data capture from participants within and between countries resulting in the ability to compare treatment patterns and outcomes across multiple regions and, since individual DSPs are repeated, over time.
Limitations of the DSP methodology must also be recognized. Participation by patients, caregivers and physicians is voluntary, and participating physicians may be more likely to be open to engaging with surveys. Similarly, the DSP criteria do not require patient samples to be representative of the population in terms of race, income, social class or age. Participating patients may not reflect the general therapy area population since the DSP only includes patients who consult with their physician and have access to healthcare. This means that patients who cannot access healthcare are not represented and those who consult more frequently might have a higher likelihood of being included. Patient eligibility is based on the judgement of the respondent physician and not on a formalized diagnostic checklist; however, it is representative of the physicians’ real-world classification of their patients. While DSPs collect retrospective data from physicians with regards to treatment patterns, clinical severity and HCRU, it must be noted that this is in the context of their cross-sectional methodology approach. Although retrospective elements of data collection, such as disease severity at different timepoints, are gathered, the fact that data are not captured longitudinally means establishing causality is not possible. However, it is possible to identify associations, for example, between variables such as treatment duration or disease severity and HRQoL. Recall bias, a common limitation of surveys, might also affect responses of both patients and physicians. However, physicians have the ability to refer to patients’ records while completing patient record forms, thus minimizing the possibility of recall bias. Furthermore, data are collected at the time of each patient’s appointment to reduce the likelihood of recall bias where the opinion of the physician is required. Compared with claims/medical records, the DSP does not contain full description of the patient journey since diagnosis and the samples sizes may be smaller. The patient self-completion element of DSPs also includes PROMs, each of which has a specific recall period that has been deemed appropriate during the measure’s design and validation process. It may well be the case that combining evidence from claims/EMR and DSPs, through the use of common variables such as demographics and clinical characteristics, can provide an even stronger source of RWE. The DSP is one of many RWD sources, and insights gathered from DSPs can complement, not replace, information and evidence generated from other types of sources and/or research.
Conclusions
RWD and RWE are established as an invaluable source of information used by patients, their caregivers and physicians, government and non-government bodies, and the pharmaceutical industry. They do not replace, but add to, the body of evidence generated via more traditional research routes. The same is true for DSPs which, for more than two decades, have collected a broad cross-section of RWD and provided RWE, showcasing how real patients, their caregivers and physicians behave in routine clinical practice. Their value and perspective, breadth of disease areas, global reach, and consistent methodology over time and between countries, combined with depth and level of detail, make the DSPs a unique, independent and invaluable source of RWD. In this paper, we have provided an update on the development of the DSP methodology since the original description published in 2008 and presented examples of the type of evidence generated through analysis of DSP data. DSPs will continue to evolve along with the RWD/RWE landscape. Future developments and directions might include examining the impact of the uptake of new products from a payor’s perspective, acting as a virtual control arm in clinical studies, and addressing disparities in healthcare between diverse population subgroups and potentially between countries, with the ultimate desire to inform and contribute to improvements in people’s lives.
Transparency
Acknowledgements
The authors would like to thank Jack Wright of Adelphi Real World for the design of the figures.
Declaration of financial/other relationships
Peter Anderson, Victoria Higgins, Jonathan de Courcy, Katerina Doslikova, Victoria A. Davis, Maria Karavali and James Piercy are employees of Adelphi Real World.
Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Additional information
Funding
References
- Food and Drug Administration (FDA). Real-world evidence. 2023 [cited 26 October 2023]; Available from: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence.
- National Institute for Health and Care Excellence (NICE). NICE real-world evidence framework. 2022 [cited April 2023]; Available from: https://www.nice.org.uk/corporate/ecd9/chapter/introduction-to-real-world-evidence-in-nice-decision-making.
- Food and Drug Administration (FDA). Framework for FDA’s Real-World Evidence Program. 2018 [cited June 2020]; Available from: https://www.fda.gov/media/120060/download.
