Abstract
Background
Influenza is associated with significant disease burden in the US and is currently best controlled by vaccination programs. Influenza vaccine effectiveness (VE) is low and may be reduced by several factors, including egg adaptations. Although non-egg-based influenza vaccines reportedly have greater VE in egg-adapted seasons, evidence for egg adaptations’ reduction of VE is indirect and dissociated, apart from two previous European consensuses.
Methods
This study replicated the methodology used in a 2020 literature review and European consensus, providing an updated review and consensus opinion of 10 US experts on the evidence for a mechanistic basis for reduction of VE due to egg-based manufacturing methods. A mechanistic basis was assumed if sufficient evidence was found for underlying principles proposed to give rise to such an effect. Evidence for each principle was brought forward from the 2020 review and identified here by structured literature review and expert panel. Experts rated the strength of support for each principle and a mechanistic basis for reduction of VE due to egg-based influenza vaccine manufacture in a consensus method (consensus for strong/very strong evidence = ≥ 3.5 on 5-point Likert scale).
Results
Experts assessed 251 references (from previous study: 185; this study: 66). The majority of references for all underlying principles were rated as strong or very strong supporting evidence (52–86%). Global surveillance, WHO candidate vaccine virus selection, and manufacturing stages involving eggs were identified as most likely to impact influenza VE.
Conclusion
After review of extensive evidence for reduction of VE due to egg-based influenza vaccine manufacture, influenza experts in the US joined those in Europe in unanimous agreement for a mechanistic basis for the effect. Vaccine providers and administrators should consider use of non-egg-based influenza vaccine manufacture to reduce the risk of egg adaptations and likely impact on VE.
Introduction
In the 2017/2018 season, influenza was estimated to be responsible for 710,000 hospitalizations and 52,000 deaths in the US aloneCitation1. Influenza vaccination is widely regarded as the most successful strategy for reducing morbidity and mortality of the disease and has been endorsed by virtually all specialty societies and public health agencies. However, influenza vaccine effectiveness (VE) is low. Mean influenza VE in the US between 2009 and 2020 was estimated at approximately 43%Citation2, with a meta-analysis suggesting lowest VE for H3N2Citation3. Older estimates have been higher but are also suboptimalCitation4. Low influenza VE has been related to host factors that are difficult to control from a public health standpoint, such as older ageCitation5, serial vaccinationCitation6, low immunocompetence and underlying comorbidityCitation7. Antigenic mismatch between vaccine and circulating strains may also reduce influenza VE and has been proposed to occur due to antigenic driftCitation8 and egg adaptations, host-adaptive antigenic changes in viral strains during egg-based influenza vaccine manufactureCitation9–18, the most common means of vaccine production in the USCitation19.
Antigenic drift is a naturally occurring evolutionary phenomenon involving selection of genetic mutations adaptive to host immunity, with mutations in circulating viral strains contributing to antigenic mismatch when affecting the conformation of proteins at antibody binding sitesCitation20. Occurrence of such antigenic drift after pre-season recommendation of vaccine virus strains by the WHO, usually in February in the northern hemisphere, may contribute to low VE in a season even if recommendations accurately predict dominant strains from previous season influenza activity and global surveillance reportsCitation20. Antigenic mismatch has also been proposed to arise during later stages of egg-based influenza vaccine manufacture, such as reassortment of recommended wild-type vaccine strains by WHO Collaborating Centres for adaptation to high growth in eggs to optimize manufacturing yieldCitation21. Reassorted strains are then provided to influenza vaccine manufacturers as candidate vaccine viruses (CVVs). Growth of seed vaccines by inoculation and incubation of CVVs in eggs may provoke adaptation of virus strains to avian rather than human host cellsCitation22 before harvest and purification to provide monovalent strains or combined polyvalent vaccine batches for release and distribution. Differences between human (a-2,6 type) and avian (avian a-2,3 type) cell sialic acid residues, to which influenza virus hemagglutinin binds, may result in antigenic mismatch between candidate viruses and circulating strainsCitation9,Citation18,Citation23–26. In seasons affected by egg adaptations, non-egg-based influenza vaccines have shown greater effectiveness than egg-based and were associated with lower health and economic burdenCitation27,Citation28.
Of all influenza subtypes, A/H3N2 appears subject to the most extensive and frequent antigenic changes arising from genetic mutation. In the US 2014/2015 season, over 80% of characterized circulating A/H3N2 virus strains varied from vaccine strains, and VE for A/H3N2 was only 13%Citation29. Low A/H3N2 VE in 2012/2013Citation30, 2016/2017Citation31,Citation32 and 2017/2018Citation33 seasons in the US has been attributed to egg adaptations. During the 2016/2017 season, egg-adapted A/H3N2 strains lost a glycosylation site, reducing their likelihood of eliciting antibodies effective against circulating strains due to the role of the site in binding influenza hemagglutininCitation31,Citation32. Antibody-mediated inhibition of circulating virus hemagglutination by ferret sera exposed to vaccine-homologous strains suggests poorer antigenic match for egg-propagated strainsCitation34. In the 2017/2018 season in the US, predominated by A/H3N2, 48.2% and 77.3% of A/H3N2 viruses tested were well-inhibited by ferret antisera raised against egg-propagated A/Hong Kong/4801/2014 and A/Singapore/INFIMH-16–0019/2016 reference viruses, respectively. This was much lower than the 93.4% well-inhibited by ferret antisera raised against cell-propagated A/Michigan/15/2014Citation33. A 2021 expert consensus estimated that egg adaptations were likely to reduce VE against A/H3N2 by up to 16% in the 18–64 years age group, and an average of 9% across all strains in all age groupsCitation35.
