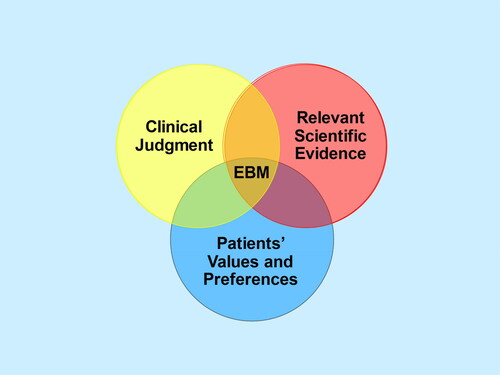
Evidence-based medicine (EBM) is all about how we use evidence in the care of individual patients, as so eloquently summarized in an editorial by Sackett et al. that appeared almost 30 years ago in the BMJCitation1. Regarding the evidence, it was made clear that “External clinical evidence can inform, but can never replace, individual clinical expertise, and it is this expertise that decides whether or not the external evidence applies to the individual patient at all and, if so, how it should be integrated into a clinical decision.” The components of EBM can be illustrated by a Venn diagramCitation2, (), where at the intersection of (a) clinical judgement (based on clinical experience and prior knowledge), (b) patients’ values and preferences (based on patient experience and prior knowledge) and (c) relevant scientific evidence, is the best available medical decision.
Figure 1. What Is evidence-based medicine (EBM)? (Reprinted with permission from Citrome L. Think Bayesian, Think Smarter! Int J Clin Pract. 2019;73(4):e13351. doi: 10.1111/ijcp.13351. PMID: 30968533).
What evidence should we use? Typically, we look to randomized controlled clinical trials (RCTs) and meta-analyses of RCTs and consider these to be at the top of the evidence hierarchy pyramidCitation3. This is subject to some modification, such as placing the N of 1 trial above all else (when engaging with individual patients) and re-examining the boundaries between the different lines of evidence in terms of usefulnessCitation3. However, RCTs are conducted under conditions that do not exist in routine clinical practice, involving inclusion/exclusion criteria that aim to avoid confounding comorbidities, application of standardized measures never used in the regular office or clinic, and financial incentives for both the investigator and subject. A complementary line of evidence can come from real-world practice, i.e. practice-based evidence. When such data is collected systematically and careful attention is made to what comparisons are conducted, it is possible to answer clinically relevant questions that are of interest to medical/surgical practitioners, patients, as well as health plans when considering what treatments to support, and governments when formulating health policies.
Of special note is that the US Food and Drug Administration (FDA) recently issued guidance for industry regarding considerations for the use of real-world data (RWD) and real-world evidence (RWE) to support regulatory decision-making for drug and biological productsCitation4. The FDA defines RWD as data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources and RWE as the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD. RWD can be collected from healthcare databases, registries, claims databases, health-related data from mobile devices, social media, and patient platformsCitation5. Study designs can include cohort studies, cross-sectional studies, case-control studies, registry analysis, and othersCitation5. RWD can be messy, “as treatment is prescribed as per marketing authorization, physician discretion, and national or regional treatment guidelines, and not as per a pre-specified protocol as in the case of RCTs”Citation5. There can be channeling or selection bias when clinicians select interventions that minimize risk, but the person is already at high risk for an undesirable outcome. An example of this is use of claims data and the inability to adequately control for differential prescribing of second-generation antipsychotics to patients at higher risk of diabetesCitation6. Nonetheless, RWE can answer many questions that have not been adequately addressed in a randomized controlled trial, like supratherapeutic dosing of medications such as vedolizumab, an antibody-based treatment used for ulcerative colitisCitation7.
Another consideration is ensuring diversity and inclusion, an issue that has been a criticism of RCTsCitation8, and that can be potentially addressed with RWD, if access to care is present and documentation of the care provided is accurate and complete. Examples include the use of RWE from integrated delivery networks illustrating potential treatment disparitiesCitation9,Citation10.
