Abstract
Objective
This Phase IV placebo-controlled clinical trial was designed to demonstrate the efficacy and safety of the product Neurodoron (Kalium phosporicum comp., KPC) in patients with neurasthenia.
Methods
This monocenter, randomized, double-blind, placebo-controlled, parallel-group clinical trial (registration number: DRKS00003261) was conducted in an outpatient German trial site. Women and men aged 18 and above were randomized to receive either KPC or placebo if they reported typical symptoms of neurasthenia and a severe psychiatric disorder could be excluded. The primary objectives were a reduction in characteristic symptoms of nervous exhaustion and perceived stress as well as improvement in general health status after 6 weeks of treatment.
Results
In total, 204 patients underwent screening, 78 were randomized in each treatment group, and 77 patients each received treatment (intention-to-treat (ITT) population = 154 patients). For none of the primary efficacy variables, an advantage in favor of KPC could be demonstrated in the pre-specified analysis (p-values between 0.505–0.773, Student’s t-test). In a post-hoc analysis of intra-individual differences after 6 weeks treatment, a significant advantage of KPC vs. placebo was shown for characteristic symptoms of nervous exhaustion (irritability (p = 0.020); nervousness (p = 0.045), Student’s t-test). Adverse event (AE) rates were similar between treatment groups, in both groups six AEs were assessed as causally related to treatment (severity mild or moderate). No AE resulted in discontinuation of treatment.
Conclusions
Trial treatment was well tolerated with only a few and minor AEs reported, confirming the markedly good safety of KPC. A significant improvement of neurasthenia was seen for the total study population at the end of the treatment period. Superiority of KPC vs. placebo could not be demonstrated with the pre-specified analysis with regards to a sum score of 12 typical symptoms, perceived stress, or general health status. However, the explorative post-hoc analysis revealed that KPC is superior to placebo in the characteristic symptoms irritability and nervousness. KPC could therefore be a beneficial treatment option for symptomatic relief of neurasthenia.
Introduction
The term “neurasthenia”, meaning exhaustion of the “nerve strength”, was introduced – presumably independently – by two American physicians, Edwin Holmes Van Deusen and George Miller Beard, at the end of the nineteenth centuryCitation1,Citation2. Nowadays, the term neurasthenia is used for patients with chronic exhaustion or fatigue who have characteristic symptoms and in whom organic causes can be excludedCitation3–5.
Data on the frequency of neurasthenia vary: an international WHO-multicenter study gives numbers between 1.3% and 10.8% in the general populationCitation6. In a WHO report on health care, the prevalence of pure neurasthenia ranged between 0.3% (Athens and Bangalore) and 3.7% (Manchester). A long-term study in Switzerland suggests that the prevalence increases with age (0.7% for 22-year-olds to 3.8% for 41-year-olds)Citation7. The persistent exhaustion often leads to absenteeism and thus causes economic costsCitation8.
Treatment options for chronic fatigue include cognitive and behavioral therapies, as well as exercise therapy or a gradual increase of activityCitation9. Henningsen, Zipfel, and Herzog propose a graded therapeutic approach including the administration of pharmacotherapy as a symptomatic treatmentCitation10 but currently there is no internationally accepted standard therapy for neurasthenia.
There is minor evidence for the efficacy of antidepressant and immunoglobulin therapies and no evidence could be found for an efficacy of corticosteroid and interferon therapiesCitation9,Citation10. Some of the benzodiazepines have shown efficacy in establishing sleep maintenance but have side-effects (e.g. next-day sedation) and can only be used to a limited extent or for a limited period of time due to the addictive and habituation effectsCitation11. Even in the absence of randomized controlled trials (RCTs), resting, sparing behavior, and inactivity are considered clearly contraindicatedCitation9.
However, drug treatment to alleviate the symptoms is feasible. The characteristic symptoms, such as nervousness, irritability, restlessness, and sleep disorders, can be well influenced with complementary and alternative medicine (CAM) approaches, such as natural compounds, which are often perceived to be a safer alternative to conventional medicineCitation12. The effect has been shown by various clinical studies for different herbal and homeopathic remediesCitation13–18. However, no data from RCTs has yet been available.