Seasonal and geographic variation in circulating strains, WHO strain selection, and agent-host and immuno-epidemiologic factorsCitation36 make it difficult to study the impact of egg adaptations on VE due to uncertainty of the presence and degree of effect in a given season. As a result, most findings on the topic remain dissociated. In addition to the consensus conducted in 2021Citation35, a structured literature review and consensus conducted with European experts in 2020 aimed to overcome lack of direct evidence for reduction of VE due to egg adaptations during influenza vaccine manufactureCitation37. Findings suggested a mechanistic basis for the relationship between egg adaptations and low VE. Since then, research on antigenic drift, egg adaptations, influenza vaccine strain selection and manufacturing methods has increased. The aim of this study was to update the 2020 European structured literature review and consensus on the mechanistic basis for an association between reduced influenza VE and egg-based manufacturing technologies with US experts to determine support for the effect with twice as many experts representing opinion in a second major vaccine market.
Method
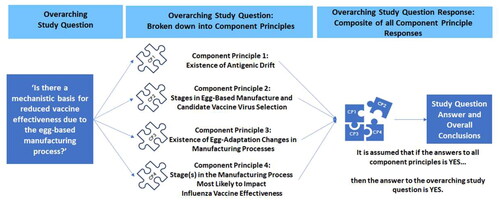
This study was conducted between June 2021 and January 2022 and replicated the methodology used in a 2020 literature review and European consensusCitation37. The aim of this body of work was to unite existing dissociated findings on which the hypothesis of reduced influenza VE due to egg-based manufacturing methods rests. The objective of this study was to provide an updated review and consensus of 10 US experts to answer the research question, “Is there a mechanistic basis for an association between reduced influenza VE and egg-based manufacturing technologies, particularly against influenza A/H3N2?” A secondary objective was to interpret these findings in light of the study conducted with European experts in 2020 to determine whether further support for the mechanistic basis was apparent.
In both studies, a mechanistic basis for reduced influenza VE due to egg-based manufacture was assumed if sufficient support was identified for underlying principles proposed to give rise to such an effect. Component principles were defined as antigenic drift, stage(s) in egg-based influenza vaccine manufacture and candidate virus selection, egg adaptation changes in influenza vaccine manufacturing processes and influenza vaccine manufacturing stages most likely to reduce VE (). Evidence for each principle was brought forward from the previous 2020 study and combined with evidence identified here by structured literature review and expert panel (AC, DHC, SD, NE, VCH, RJh, ACN, MV, RV and LBA). Experts assessed strength of support for component principles to determine extent of consensus on a mechanistic basis for reduced VE due to egg-based influenza vaccine manufacture. Expert rating of the evidence was interrogated using statistical methods.
Structured literature review
The review was guided by the Cochrane Handbook for Systematic Reviews of Interventions where applicable. The aim of the review was to generate a list of references for each component principle. A protocol, including search strategy, was developed a priori by two authors (RJa, RW) independently and was reviewed by two authors (KA, AS). The search strategy was the same as in the previous consensus conducted with European experts in 2020Citation37 but was limited to studies published between 2019 and 2021, as references identified by the previous review were carried over. Search strings and inclusion/exclusion criteria were developed using the PICOS framework (Supplemental Table 1). The search was limited to studies on human influenza published in English without geographical limitation. Separate searches were completed for each component principle with specific limiters (Supplemental Table 1). Searches were conducted in PubMed and the Cochrane Library with date of last access June 2021. Working independently, two research analysts screened titles and abstracts with disputes settled by discussion. One analyst screened full-text articles for eligibility and extracted data on author, year of publication, title and journal into an extraction table developed a priori. This was quality checked by the other analyst with disputes settled by discussion. Assessment of study quality was not carried out at this stage, as experts assessed quality of evidence in the consensus exercise.
Expert panel
Eleven US influenza vaccine specialists were recruited to the expert panel using purposive sampling via professional networks, publication scanning, and through a global pharmaceutical company’s US medical department. Experts were healthcare professionals or virologists in the US with a special interest in influenza vaccination evidenced by publications on influenza burden and/or experience as a medical committee chair, clinical commissioning group lead, or in other senior roles that require specialist knowledge of influenza vaccination.
Assessment of expert opinion
Opinion of the expert panel was assessed in the two-stage Jandhyala method, which combines literature review and a consensus exercise to develop highly accurate disease-specific construct measures and core datasets in contexts with paucity of data or where generic measures are inaccurateCitation38–42. It has been used for research questions that cannot be addressed by traditional methods, such as in this case, where the effect occurs unpredictably in the real-world context such that direct observation is impracticable in the usual course of planned researchCitation35,Citation37.