- Food and Drug Administration (FDA). Use of electronic health record data in clinical investigations guidance for industry. 2018 [cited June 2020]; Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-electronic-health-record-data-clinical-investigations-guidance-industry.
- Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33(34):e213. doi: 10.3346/jkms.2018.33.e213.
- Tan YY, Papez V, Chang WH, et al. Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longev. 2022;3(10):e674–e89. doi: 10.1016/S2666-7568(22)00186-6.
- Schroeder M, Lim YMF, Savarese G, et al. Sex differences in the generalizability of randomized clinical trials in heart failure with reduced ejection fraction. Eur J Heart Fail. 2023;25(6):912–921. doi: 10.1002/ejhf.2868.
- Berger M, Daniel G, Frank K, et al. A framework for regulatory use of real-world evidence 2017 [cited 22 May 2023]; Available from: https://healthpolicy.duke.edu/sites/default/files/2020-08/rwe_white_paper_2017.09.06.pdf.
- Anderson P, Benford M, Harris N, et al. Real-world physician and patient behaviour across countries: disease-Specific programmes – a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040.
- Babineaux SM, Curtis B, Holbrook T, et al. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the disease specific programme. BMJ Open. 2016;6(8):e010352. doi: 10.1136/bmjopen-2015-010352.
- Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–380. doi: 10.2147/DMSO.S120101.
- US Department of Health and Human Services. Summary of the HIPAA privacy rule. 2003;2020(May).
- Health Information Technology (HITECH). Health Information Technology Act. 2009;2020(May).
- European Pharmaceutical Market Research Association (EphMRA). Code of Conduct. 2021 [cited January 2023]; Available from: https://www.ephmra.org/sites/default/files/2022-03/2021%2520EPHMRA%2520Code%2520of%2520Conduct%25202.11.21.pdf.
- International Society for Pharmacoeconomics and Outcomes Research (ISPOR). 2022–2023 Top 10 HEOR trends. 2022 cited January 2023]; Available from: https://www.ispor.org/docs/default-source/ispor-good-practices-for-outcomes-research-index/ispor_top10-2022-2023_online.pdf?sfvrsn=61a9ec28_2.
- Hall JP, Chang J, Moon R, et al. Real-world treatment patterns in patients with advanced (stage III-IV) ovarian cancer in the USA and Europe. Future Oncol. 2020;16(15):1013–1030. doi: 10.2217/fon-2020-0083.
- Rane PB, Patel J, Harrison DJ, et al. Patient characteristics and real-world treatment patterns among early users of PCSK9 inhibitors. Am J Cardiovasc Drugs. 2018;18(2):103–108. doi: 10.1007/s40256-017-0246-z.
- Patel V, Horn EJ, Lobosco SJ, et al. Psoriasis treatment patterns: results of a cross-sectional survey of dermatologists. J Am Acad Dermatol. 2008;58(6):964–969. doi: 10.1016/j.jaad.2008.02.048.
- Pillas D, Klein A, Gasalla T, et al. The burden of progressive supranuclear palsy on patients, caregivers, and healthcare systems by PSP phenotype: a cross-sectional study. Front Neurol. 2022;13:821570. doi: 10.3389/fneur.2022.821570.
- Iyer S, Taylor-Stokes G, Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer. 2013;81(2):288–293. doi: 10.1016/j.lungcan.2013.03.008.
- Wei W, Anderson P, Gadkari A, et al. Extent and consequences of inadequate disease control among adults with a history of moderate to severe atopic dermatitis. J Dermatol. 2018;45(2):150–157. doi: 10.1111/1346-8138.14116.
- Sadosky AB, Bushmakin AG, Cappelleri JC, et al. Relationship between patient-reported disease severity in osteoarthritis and self-reported pain, function and work productivity. Arthritis Res Ther. 2010;12(4):R162. doi: 10.1186/ar3121.