The present drug, NeurodoronFootnotei (Kalium phosporicum comp., KPC), is a CAM representative from anthroposophic medicine. Data from a non-interventional study (NIS) performed with physicians in Germany from 2008 to 2009 confirmed that it is a valuable alternative for treatment of stress-related nervous fatigueCitation18. Based on these findings, the objectives of the presented clinical trial were to demonstrate the efficacy and safety of KPC by means of an RCT, a method regarded as gold standard in clinical research. A preprint of this RCT has previously been publishedCitation19. Following the RCT, a pharmacy-based NIS was conducted between 2014 and 2016, which confirmed the beneficial effects of KPC in self-medicationCitation20.
Anthroposophic medicine, as part of CAM, is an integrative multimodal treatment system based on a holistic understanding of disease and treatment, which was developed in the early 1920s in Germany and SwitzerlandCitation21,Citation22. Meanwhile, a curriculum for becoming a certified anthroposophic physician is available in numerous countries. Europe is most strongly represented, but also countries from all other continents, except for Africa and the Antarctic. The training takes different lengths of time (e.g. 3 years in Germany) and a conventional medical degree is a prerequisite. Anthroposophic medicines are also prescribed by conventional physicians without special trainingCitation22. Recently, the WHO has also confirmed the global importance of anthroposophic medicine by issuing benchmarks for its trainingCitation23.
According to anthroposophic pharmaceutical principles, Dr. Kurt Magerstaedt developed KPC in the early 1950s to support people to better cope with exam stress. In 1954, the product was first launched in Germany, followed by Switzerland, Austria, France, Ukraine, Italy, and the UK. Worldwide, ∼ 300,000 units are sold per year. Since 2007, KPC has been listed in Germany with the indication nervous exhaustion and metabolic weakness, based on case reports from medical practitioners indicating that KPC seems to be efficient in treating stress-related health complaintsCitation24. KPC is taken orally in tablet form and can either be left to dissolve in the mouth or can be taken with a little liquid (3–4 tablets daily). It contains Aurum metallicum praeparatum, Kalium phosphoricum, and Ferrum-Quarz (Weleda AG, Schwäbisch Gmünd, Germany) in homeopathic dilutions.
Methods
Trial design
This clinical trial was a monocentric, randomized, double-blind, placebo-controlled parallel group phase IV superiority trial. Prior to enrollment of the patients, the trial was approved by the relevant competent authorities and the ethics, and registered at the “German Clinical Trials Register” (GCTR-ID: DRKS00003261). Written informed consent was obtained from each patient at the screening visit, prior to trial enrollment.
Four trial visits and a 6-weeks treatment phase were scheduled. If a patient was eligible to participate in the trial, randomization and initiation of treatment occurred at baseline (Visit 1).
Participants
The investigator interviewed potential trial patients and checked the inclusion and exclusion criteria. Main eligibility criteria were based on the diagnosis neurasthenia as defined in ICD-10 F48.0Citation25, i.e. persistent and distressing complaints of feelings of exhaustion after minor mental effort or persistent and distressing complaints of feelings of fatigue and bodily weakness after minor physical effort for at least 3 months. Furthermore, at least one of the following characteristic symptoms had to be present: muscular aches and pains, dizziness, tension headaches, sleep disturbance, inability to relax, and irritability. Male and female patients aged at least 18 years were excluded if they reported an organic cause for the complaints or presented with a certain severe psychiatric disorder. All procedures per trial visits and the flow chart as well as a full list of inclusion and exclusion criteria are shown in Supplementary Appendices I and II.
The trial was conducted at a dedicated clinical trial site in Berlin, Germany, and was coordinated by the contract research organization ACM Allied Clinical Management GmbH.
Interventions
Patients received either KPC (Manufacturer: Weleda AG, Schwäbisch Gmünd, Germany; German registration number 6646311.00.00, German Commission C monographCitation26) or placebo. Tablets were to be taken orally three times a day with a little liquid for 6 weeks. One tablet of KPC contains the following active ingredients: 83.3 mg Aurum metallicum praeparatum trituration (trit.) D10 (corresponding to 0.0092 ng gold), 83.3 mg Kalium phosphoricum trit. D6 (corresponding to 0.0883 µg potassium dihydrogen phosphate (corresponding to 0.062 µg phosphate and 0.025 µg potassium)), 8.3 mg Ferrum-Quarz trit. D2 (corresponding to 31.82 µg ferric sulfate (corresponding to 11.7 µg iron, 20.12 µg sulfate and 6.72 µg sulfur) and 15 µg quartz). The excipients are wheat starch and lactose. The corresponding placebo was identical in form, color, size, taste, and smell and contained the same ingredients, except for the active ingredients.