The Jandhyala method was delivered in two rounds using the qualitative online survey from the consensus study conducted with European experts in 2020Citation37 with updates to include evidence identified in the present study. Awareness Round (1) determined experts’ baseline knowledge of the component principles and supporting evidence. Participants gave free-text responses to open-ended questions asking for their opinion on each component principle and a list of references to support their stance. Experts could conduct independent searches of the literature during survey completion. Responses and references were coded by a research analyst, and after removing duplicates and applying inclusion criteria, expert-generated references were added to those from the structured literature review and the previous European study.
Consensus Round (2) assessed consensus opinion on the support for each component principle and the mechanistic basis for the effect. Text extracts from the structured literature review and expert-generated references were presented to the panel for appraisal. Text extracts were selected from full-text articles identified as most relevant to answering the research question by a research analyst and quality checked by a systematic reviewer experienced in influenza virology. To manage the large number of references, publications were divided into five groups; each group was presented to two experts. Experts had access to full-text articles and were asked to rate the strength of each reference as support for the respective component principle on a 5-point Likert scale from “not evidence” to “very strong evidence.”
Cumulative frequency and evidence rating for each reference were recorded and analyzed from all survey answers. A reference was considered supportive of a component principle if the median agreement level on the Likert scale was ≥ 3.5. Summary statistics were generated with equal weighting given to all references and presented to experts in a final round in which they were asked whether cumulative evidence supported a potential mechanistic basis for an association between egg-based influenza vaccine manufacturing and reduced VE.
Statistical analyses
To determine relative growth in the body of knowledge on each component principle, average publication rate per annum of references included in the review was assessed. Experts’ rating of the evidence was assessed visually by density plots. To account for observed findings, rating scores were subjected to statistical analysis by expert and time of publication. Significant difference between paired US experts’ rating of the same evidence was determined by two-sample t-test. Experts’ median graded scores for each reference were computed for literature published from 1969–2018 and 2019–2021, and a Wilcoxon rank sum test with continuity correction determined significant difference between the calendar ranges. STATA version 14 was used for all statistical analyses.
Results
Due to attrition of one expert for reason of unavailability, 10 experts completed the study, comprising two virologists, one expert in public health, one expert in pharmacy-based immunization practices, and six infectious disease specialists based throughout the US.
Identification and availability of evidence for component principles
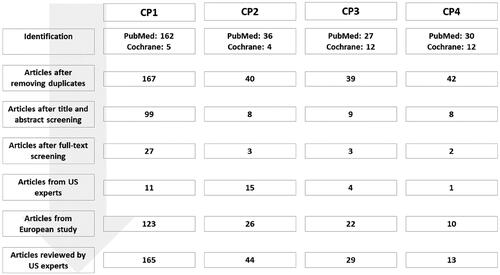
Modified PRISMA flow charts for all component principles () and each component principle (Supplemental Figures 1–4) show identification of references reviewed in this study. Of the 288 identified by this review, 35 were included. Unique references from the previous consensus (n = 185) and US expert panel (n = 31) were added to these, giving 251 overall. Mean references provided by US experts were generally equivalent except for component principle four (CP1: n = 6.4; CP2: n = 4.1; CP3: n = 5.3; CP4: n = 3.6). The 251 references were divided among five groups (n = 47–55 references each). Number of references was highest for component principle one with stepwise sequential decrease through subsequent component principles (CP1: n = 165, 66%; CP2: n = 44, 17%; CP3: n = 29, 12%; CP4: n = 13, 5%). Most references comprised historical modelling of genetic and antigenic data, studies that genetically and antigenically characterized the virus, and surveillance and effectiveness reports by national and global agencies. Mean publication rate per annum of references included in this study showed substantial growth between 2004 and 2021; greatest increase was observed in component principle one, antigenic drift, with sequential decrease observed in each subsequent component principle (Supplemental Figure 5).
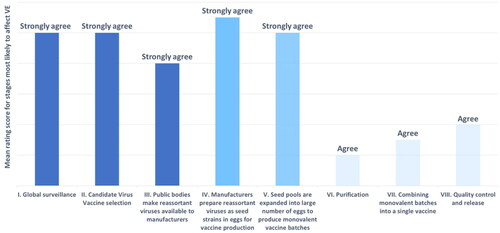
Figure 3. Expert rated stages in influenza vaccine manufacture most likely to impact VE. Strongly agree (dark blue, score 3.5–4): global surveillance and WHO CVV selection; Strongly agree (blue, score 4–4.5): manufacturing stages involving eggs; agree (light blue, score 2–2.5): purification, batching, quality control/release.
Expert assessment of evidence for component principles
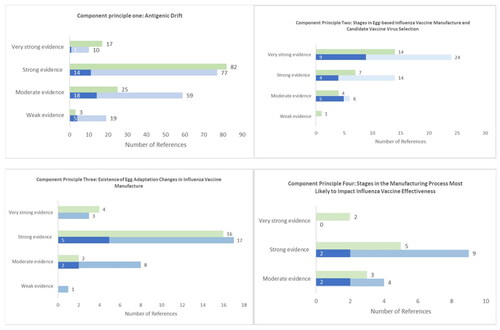
The majority of references for all component principles were rated as strong or very strong supporting evidence (CP1: 87/165 (53%); CP2: 38/44 (86%); CP3: 20/29 (69%); CP4: 9/13 (69%). Experts scored evidence from the literature reviews as stronger than evidence sourced by fellow experts, except for component principle four. Abbreviated lists of the highest ranked references for each component principle can be found in Supplemental Table 2. Of stages in influenza vaccine manufacture, experts identified global surveillance, WHO CVV selection, and manufacturing stages involving eggs as most likely to impact VE (). Experts gave unanimous support for a mechanistic basis for an association between reduced influenza VE and egg-based manufacturing technologies.