- Canonica GW, Bousquet J, Mullol J, et al. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007;85(62 Suppl):17–25.
- Christophers E, Barker JN, Griffiths CE, et al. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European Dermatology Clinics. J Eur Acad Dermatol Venereol. 2010;24(5):548–554. doi: 10.1111/j.1468-3083.2009.03463.x.
- Ford JH, Jackson J, Milligan G, et al. A real-world analysis of migraine: a cross-sectional study of disease burden and treatment patterns. Headache. 2017;57(10):1532–1544. doi: 10.1111/head.13202.
- Small M, Vickers A, Anderson P, et al. The patient-physician partnership in asthma: real-world observations associated with clinical and patient-reported outcomes. Adv Therapy. 2010;27(9):591–599. doi: 10.1007/s12325-010-0054-1.
- Martinez-Martin P, Skorvanek M, Henriksen T, et al. Impact of advanced Parkinson’s disease on caregivers: an international real-world study. J Neurol. 2023;270(4):2162–2173. doi: 10.1007/s00415-022-11546-5.
- Vilsboll AW, Anderson P, Piercy J, et al. Extent and impact of inadequate disease control in US adults with a history of moderate to severe atopic dermatitis following introduction of new treatments. Dermatol Ther (Heidelb). 2021;11(2):475–486. doi: 10.1007/s13555-021-00488-x.
- Anstee QM, Hallsworth K, Lynch N, et al. Real-world management of non-alcoholic steatohepatitis differs from clinical practice guideline recommendations and across regions. JHEP Rep. 2022;4(1):100411. doi: 10.1016/j.jhepr.2021.100411.
- Small M, Anderson P, Vickers A, et al. Importance of inhaler-device satisfaction in asthma treatment: real-world observations of physician-observed compliance and clinical/patient-reported outcomes. Adv Therapy. 2011;28(3):202–212. doi: 10.1007/s12325-010-0108-4.
- Lautsch D, Boggs R, Wang T, et al. Individualized HbA(1c) goals, and patient awareness and attainment of goals in type 2 diabetes mellitus: a real-world multinational survey. Adv Ther. 2022;39(2):1016–1032. doi: 10.1007/s12325-021-01985-3.
- Malaty IA, Martinez-Martin P, Chaudhuri KR, et al. Does the 5-2-1 criteria identify patients with advanced Parkinson’s disease? Real-world screening accuracy and burden of 5-2-1-positive patients in 7 countries. BMC Neurol. 2022;22(1):35. doi: 10.1186/s12883-022-02560-1.
- National Institute for Health and Care Excellence (NICE). Axicabtagene ciloleucel for treating relapsed or refractory follicular lymphoma. 2023 [cited September 2023]; Available from: https://www.nice.org.uk/guidance/TA894.
- European Medicines Agency (EMA). Global regulators call for international collaboration to integrate real-world evidence into regulatory decision-making. 2022; Available from: https://www.ema.europa.eu/en/news/global-regulators-call-international-collaboration-integrate-real-world-evidence-regulatory-decision#:∼:text=EMA%20has%20endorsed%20a%20joint,Medicines%20Regulatory%20Authorities%20(ICMRA).
- Food and Drug Administration (FDA). 21st Century Cures Act. 2016 [cited 08 June 2020]; Available from: https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act.
- Khosla S, White R, Medina J, et al. Real world evidence (RWE) – a disruptive innovation or the quiet evolution of medical evidence generation? F1000Res. 2018;7:111.
- Conaghan PG, Abraham L, Viktrup L, et al. Impact of osteoarthritis disease severity on treatment patterns and healthcare resource use: analysis of real-world data. Scand J Rheumatol. 2023;52(4):353–363. doi: 10.1080/03009742.2022.2058168.