Outcomes
Primary outcome measures
The following three primary outcome measures were used to show efficacy of KPC. For the a priori analysis, the respective patient assessments at treatment end (visit 3) were considered. For the post-hoc analysis, all assessments were considered (baseline/visit 1, visit 2, end of treatment/visit 3).
Characteristic symptoms of nervous exhaustion were rated by the patient on a four-level Likert scale ranging from “0 = absent” to “3 = severe” and a symptom sum score was calculated (maximum value 36). Twelve symptoms were assessed: irritability, restlessness, nervousness, listlessness, depressive mood, mood swings, anxiety states, troubles to concentrate/lack of concentration, headache, sleep disorders, digestive disorders, muscular pain/tensions.
Perceived Stress Questionnaire (PSQ):Citation27 Individually perceived stress was assessed by the patients via the revised German version of the PSQ-20Citation28,Citation29. It contains 20 items which can be assigned to four subscales (worries, tension, joy, and demands). The assessment is done on a four-level Likert scale ranging from “1 = almost never” to “4 = usually”. Scores for the subscales and the total score are obtained by calculating the mean and a subsequent linear transformation to values between 0 and 100. For calculation of the total score the values for “joy” are inverted. Thus, higher total scores indicate more severe perceived stress.
Short Form-36 Health Survey (SF-36):Citation30 Health-related quality-of-life was evaluated by the patient using the validated SF-36. This questionnaire is composed of 36 items and assesses mental, physical, and social health aspects. The 36 items of the SF-36 are summarized in eight subscales (vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning, and mental health). In addition, the subscales are computed into a physical and mental sum score. For calculation of the scores, the recorded numeric values are linearly transformed to a 0–100 range (all items are converted in the same direction, so the higher the score the better the quality-of-life). In a second step, items in the same scale are averaged.
Secondary outcome measures
The following secondary efficacy and safety measures were assessed:
The Tedium MeasureCitation31 was assessed by the patient at baseline (visit 1) through treatment end (visit 3) and is used for self-rating of burnout. This tool consists of 21 items that are scored on a 7-level Likert scale ranging from “never” to “always”, assessing the degree of physical, emotional, and mental exhaustion. The Tedium Measure is calculated according to the authors’ specifications and can reach values between 2.1 and 5.9. High values of the Tedium Measure describe a high extent of tedium or burnout. Pines et al.Citation32 offer interpretations for the test scores: values ≤ 3 suggest no problems, values between 3 and 4 indicate a high risk, and values > 5 an acute crisis.
Efficacy and tolerability rating by patient and physician: Trial patients and investigators rated the efficacy and tolerability of the IMP at treatment end (visit 3) separately on a four-level Likert scale ranging from “very good” to “unsatisfactory”.
Safety: The number and severity of (serious) adverse events (AEs) after intake of IMP was recorded by the physician for both groups independently from the causality at all trial visits. The type and frequency of clinically relevant laboratory value changes at treatment end vs. screening were assessed.
Sample size
The calculation of the sample size was based on the efficacy results of a physician-based NIS performed with KPC in 2008/2009Citation24 regarding the characteristic symptoms associated with nervous exhaustion. The assumptions were a 25% advantage of KPC over placebo at treatment end, with a mean sum symptom score of 7.1 and an assumed SD of ±5.1 for KPC, and a mean sum symptom score of 9.5 and an assumed SD of ±5.1 for placebo. In total, 182 patients had to be enrolled to obtain 146 evaluable patients, 73 in each treatment group using the Student’s t-test with α = 0.05, β = 0.2, a medium effect size of Cohens d of 0.47, and a drop-out rate of 20%.
Randomization
Trial patients were randomly assigned to either KPC or placebo in a 1:1 ratio. The randomization list was generated using a validated computerized system (EDP random number generator (SAS Version 9.2; RANUNI function)) by an independent institute. The block size of eight was not revealed to the investigator.