Comparison between US experts
Two-sample t-test showed no significant differences between paired US experts rating the same evidence in each assigned group (). Density plots for rating of references published in 1969–2018 and 2019–2021 showed varying distribution of scores between the time periods for some experts (E1, E3, E6, E8, E9) with a tendency towards lower scores for more recent references (Supplemental Figure 7). However, Wilcoxon rank sum test with continuity correction showed no significant differences between the time periods (W = 59, p-value = 0.4779).
Table 1. Two sample t-test of difference between two experts reviewing the same literature.
Comparison with the 2020 European review and consensus
Patterns of evidence rating and availability were largely similar to the 2020 European consensus apart from lower ratings for US experts for component principle one (). Matched comparison of US and European expert rating of references for component principle one confirmed a lower percentage rated as strong or very strong for US experts (US: 69/123, 56%; Europe: 95/123, 77%). An overall agreement rate of 60% (74/123) between US and European experts was observed (agree: strong or very strong n = 58/123, moderate n = 11/123, weak n = 5/123; disagree: n = 48). Of the 48 disagreed references, US experts rated more as weak or moderate than European experts (US w/m, Europe s/vs: n = 37; US s/vs, Europe w/m: n = 11). With statistical within-group comparisons for US experts, this suggests a slight tendency for US experts to rate some evidence for antigenic drift as lower than European experts. Number of references identified for other component principles were insufficient for robust direct comparison.
Discussion
There is a need for more effective influenza vaccines. Findings support the hypothesis that egg adaptations may contribute to variation in VE, but evidence for the effect is predominantly indirect and dissociated. Lack of direct evidence was addressed by a review and consensus of European experts in 2020Citation37. This update included 66 references published since and validated findings with experts from the US, a major global vaccine market. The majority of evidence for each component principle was rated as strong or very strong, and US experts unanimously agreed there is potential for a mechanistic association between egg-adaptations and reduced VE.
Component principle one, antigenic drift, had the highest number of references rated as strong or very strong evidence and the highest growth in publication rate since 1969 of all component principles. This was supported by previous findings. Antigenic drift was first identified in 1965 and has been well-evidenced, resulting in a generally accepted understanding of theory and mechanismCitation43–45. US experts had a tendency to rate some references less highly than European experts. This may be due to a preference for certain types of evidence. For example, US experts rated historical variation in the genetic diversity of various subtypesCitation46–49 as most supportive of antigenic drift. While European experts also rated these references as strong or very strong, they were not among their most highly rated references, which included greater variation in type of evidence than US experts. Both groups rated surveillance evidence across various geographical regions, including phylodynamic reports and antigenic mapping, as highly supportiveCitation50–53.
For component principle two, experts agreed that eight steps in egg-based manufacture and CVV selection provided a level of detail appropriate for the purpose of this research (). This component principle had fewer references, likely because manufacturing processes are not generally addressed by scientific research. Results were largely in line with the European consensus despite some minor variation in references cited. This was expected, as egg-based manufacturing has remained unchanged in recent years. US experts rated highly reports by governing bodiesCitation54, analyses of egg- and cell-based manufacturing methodsCitation55,Citation56, and accounts of the manufacturing process and its challengesCitation57–62. Findings emphasized the need for processes to maximize strain specificity and antigenic match.
The most highly rated references for component principle three, egg-adaptation changes in the influenza vaccine manufacture process were in line with the European consensus and included antigenic analyses comparing cell- and egg-grown virusesCitation26,Citation63, studies showing altered antibody receptor binding arising from egg-adapted genetic mutationsCitation33,Citation64,Citation65, and studies that linked genetic variation in egg-adapted strains to reduced VE in the 2012/2013 season in CanadaCitation25.
For component principle four, experts agreed that global surveillance, strain selection, and manufacturing steps involving eggs were the most likely to impact VE. The first two stages here may influence the antigenic match of CVVs to circulating strains, and manufacturing steps involving eggs include proliferation and reassortment processes that may result in egg-adapted virus strainsCitation56,Citation62. Similar references were rated by US and European experts as the strongest support for this component principle. Of these, one showed antigenic mismatch between a circulating strain and the receptor binding sites of patients immunized with an egg-adapted strainCitation6. The other showed the need for more effective influenza vaccines, the complex and multi-factorial nature of VE and challenges in developing solutionsCitation5. Few solutions have been identified to control the impact of host factors on influenza VE, and the ability of global surveillance and strain selection to ensure VE is limited due to antigenic driftCitation52. Thus, avoiding manufacturing steps involving eggs may be the most feasible way to improve influenza VE.