- Fitzgerald HM, Shepherd J, Bailey H, et al. Treatment goals in schizophrenia: a real-world survey of patients, psychiatrists, and caregivers in the United States, with an analysis of current treatment (long-acting injectable vs oral antipsychotics) and goal selection. NDT. 2021;ume 17:3215–3228. doi: 10.2147/NDT.S330936.
- Black CM, Ritchie CW, Khandker RK, et al. Non-professional caregiver burden is associated with the severity of patients’ cognitive impairment. PLoS ONE. 2018;13(12):e0204110. doi: 10.1371/journal.pone.0204110.
- Lahoz R, Proudfoot C, Fonseca AF, et al. Caregivers of patients with heart failure: burden and the determinants of Health-Related quality of life. PPA. 2021;ume 15:1153–1164. doi: 10.2147/PPA.S297816.
- Chhabra A, Spurden D, Fogarty PF, et al. Real-world outcomes associated with standard half-life and extended half-life factor replacement products for treatment of haemophilia a and B. Blood Coagul Fibrinolysis. 2020;31(3):186–192. doi: 10.1097/MBC.0000000000000885.
- Sullivan E, Piercy J, Waller J, et al. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLoS ONE. 2017;12(4):e0175826. doi: 10.1371/journal.pone.0175826.
- Neumann PJ, Garrison LP, Willke RJ. The history and future of the "ISPOR value flower": addressing limitations of conventional cost-effectiveness analysis. Value Health. 2022;25(4):558–565. doi: 10.1016/j.jval.2022.01.010.
- Lakdawalla DN, Doshi JA, Garrison LP, Jr., et al. Defining elements of value in health care – a health economics approach: an ISPOR special task force report [3]. Value Health. 2018;21(2):131–139. doi: 10.1016/j.jval.2017.12.007.
- Richesson R, Vehik K. Patient registries: utility, validity and inference. Adv Exp Med Biol. 2010;686:87–104.
- Tan MH, Thomas M, MacEachern MP. Using registries to recruit subjects for clinical trials. Contemp Clin Trials. 2015;41:31–38. doi: 10.1016/j.cct.2014.12.012.
- Wirta SB, Balas B, Proenca CC, et al. Perceptions of heart failure symptoms, disease severity, treatment decision-making, and side effects by patients and cardiologists: a multinational survey in a cardiology setting. TCRM. 2018;ume 14:2265–2272. doi: 10.2147/TCRM.S183200.
- Soriano ER, Zazzetti F, Alves Pereira I, et al. Physician-patient alignment in satisfaction with psoriatic arthritis treatment in Latin America. Clin Rheumatol. 2020;39(6):1859–1869. doi: 10.1007/s10067-019-04870-1.
- Sikirica MV, Martin AA, Wood R, et al. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. DMSO. 2017;ume 10:403–412. doi: 10.2147/DMSO.S141235.
- Wang C, Aranishi T, Reed C, et al. Impact of patient and physician disconnect on satisfaction with treatment for atopic dermatitis in Japan. Dermatol Ther (Heidelb). 2023;13(2):505–522. doi: 10.1007/s13555-022-00866-z.
- Hubbard TE, Paradis R. Real-World evidence: a new era for health care innovation the network for excellence in health innovation. 2015.
- Vestbo J, Vogelmeier C, Small M, et al. Understanding the GOLD 2011 strategy as applied to a real-world COPD population. Respir Med. 2014;108(5):729–736.
- Smid DE, Franssen FME, Gonik M, et al. Redefining cut-points for high symptom burden of the global initiative for chronic obstructive lung disease classification in 18,577 patients with chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2017;18(12):1097 e11–97 e24.
- Black CM, Woodward M, Ambegaonkar BM, et al. Quantifying the diagnostic pathway for patients with cognitive impairment: real-world data from Australia. Int Psychogeriatr. 2020;32(5):601–610. doi: 10.1017/S1041610219001856.
- Horvath Walsh LE, Rider A, Piercy J, et al. Real-world impact of physician and patient discordance on health-related quality of life in US patients with acute myeloid leukemia. Oncol Ther. 2019;7(1):67–81. doi: 10.1007/s40487-019-0094-x.