Blinding
Investigational medicinal product (IMP) containers were numbered sequentially. Each eligible patient was assigned to the next available IMP container number by the investigator at the investigational site. Neither investigator nor patient could identify group assignment based on the IMP container. A blind data review meeting was conducted and the randomization list was broken only after finalization of the statistical analysis plan and the subsequent database lock. Post-hoc analysis was defined after unblinding and assessors performing the analysis were unblinded.
Statistical methods
A priori statistical analysis was conducted using SAS Version 9.2 (SAS Institute Inc., Cary, NC), and exploratory post-hoc analysis was conducted using SPSS 20 (SPSS Inc., Chicago, IL).
All demographic and baseline data were descriptively analyzed. The homogeneity of treatment groups was tested.
For confirmatory analysis, the three primary efficacy variables were tested in hierarchical order in the intention-to-treat (ITT) population, i.e. in all patients who had administered at least one dose of the IMP and had no missing values in the respective measure. Therefore, depending on the variable analyzed, the number of patients considered for analyses varied slightly (max. n = 154). All analyses were carried out using Student’s t-test for two independent samples on the same one-sided alpha-level of 0.025 (equivalent to 0.05 two-sided). All analyses performed for the primary endpoints were also performed with the per-protocol (PP) population (n = 131). Patients of the PP had respective data at the final visit after 6 weeks treatment and were treated according to the trial protocol (i.e. compliance with the inclusion and exclusion criteria, IMP compliance ≥70% and ≤130%, no intake of not permitted concomitant medication).
A post-hoc analysis was performed for the three primary efficacy variables. While the original analysis was planned on the sum scores, the post-hoc analysis was performed for evaluation of intra-individual differences based on single symptoms (characteristic symptoms), and subscales (PSQ, SF-36), respectively (Student’s t-test for two independent samples). The two treatment groups were also analyzed over time using analysis of variance with repeated measures (repeated measures ANOVA) with three time points (visit 1, visit 2, visit 3).
The secondary efficacy variables were exploratively analyzed. For this purpose, group comparisons were carried out using statistical tests (Tedium Measure: Student’s t-test for two independent samples, efficacy rating by patient and physician: chi-square test); the results of these analyses were indicative yet not confirmatory.
Additionally, supportive analyses with replaced missing values were performed for the ITT and the PP population applying Mixed Models for repeated measures (MMRM)Citation33.
Results
Between September 2011 and July 2012, 204 patients were screened, 156 patients were invited for baseline/visit 1, 154 patients underwent treatment with n = 154 in the ITT and safety population (SP), and n = 131 in the PP population (). The last trial visit took place in September 2012. In this trial, 154 patients were randomized and received allocated treatment, of which 113 were female (73.4%) and 41 male (26.6%). The mean age of the patients was 53.1 ± 13.1. The mean treatment duration was 41.9 ± 4.5 days (KPC = 41.7 ± 5.0 days, Placebo = 42.1 ± 3.9 days), the median treatment duration was 42.0 days, range 10.0 − 50.0 days (KPC = 42.0 days, range 10.0 − 48.0 days, Placebo = 42.0 days, range 19.0 − 50.0 days). One patient of each treatment group withdrew their consent during the trial. The patients gave no further reason and the withdrawals were not related to an AE. When comparing baseline demographic characteristics between the KPC and the placebo group, patients of the placebo group were more often ex-smokers (32.5%) compared to patients of the KPC group (22.1%), but there were more smokers in the KPC group (26.0% vs. 15.6%). Patients of the KPC group were more often employed workers compared to patients of the placebo group (40.3% vs. 27.3%). The residual baseline characteristics did not differ >10% between both groups (). The difference in severity of neurasthenia at visit 1/Baseline was not significant except for the PSQ ().
Figure 1. CONSORT flowchart of the trial patients showing the distribution of the patients from initial assessment to analysis of trial data.
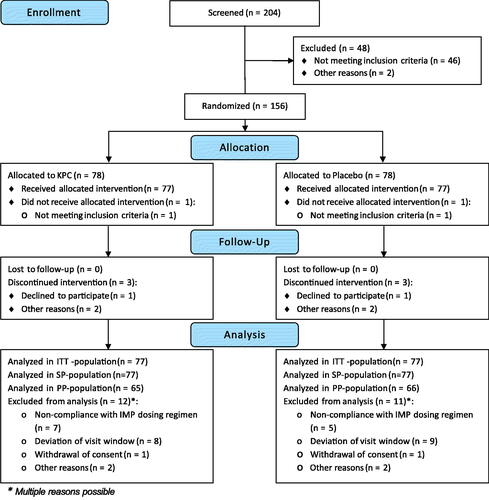
Table 1. Characteristics of trial patients, ITT-population (n = 154) – demographics.