This study adds to evidence challenging established vaccine production methods and supports the widespread adoption of newer vaccine-manufacturing technologies that can provide a better match to circulating strains. Methods for manufacture of cell-based, recombinant, and next-generation mRNA influenza vaccines avoid egg-based methods and likely associated reduction in VE. Influenza vaccines using this technology are in developmentCitation66–69. European experts have estimated that egg adaptations may reduce VE by up to 16%Citation35. A 5% absolute increase in VE may have prevented an additional 1,050,000 illnesses and 25,000 hospitalizations in the US during a single seasonCitation70. Avoiding egg-based influenza vaccine manufacture may therefore substantially decrease disease burden and healthcare-associated costs. Despite this, available evidence in support of egg-adaptations was approximately four times lower than that for antigenic drift despite similar estimated reduction of influenza VECitation35, suggesting a need for further research. Findings here suggest that further updates and replications of this review and consensus may be useful.
Limitations
Strength of evidence against component principles was not explicitly measured by the Likert scale used for expert rating, as the variable of interest in this study was support for component principles. This was unlikely to impact overall findings, as evidence ‘against’ a principle has tended not to disprove the phenomena itself but to preference one effect over others as a reason for low VE in certain seasons. In this study, mean scores suggested that such evidence was rated ‘weak’ for the disfavoured principle (for example, Skowronski et al. 2014 for component principle one). Publication bias could be considered a factor in higher relative frequency of supportive evidence.
Inclusion of only US experts may limit the generalizability of these findings; however, the experts in this study doubled the 10 who completed the previous European consensus. Though purposive sampling may have introduced potential sampling bias, expert affiliations were sufficiently diverse to consider them representative of the population of US influenza vaccination experts. As with all consensus methods, there was a risk of oversimplification of the topic of study due to inherent methodological limitationsCitation71. However, expert consensus was appropriate for the research question due to the need to unite dissociated and methodologically diverse findings. Finally, the study did not investigate the impact of egg-adaptation changes on different strains of influenza virus, even though reduction of influenza VE due to egg-adaptations may vary by strainCitation35. Further research could usefully address this topic.
Conclusion
Experts from the US agreed that there is a mechanistic basis for an association between reduced influenza VE and egg-based manufacturing technologies. Evidence rated as strong or very strong by the US experts for the four component principles suggested that, of the steps identified as most likely to impact influenza VE, steps involving eggs were the only ones that were both likely to reduce VE and be immediately manageable through human intervention. There is now US and European expert agreement for the increased risk of reduced influenza VE resulting from egg-based manufacturing techniques. Increasing the use of non-egg-based manufacturing that avoids egg-adaptations is a currently available strategy that may improve influenza VE.
Transparency
Declaration of funding
This research was carried out by Medialis Ltd, a medical affairs consultancy and contract research organisation. CSL Seqirus, Inc. funded this work but did not participate in the design of the study; the collection, analysis or interpretation of the data; the writing of the manuscript, apart from review and approval; or the decision to publish the results. This study received no external grants.
Declaration of financial/other relationships
AC, SD, NE, VCH, ACN, and RV declare no conflicts of interest. KA and AS are paid employees and shareholders of CSL Seqirus, Inc. RJa and RW are employees of Medialis Ltd. The expert panel (AC, DHC, SD, NE, VCH, RJh, ACN, MV, RV, LBA) were remunerated by CSL Seqirus, Inc. for their involvement in this study. DHC has completed consulting work for CSL Seqirus, Inc and is a recipient of investigator-initiated grants to his university from Pfizer to study pneumococcal vaccines and Sanofi Pasteur and CSL Seqirus, Inc. to study influenza vaccines. RJa is the founder and CEO of Medialis Ltd., and RW is a paid employee of Medialis Ltd., which was commissioned and funded by CSL Seqirus, Inc. to conduct the research. RJh is a consultant for AstraZeneca, CSL Seqirus, Inc. and Dynavax, and he receives an editorial stipend from the Pediatric Infectious Diseases Society and royalties from UpToDate. MV is on an advisory board for Sanofi, which makes influenza vaccine; has done clinical trials with Pfizer, GSK, Sanofi, and others; and has received honorarium from CSL Seqirus, Inc. The Jandhyala method was developed by Dr Jandhyala (RJa) but is free of commercial licensing restrictions and while used as part of proprietary methodology, is not a direct means of commercial gain for the author.
Peer reviewers on this manuscript have received an honorarium from CMRO for their review work. A reviewer on this manuscript also disclosed that they occasionally consult for CSL Seqirus Vaccines plc. and Sanofi Pasteur who both manufacture egg-based influenza vaccine. The former also manufacture cell-grown vaccines and the latter recombinant vaccines. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Conceptualization, RJa and RW; Formal analysis, RJa and RW; Investigation, AC, DHC, SD, NE, VCH, RJh, ACN, MV, RV; Methodology, RJa and RW; Writing – original draft, RJa and RW; Writing – review & editing, AC, KA, DHC, SD, NE, VCH, RJa, RJh, ACN, AS, MV, RV and RW. All authors were involved in final approval of the published version and agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (64.9 KB)Supplemental Material
Download MS Word (164 KB)Acknowledgements
The authors would like to acknowledge Dr Lauren B. Angelo for her contributions as an expert participant in the literature review process. Employees of Medialis Ltd. provided biostatistics, medical writing and research analytics for this work as part of their roles.