- Korman NJ, Zhao Y, Pike J, et al. Satisfaction with current psoriasis treatment: misalignment between physician and patient perceptions. Dermatol Online J. 2016;22(7).
- Wei W, Anderson P, Gadkari A, et al. Discordance between physician- and patient-reported disease severity in adults with atopic dermatitis: a US cross-sectional survey. Am J Clin Dermatol. 2017;18(6):825–835. doi: 10.1007/s40257-017-0284-y.
- Amin AN, Ganapathy V, Roughley A, et al. Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease. PPA. 2017;ume 11:1205–1212. doi: 10.2147/PPA.S140139.
- Chrystyn H, Small M, Milligan G, et al. Impact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med. 2014;108(2):358–365. doi: 10.1016/j.rmed.2013.09.021.
- Waller J, Sullivan E, Piercy J, et al. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. PPA. 2017;ume 11:519–530. doi: 10.2147/PPA.S129333.
- Benford M, Milligan G, Pike J, et al. Fixed-dose combination antidiabetic therapy: real-world factors associated with prescribing choices and relationship with patient satisfaction and compliance. Adv Ther. 2012;29(1):26–40. doi: 10.1007/s12325-011-0096-z.
- Cook NS, Criner GJ, Burgel PR, et al. People living with moderate-to-severe COPD prefer improvement of daily symptoms over the improvement of exacerbations: a multicountry patient preference study. ERJ Open Res. 2022;8(2):00686-2021. doi: 10.1183/23120541.00686-2021.
- Pike J, Dong Y, Piercy J, et al. Cross-walk of the assessment of spondyloarthritis international society health index and ankylosing spondylitis quality of life scores in ankylosing spondylitis and non-radiographic axial spondyloarthritis patients. Rheumatol Ther. 2021;8(2):849–862. doi: 10.1007/s40744-021-00306-y.
- Kay S, Tolley K, Colayco D, et al. Mapping EQ-5D utility scores from the incontinence quality of life questionnaire among patients with neurogenic and idiopathic overactive bladder. Value Health. 2013;16(2):394–402. doi: 10.1016/j.jval.2012.12.005.
- Fishman J, Higgins V, Piercy J, et al. Cross-walk of the chronic liver disease questionnaire for nonalcoholic steatohepatitis (CLDQ-NASH) and the EuroQol EQ-5D-5L in patients with NASH. Health Qual Life Outcomes. 2023; Oct 1421(1):113.
- Andersson F, Anderson P, Holm-Larsen T, et al. Assessing the impact of nocturia on health-related quality-of-life and utility: results of an observational survey in adults. J Med Econ. 2016;19(12):1200–1206. doi: 10.1080/13696998.2016.1211136.
- Leith A, Ribbands A, Kim J, et al. Real-world homologous recombination repair mutation testing in metastatic castration-resistant prostate cancer in the USA, Europe and Japan. Future Oncol. 2022;18(8):937–951. doi: 10.2217/fon-2021-1113.
- Gairy K, Knight C, Anthony P, et al. Burden of illness among subgroups of patients with primary Sjogren’s syndrome and systemic involvement. Rheumatology (Oxford). 2021;60(4):1871–1881. doi: 10.1093/rheumatology/keaa508.
- Gossec L, Walsh JA, Michaud K, et al. Women with psoriatic arthritis experience higher disease burden than men: findings from a Real-World survey in the United States and Europe. J Rheumatol. 2023;50(2):192–196. doi: 10.3899/jrheum.220154.
- Ueda K, Ye W, Lombard L, et al. Real-world treatment patterns and patient-reported outcomes in episodic and chronic migraine in Japan: analysis of data from the Adelphi migraine disease specific programme. J Headache Pain. 2019;20(1):68. doi: 10.1186/s10194-019-1012-1.