Table 2. Characteristics of trial patients, ITT-population (n = 154) – neurasthenia severity at visit 1 (baseline).
Reasons for neurasthenia
Reasons for neurasthenia were comparable between the treatment groups (). Patients (n = 154) reported work or school as the most common cause of neurasthenia (KPC: 53.2%, Placebo: 49.4%), followed by housekeeping (KPC: 33.8%, Placebo: 31.2%). Apart from reasons associated with work/school or all reasons associated with the familial situation, other not further specified reasons were frequently documented (KPC: 45.5%, Placebo: 37.7%).
Table 3. Reasons for neurasthenia, ITT-population (n = 154).
Primary efficacy analysis
Efficacy on 12 characteristic symptoms of nervous exhaustion
The mean symptom sum score at visit 3 did not differ significantly in the a priori analysis between the placebo (10.9 ± 6.0) and the KPC group (10.6 ± 5.7, p = 0.728, and ).
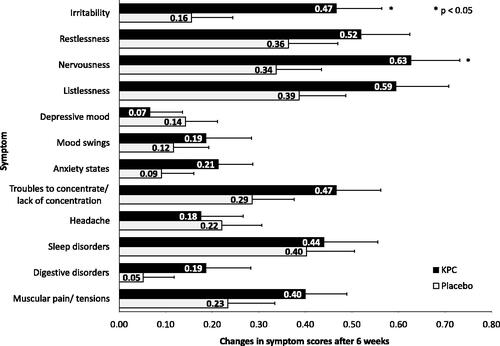
Figure 2. Characteristic symptoms of neurasthenia: symptom sum score (12 symptoms) of nervous exhaustion over trial period shown as means and standard error (SE), ITT-population (n = 154); ratings 0 = absent, 1 = mild, 2 = moderate, 3 = severe, max. score: 36; V1: visit 1, V2: visit 2, V3: visit 3.
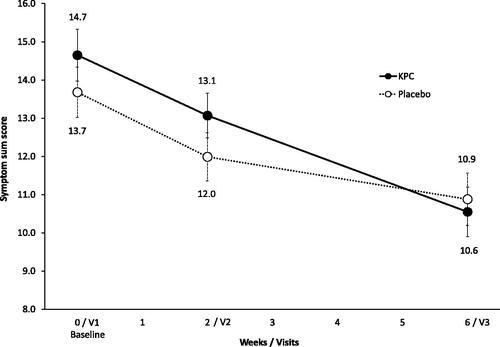
Table 4. Primary efficacy results at visit 3 (after 6 weeks of treatment), ITT population (n = 154).
A post-hoc analysis showed that the symptom sum score decreased significantly in both groups over time. Additionally, 10 out of 12 characteristic symptoms abated to a larger extent in the KPC group when compared to placebo. Two of them, the symptoms irritability (p = 0.020) and nervousness (p = 0.045), were significantly reduced (). Also, the change in the sum score of the KPC group (baseline minus visit 3 = 4.3 ± 0.6) was more pronounced than in the placebo group (2.8 ± 0.7), without reaching statistical significance (p = 0.091).
Efficacy on perceived stress
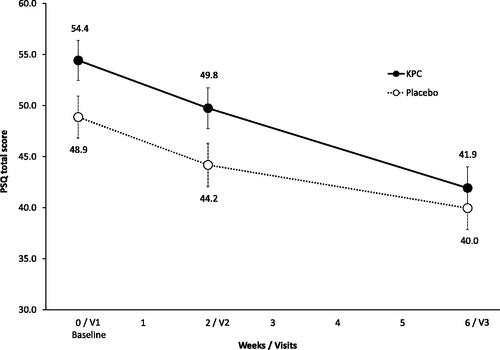
At baseline, perceived stress as measured with the PSQ was significantly higher in subjects of the KPC arm (54.4 ± 17.1) when compared to placebo (48.7 ± 17.9, p = 0.044, , ) in the a priori analysis. Post-treatment, no difference between groups was observed any more in the pre-specified analysis regarding mean PSQ (KPC: 41.9 ± 18.0, placebo: 40.0 ± 18.3, p = 0.505, and ).