References
- Centers for Disease Control and Prevention. Past seasons estimated influenza disease burden. 2022. https://www.cdc.gov/flu/about/burden/past-seasons.html
- Centers for Disease Control and Prevention. Past seasons VE estimates. 2022. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm#figure
- Belongia EA, Simpson MD, King JP, et al. Variable influenza VE by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–951. doi:10.1016/S1473-3099(16)00129-8.
- Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi:10.1016/S1473-3099(11)70295-X.
- Okoli GN, Racovitan F, Abdulwahid T, et al. Variable seasonal influenza vaccine effectiveness across geographical regions, age groups and levels of vaccine antigenic similarity with circulating virus strains: a systematic review and meta-analysis of the evidence from test-negative design studies after the 2009/10 influenza pandemic. Vaccine. 2021;39(8):1225–1240. doi:10.1016/j.vaccine.2021.01.032.
- Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014-2015 season. Clin Infect Dis. 2016;63(1):21–32. doi:10.1093/cid/ciw176.
- Tosh PK, Jacobson RM, Poland GA. Influenza vaccines: from surveillance through production to protection. Mayo Clin Proc. 2010;85(3):257–273. doi:10.4065/mcp.2009.0615.
- Flannery B, Zimmerman RK, Gubareva LV, et al. Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014–2015. J Infect Dis. 2016;214(7):1010–1019. doi:10.1093/infdis/jiw181.
- Raymond DD, Stewart SM, Lee J, et al. Influenza immunization elicits antibodies specific for an egg-adapted vaccine strain. Nat Med. 2016;22(12):1465–1469. doi:10.1038/nm.4223.
- Centers for Disease Control and Prevention. Vaccine effectiveness: how well do the flu vaccines work? 2021. 2022. https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm
- Oxford JS, Newman R, Corcoran T, et al. Direct isolation in eggs of influenza A (H1N1) and B viruses with haemagglutinins of different antigenic and amino acid composition. J Gen Virol. 1991;72(Pt 1):185–189. doi:10.1099/0022-1317-72-1-185.
- Kishida D, Fujisaki S, Yokoyama M, et al. Evaluation of influenza virus a/H3N2 and B vaccines on the basis of crossreactivity of postvaccination human serum antibodies against influenza viruses a/H3N2 and B isolated in MDCK cells and embryonated hen eggs. Clin Vaccine Immunol. 2012;19(6):897–908. doi:10.1128/CVI.05726-11.
- Kodihalli S, Justewicz DM, Gubareva LV, et al. Selection of a single amino acid substitution in the hemagglutinin molecule by chicken eggs can render influenza a virus (H3) candidate vaccine ineffective. J Virol. 1995;69(8):4888–4897. doi:10.1128/JVI.69.8.4888-4897.1995.
- Katz JM, Webster RG. Efficacy of inactivated influenza a virus (H3N2) vaccines grown in mammalian cells or embryonated eggs. J Infect Dis. 1989;160(2):191–198. doi:10.1093/infdis/160.2.191.
- Robertson JS, Cook P, Nicolson C, et al. Mixed populations in influenza virus vaccine strains. Vaccine. 1994;12(14):1317–1322. doi:10.1016/s0264-410x(94)80058-8.
- Wood JM, Oxford JS, Dunleavy U, et al. Influenza A (H1N1) vaccine efficacy in animal models is influenced by two amino acid substitutions in the hemagglutinin molecule. Virology. 1989;171(1):214–221. doi:10.1016/0042-6822(89)90528-x.
- Wu NC, Zost SJ, Thompson AJ, et al. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017;13(10):e1006682–e1006682. doi:10.1371/journal.ppat.1006682.
- Barr IG, Russell C, Besselaar TG, et al. WHO recommendations for the viruses used in the 2013–2014 Northern hemisphere influenza vaccine: epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from october 2012 to january 2013. Vaccine. 2014;32(37):4713–4725. doi:10.1016/j.vaccine.2014.02.014.
- Centers for Disease Control and Prevention. How influenza (flu) vaccines are made. 2022. https://www.cdc.gov/flu/prevent/how-fluvaccine-made.htm
- Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25(39-40):6852–6862. doi:10.1016/j.vaccine.2007.07.027.
- Weir JP, Gruber MF. An overview of the regulation of influenza vaccines in the United States. Influenza Other Respir Viruses. 2016;10(5):354–360. doi:10.1111/irv.12383.
- Park YW, Kim YH, Jung HU, et al. Comparison of antigenic mutation during egg and cell passage cultivation of H3N2 in-fluenza virus. Clin Exp Vaccine Res. 2020;9(1):56–63. doi:10.7774/cevr.2020.9.1.56.
- Parker L, Wharton SA, Martin SR, et al. Effects of egg adaptation on receptor-binding and antigenic properties of recent influenza A (H3N2) vaccine viruses. J Gen Virol. 2016;97(6):1333–1344. doi:10.1099/jgv.0.000457.
- Harding AT, Heaton NS. Efforts to improve the seasonal influenza vaccine. Vaccines. 2018;6(2):19. doi:10.3390/vaccines6020019.
- Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One. 2014;9(3): e92153. doi:10.1371/journal.pone.0092153.