In this special issue of Current Medical Research and Opinion, our guest editor, Ravi Jandhyala, has assembled 20 papers from an array of authors describing various aspects of RWECitation11. A key issue is building trust in this process, as reviewed by Richard WhiteCitation12. In circling back to , RWE is indeed relevant scientific evidence, and the data itself is driven by clinical judgement (the health care provider decided to use the intervention in question) and the patients’ values and preferences (as demonstrated by accepting the intervention). This is beyond anecdotal evidence, and when research using RWD is properly conducted, it deserves our serious and thoughtful consideration.
Transparency
Declaration of funding
This article was not funded.
Declaration of financial/other relationships
In the past 5 years Leslie Citrome has served as consultant to AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, Biogen, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Delpor, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Luye, Lyndra, Maplight, Marvin, Medavante-ProPhase, Merck, Mitsubishi-Tanabe Pharma, Neumora, Neurocrine, Neurelis, Noema, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sumitomo/Sunovion, Supernus, Teva, University of Arizona, Vanda, Wells Fargo, and one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, Teva, and CME activities organized by medical education companies such as Medscape, NACCME, NEI, Vindico, and Universities and Professional Organizations/Societies; owns stock (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, J & J, Merck, Pfizer purchased > 10 years ago, and stock options in Reviva; earns royalties/publishing income from Taylor & Francis (Editor-in-Chief, Current Medical Research & Opinion, 2022-date), Wiley (Editor-in-Chief, International Journal of Clinical Practice, through end 2019), UpToDate (reviewer), Springer Healthcare (book), Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics).
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–72. doi:10.1136/bmj.312.7023.71.
- Citrome L. Think bayesian, think smarter!. Int J Clin Pract. 2019;73(4):e13351. doi:10.1111/ijcp.13351.
- Murad MH, Asi N, Alsawas M, et al. New evidence pyramid. Evid Based Med. 2016;21(4):125–127. doi:10.1136/ebmed-2016-110401.
- US Department of Health and Human Services, Food and Drug Administration. Considerations for the use of real-world data and real-world evidence to support regulatory decision-making for drug and biological products. guidance for industry. August 2023; [cited 2023 Oct 29]. Available from: https://www.fda.gov/media/171667/download.
- Dang A. Real-world evidence: a primer. Pharmaceut Med. 2023;37(1):25–36. doi:10.1007/s40290-022-00456-6.
- Citrome L, Collins JM, Nordstrom BL, et al. Incidence of cardiovascular outcomes and diabetes mellitus among users of second-generation antipsychotics. J Clin Psychiatry. 2013;74(12):1199–1206. doi:10.4088/JCP.13m08642.
- Gisbert JP, Streit P, Redondo I, et al. Clinical profiles and outcomes in patients with ulcerative colitis receiving standard and higher-than-standard doses of vedolizumab: findings from a real-world study in Europe. Curr Med Res Opin. 2023;39(9):1205–1214. doi:10.1080/03007995.2023.2244414.
- Versavel S, Subasinghe A, Johnson K, et al. Diversity, equity, and inclusion in clinical trials: a practical guide from the perspective of a trial sponsor. Contemp Clin Trials. 2023;126:107092. doi:10.1016/j.cct.2023.107092.
- Mahabaleshwarkar R, Lin D, Joshi K, et al. Characteristics and healthcare burden of patients with schizophrenia treated in a US integrated healthcare system. J Ment Health Policy Econ. 2021;24(2):47–59.
- Seo S, Healey B, McLin R, et al. Health disparities among patients with schizophrenia in an integrated healthcare system. Presented at Psych Congress Elevate; June 1–4, 2023; Las Vegas, Nevada; [cited 2023 Oct 18]. Available from: http://qr.apothecomcx.com/review/qrcodes/150203951/PsychElevate2023_PALM_1462_Poster.pdf.
- Jandhyala R. Guest editor foreword: real-world evidence and medical affairs. Curr Med Res Opin 2023;39(12):1537–1539. doi:10.1080/03007995.2023.2286091.
- White R. Building trust in real world evidence (RWE): moving transparency in RWE towards the randomized controlled trial standard. Curr Med Res Opin. 2023;39(12):1737–1741. doi:10.1080/03007995.2023.2263353.