Figure 4. Perceived stress: PSQ total score over treatment period of 6 weeks shown as means and SE, ITT-population (n = 154); total score between 0 to 100 (considering four subscales (worries, tension, joy, demands)). V1: visit 1, V2: visit 2, V3: visit 3.
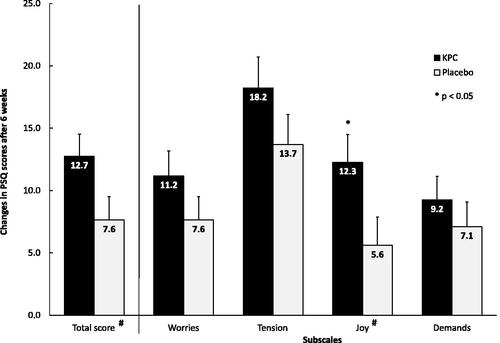
In both groups, perceived stress decreased significantly over time on the overall score and on all subscales, as shown in the post-hoc analysis. A comparison of the PSQ subscales for pre–post treatment differences revealed a significantly greater increase of joy under KPC treatment (p = 0.037, ).
Figure 5. PSQ total score and subscales: post-hoc analysis, intra-individual differences (baseline minus visit 3) in the four PSQ subscales shown as mean and SE, ITT-population (n = 154); scores of subscales between 0 and 100. # “joy” values are inverted for calculation of total score and are shown inverted for better understanding, respectively.
Health-related quality-of-life (SF-36)
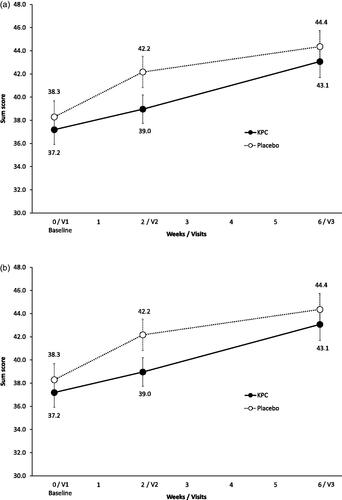
Results of the SF-36 showed no significantly stronger improvement at visit 3, neither of the physical nor the mental sum scores for the KPC arm when compared to placebo (KPC: 46.9 ± 9.0, placebo: 47.3 ± 9.0, p = 0.773 (physical sum score), KPC: 43.1 ± 12.0, placebo: 44.4 ± 12.0, p = 0.510 (mental sum score), and ).
Figure 6. SF-36: physical (a) and mental (b) sum scores (score range 0–100) over trial period shown as means and SE, ITT-population (n = 154). V1: visit 1, V2: visit 2, V3: visit 3.
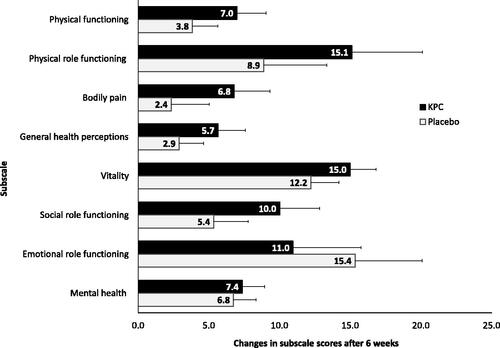
A post-hoc analysis showed that all subscales improved significantly over the treatment period, but the extent of improvement did not reach levels of statistical significance when comparing groups. Descriptively, patients treated with KPC had a greater improvement on the subscales social role functioning, vitality, mental health, general health perceptions, physical role functioning, and physical functioning, while patients treated with placebo improved more regarding emotional role functioning and reported less bodily pain ().
Figure 7. SF-36 subscales: post-hoc analysis, SF-36: changes in subscale scores (score range 0–100) of eight subscales over treatment period of 6 weeks shown as means and SE, ITT-population (n = 154).
The sensitivity analyses performed with the PP-population for all primary variables showed an equivalent outcome to the ITT analyses. The additionally performed supportive analyses with replaced missing values also confirmed the primary analysis.