- Wang ML, Katz JM, Webster RG. Extensive heterogeneity in the hemagglutinin of egg-grown influenza viruses from different patients. Virology. 1989;171(1):275–279. doi:10.1016/0042-6822(89)90538-2.
- Boikos C, Fischer L, O'Brien D, et al. Relative effectiveness of the cell-derived inactivated quadrivalent influenza vaccine versus egg-derived inactivated quadrivalent influenza vaccines in preventing influenza-related medical encounters during the 2018–2019 influenza season in the United States. Clin Infect Dis. 2021;73(3):e692–e698. doi:10.1093/cid/ciaa1944.
- Divino V, Krishnarajah G, Pelton SI, et al. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season. Vaccine. 2020;38(40):6334–6343. doi:10.1016/j.vaccine.2020.07.023.
- Paules CI, Sullivan SG, Subbarao K, et al. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med. 2018;378(1):7–9. doi:10.1056/NEJMp1714916.
- McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis. 2015;211(10):1529–1540. doi:10.1093/infdis/jiu647.
- Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A. 2017;114(47):12578–12583. doi:10.1073/pnas.1712377114.
- Cobey S, Gouma S, Parkhouse K, et al. Poor immunogenicity, not vaccine strain egg adaptation, may explain the low H3N2 influenza vaccine effectiveness in 2012–2013. Clin Infect Dis. 2018;67(3):327–333. doi:10.1093/cid/ciy097.
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67(22):634–642. doi:10.15585/mmwr.mm6722a4externalicon.
- Petrie JG, Gordon A. Epidemiological studies to support the development of next generation influenza vaccines. Vaccines. 2018;6(2):17. doi:10.3390/vaccines6020017.
- Ortiz de Lejarazu-Leonardo R, Montomoli E, Wojcik R, et al. Estimation of reduction in influenza vaccine effectiveness due to egg-adaptation changes—systematic literature review and expert consensus. Vaccines. 2021;9(11):1255. doi:10.3390/vaccines9111255.
- Skowronski DM, Chambers C, Sabaiduc S, et al. Beyond antigenic match: possible agent-host and immuno-epidemiological influences on influenza vaccine effectiveness during the 2015–2016 season in Canada. J Infect Dis. 2017;216(12):1487–1500. doi:10.1093/infdis/jix526.
- Rajaram S, Wojcik R, Moore C, et al. The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: literature review and expert consensus. Vaccine. 2020;38(38):6047–6056. doi:10.1016/j.vaccine.2020.06.021.
- Jandhyala R. A novel method for observing proportional group awareness and consensus of items arising from list-generating questioning. Curr Med Res Opin. 2020;36(5):883–893. doi:10.1080/03007995.2020.1734920.
- Jandhyala R. Delphi, non-RAND modified delphi, RAND/UCLA appropriateness method and a novel group awareness and consensus methodology for consensus measurement: a systematic literature review. Curr Med Res Opin. 2020;36(11):1873–1887. doi:10.1080/03007995.2020.1816946.
- Jandhyala R. Concordance between the schedule for the evaluation of individual quality of life – direct weighting (SEIQoL-DW) and the EuroQoL-5D (EQ-5D) measures of quality of life outcomes in adults with X-linked hypophosphatemia. Orphanet J Rare Dis. 2022;17(1):81. doi:10.1186/s13023-022-02250-8.
- Damy T, Conceição I, García-Pavía P, et al. A simple core dataset and disease severity score for hereditary transthyretin (ATTRv) amyloidosis. Amyloid. 2021;28(3):189–198. Epub 2021 May 27.PMID: 34042016 doi:10.1080/13506129.2021.1931099.
- Freedman S, de-Madaria E, Singh VK, et al. A simple core dataset for triglyceride-induced acute pancreatitis. Curr Med Res Opin. 2023;39(1):37–46. doi:10.1080/03007995.2022.2144054.
- Altman MO, Angeletti D, Yewdell JW. Antibody immunodominance: the key to understanding influenza virus antigenic drift. Viral Immunol. 2018;31(2):142–149. doi:10.1089/vim.2017.0129.
- Shen J, Ma J, Wang Q. Evolutionary trends of A(H1N1) influenza virus hemagglutinin since 1918. PLoS One. 2009;4(11):e7789. doi:10.1371/journal.pone.0007789.
- Hardy I, Li Y, Coulthart MB, et al. Molecular evolution of influenza a/H3N2 viruses in the province of québec (Canada) during the 1997–2000 period. Virus Res. 2001;77(1):89–96. doi:10.1016/S0168-1702(01)00269-6.
- Bragstad K, Nielsen LP, Fomsgaard A. The evolution of human influenza a viruses from 1999 to 2006: a complete genome study. Virol J. 2008;5(1):40. doi:10.1186/1743-422X-5-40.
- Venter M, Naidoo D, Pretorius M, et al. Evolutionary dynamics of 2009 pandemic influenza a virus subtype H1N1 in South Africa during 2009-2010. J Infect Dis. 2012;20(suppl_1):S166–S172. doi:10.1093/infdis/jis539.
- Yohannes K, Roche P, Hampson A, et al. Annual report of the national influenza surveillance scheme. Commun Dis Intell Q Rep. 2004;28(2):160–168.