Secondary efficacy and safety analysis
Tedium measure
In the a priori analysis, statistically significant differences between both groups were seen at baseline (p = 0.032) and visit 2 (p = 0.030) (), with lower values for the placebo group, indicating a lower level of exhaustion. At visit 3, however, no statistical significance regarding group differences was seen.
Figure 8. Level of exhaustion: Tedium measure (21 items) of level of exhaustion over trial period shown as means and SE, ITT-population (n = 154); tedium measure (min/max score: 2.1/5.9). V1: visit 1, V2: visit 2, V3: visit 3.
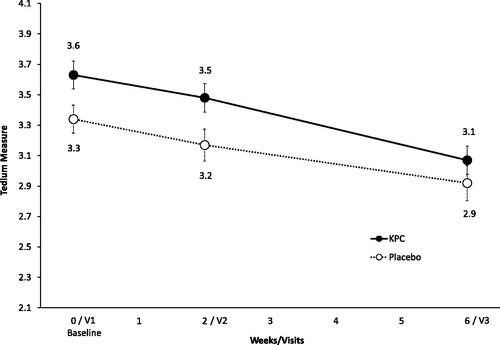
In a post-hoc analysis, results for the Tedium Measure showed a significant improvement for both groups over the course of the trial.
Efficacy from patients and physicians’ point of view
In total, 51.3% of the patients evaluated the efficacy of KPC as good or very good. The efficacy of KPC was rated more frequently as good or very good by the patients than placebo (37.7%) without reaching statistical significance (see Supplementary Appendix III). The assessment of efficacy from the physicians’ perspective is very similar to that of the patients: 51.3% of physicians assessed KPC as good or very good, compared with 36.4% for placebo.
Subjective assessment of efficacy
Subjective assessment of safety from patients and physicians’ point of view
In total, 96.1% of the patients evaluated the safety of KPC as good or very good vs. 97.4% in the placebo group (see Supplementary Appendix IV). The physicians’ assessment was also in line with that of the patients: 97.3% of the physicians evaluated KPC as well or very well tolerated (97.4% for placebo treatment).
Of the HPC patients, 72.4% would recommend the product (placebo group: 62.3%).
Safety evaluation of KPC
Forty-six AEs (number of patients (n) = 32) were reported in the KPC group vs. 33 in the placebo group (n = 28). For both groups, six AEs (KPC: n = 6, placebo: n = 5) were rated as causally related to the IMP. These AEs were all assessed as mild or moderate and mainly affected the gastrointestinal tract. One patient in the placebo group interrupted the IMP-intake. One serious AE occurred in the KPC group (postoperative joint dislocation). There was no causal relationship to the IMP. Overall, the most frequently reported AEs were diseases of the respiratory tract, chest and mediastinum (KPC: n = 13, placebo: n = 9), gastrointestinal tract (KPC: n = 4; placebo: n = 7), nervous system (KPC: n = 5, placebo: n = 4) and diseases of the skeletal muscles, connective tissue, and bones (KPC: n = 6, placebo: n = 4). There were no signs for abnormalities in the blood tests. In either group, there were no dropouts or withdrawals of informed consent due to AEs or lack of efficacy.
Discussion
The efficacy results of this double-blind RCT showed a significant improvement of neurasthenia after treatment of 6 weeks in both treatment groups for the investigated efficacy parameters. This is remarkable, as patients were suffering from persistent and distressing complaints of neurasthenia for more than 3 months prior to trial enrollment according to the ICD-10 criteria F48.0Citation25. However, the results did not achieve showing superiority of KPC over placebo in any of the pre-specified outcome measures. When looking at the course of the efficacy endpoints over the 6 weeks treatment time, KPC seems to be associated with a more pronounced improvement compared to placebo, without reaching significance in any of the a priori defined endpoints. Exemplary, 10 out of 12 characteristic symptoms of neurasthenia decreased more in the KPC group. Interestingly, a post-hoc analysis revealed some statistically significant group differences in favor of KPC, e.g. it showed that this anthroposophic medicinal product successfully reduced the characteristic stress symptoms nervousness and irritability. Furthermore, KPC improved the globally perceived stress in the subscale “joy”. It may well be that a larger sample size would have shown superiority of KPC over placebo in the pre-specified efficacy parameters.