- Vijaykrishna D, Holmes EC, Joseph U, et al. The contrasting phylodynamics of human influenza B viruses. Elife. 2015;4:e05055. doi:10.7554/eLife.05055.
- Smith DJ, Lapedes AS, de Jong JC, et al. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004;305(5682):371–376. doi:10.1126/science.1097211.
- Bedford T, Suchard MA, Lemey P, et al. Integrating influenza antigenic dynamics with molecular evolution. Elife. 2014;3:e01914. doi:10.7554/eLife.01914e01914.
- Sleigh MJ, Both GW, Underwood PA, et al. Antigenic drift in the hemagglutinin of the Hong Kong influenza subtype: correlation of amino acid changes with alterations in viral antigenicity. J Virol. 1981;37(3):845–853. doi:10.1128/JVI.37.3.845-853.1981.
- Tenforde MW, Kondor RJG, Chung JR, et al. Effect of antigenic drift on influenza vaccine effectiveness in the United States—2019–2020. Clin Infect Dis. 2021;73(11):e4244–e4250. doi:10.1093/cid/ciaa1884.
- Ampofo WK, Baylor N, Cobey S, WHO Writing Group., et al. Improving influenza vaccine virus selection: report of a WHO informal consultation held at WHO headquarters, Geneva, Switzerland, 14-16 june 2010. Influenza Resp Viruses. 2012;6(2):142–152, e1-5. doi:10.1111/j.1750-2659.2011.00277.x.
- Milián E, Kamen AA. Current and emerging cell culture manufacturing technologies for influenza vaccines. Biomed Res Int. 2015;2015:504831–504811. doi:10.1155/2015/504831.
- Shin D, Park KJ, Lee H, et al. Comparison of immunogenicity of cell-and egg-passaged viruses for manufacturing MDCK cell culture-based influenza vaccines. Virus Res. 2015;204:40–46. doi:10.1016/j.virusres.2015.04.005.
- Wei CJ, Crank MC, Shiver J, et al. Next-generation influenza vaccines: opportunities and challenges. Nat Rev Drug Discov. 2020;19(4):239–252. doi:10.1038/s41573-019-0056-x.
- Wahid R, Holt R, Hjorth R, et al. Chemistry, manufacturing and control (CMC) and clinical trial technical support for influenza vaccine manufacturers. Vaccine. 2016;34(45):5430–5435. doi:10.1016/j.vaccine.2016.07.046.
- Trombetta CM, Marchi S, Manini I, et al. Challenges in the development of egg-independent vaccines for influenza. Expert Rev Vaccines. 2019;18(7):737–750. doi:10.1080/14760584.2019.1639503.
- Treanor J. Weathering the influenza vaccine crisis. N Engl J Med. 2004;351(20):2037–2040. doi:10.1056/NEJMp048290.
- Weir JP, Gruber MF. An overview of the regulation of influenza vaccines in the United States. Influenza Other Respir Viruses. 2016;10(5):354–360. doi:10.1111/irv.12383.
- Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21(16):1776–1779. doi:10.1016/S0264-410X(03)00071-9.
- Rocha E, Xu X, Hall H, et al. Comparison of 10 influenza A (H1N1 and H3N2) haemagglutinin sequences obtained directly from clinical specimens to those of MDCK cell- and egg-grown viruses. J Gen Virol. 1993;74 (Pt 11)(11):2513–2518. online doi:10.1099/0022-1317-74-11-2513.
- Robertson JS, Bootman JS, Newman R, et al. Structural changes in the haemagglutinin which accompany egg adaptation of an influenza A(H1N1) virus. Virology. 1987;160(1):31–37. doi:10.1016/0042-6822(87)90040-7.
- Stevens J, Chen LM, Carney PJ, et al. Receptor specificity of influenza a H3N2 viruses isolated in mammalian cells and embryonated chicken eggs. J Virol. 2010;84(16):8287–8299. doi:10.1128/JVI.00058-10.
- Feldman RA, Fuhr R, Smolenov I, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37(25):3326–3334. doi:10.1016/j.vaccine.2019.04.074.
- ModernaTX I. A Phase 1/2, randomized, stratified, observer-blind, dose-ranging study to evaluate the safety, reactogenicity, and immunogenicity of mRNA-1010 seasonal influenza vaccine in healthy adults 18 years and older. 2021. https://clinicaltrials.gov/ct2/show/NCT04956575.
- Dolgin E. mRNA flu shots move into trials. Nat Rev Drug Discov. 2021;20(11):801–803. doi:10.1038/d41573-021-00176-7.
- Jeeva S, Kim KH, Shin CH, et al. An update on mRNA-based viral vaccines. Vaccines (Basel). 2021;9(9):965. doi:10.3390/vaccines9090965.
- Hughes MM, Reed C, Flannery B, et al. Projected population benefit of increased effectiveness and coverage of influenza vaccination on influenza burden in the United States. Clin Infect Dis. 2020;70(12):2496–2502. doi:10.1093/cid/ciz676.
- Hohmann E, Brand JC, Rossi MJ, et al. Expert opinion is necessary: delphi panel methodology facilitates a scientific approach to consensus. Arthroscopy. 2018;34(2):349–351. doi:10.1016/j.arthro.2017.11.022.