Three major differences between the two clinical study designs (RCT vs. NISCitation18,Citation20) are likely to have contributed to the finding that superiority of KPC over placebo couldn’t be shown. First of all, the NIS were not controlled. Therefore, the placebo effect is likely to reduce the magnitude of the KPC efficacy in the RCT. The placebo effect is particularly well documented for pain treatmentCitation34–39. In an inverse placebo RCT the placebo effect size was investigated. Patients who were told that they were being treated with a verum showed a decrease in pain perception, whereas patients who knew that they were receiving placebo had an unchanged pain perception 6 weeks after baselineCitation40. As neurasthenia has a strong mental component, it is likely that this indication is also prone for a strong placebo response. The effect size of placebo cannot be determined by the chosen design of the clinical trial. However, the results of the characteristic symptoms of neurasthenia observed in the RCT and the pharmacy-based NIS might be interpreted in this way. Aside from the placebo effect, the intra-individual change after treatment with KPC is similar for irritability (RCT: 0.5 scores, NIS: 0.6 scores)Citation20 and nervousness (RCT: 0.6 scores, NIS: 0.5 scores). This indicates that the effects between RCT and NIS are comparable for these two symptoms, which is in line with Concato’s conclusion that effects are not necessarily overestimated in NISCitation41. A second difference in the study design is the indication. Typical for an RCT, the inclusion diagnosis had clear and narrow criteria (F48.0 ICD-10)Citation25 resulting in a more controlled setting. For the past physician-based NISCitation18 as well as for the pharmacy-based NIS that was performed after this RCTCitation20, the inclusion indication was broader and referred to patient-reported stress-related disorders, according to the authorized indication. Therefore, patients may have presented to the NIS with both less severe as well as more severe symptoms of nervous fatigue. As KPC is an OTC product, it can be assumed that the patients of the pharmacy-based NIS best represent the affected patient population. Due to the rigorous patient selection in the RCT, it is possible that the treatment effect of KPC in the RCT differs from the effect seen in the two NIS. Lastly, the design of the NIS allowed a holistic treatment of this complex indication. From a CAM perspective, disease management pursues an overall therapeutic approach including recommendations concerning lifestyle changes to improve patient’s quality-of-life as a whole. In the RCT, patients were instructed to keep medication as for baseline, and they were prompted not to change their usual habits regarding daily activities such as sports, non-medicamentous therapies, etc. As neurasthenia, or nervous exhaustion, is often due to mental complaints/chronic stress, a holistic approach that addresses the root of the complaints is likely to show a stronger effect than treating the symptoms alone.
In the present RCT from 2011/2012, 204 patients were screened and 154 patients (77 in each group) were analyzed. Regarding baseline demographics, the two treatment groups differed only in terms of employment status and smoking behavior. Neither factor has yet been shown or is expected to be the sole cause of nervous exhaustion. Therefore, homogeneity of both groups at the beginning of treatment concerning demographics can be assumed. The proportion of women (73.4%) and the mean age of enrolled patients (53.1 years) was comparable to the two NIS conducted with KPC (physician-based NIS:Citation18 78.0% and 50.3 years vs. 73.9% and 44.8 years in the pharmacy-based NISCitation20) Regarding symptom severity at baseline, patients of the KPC group showed a slightly more severe symptomatology of neurasthenia compared to the placebo group, with significant differences for the parameters perceived stress (PSQ) and Tedium Measure. This may have impacted the efficacy analysis.
As mentioned above, the positive results of both NIS could be reproduced in the KPC group in the pre-specified primary efficacy evaluation of the RCT. However, as neurasthenia also improved in patients of the placebo group, the primary efficacy endpoint was not met. For the secondary efficacy parameters, the results are similar: The patients’ constitution significantly improved in both groups until treatment end, but no significant differences were found between groups. For patients in the KPC group, changes over the course of the trial indicate greater improvement in symptoms for all efficacy endpoints. For example, though not as convincing as efficacy ratings in the NIS, KPC’s subjective efficacy rating was more frequently considered by physicians and patients to be “good” or “very good” (both 51.3%) than placebo (37.7% and 36.4%).
Regarding safety, very few and non-severe side-effects were reported for the entire trial. Overall, no differences were observed between KPC and placebo in the safety evaluation. The subjective safety rating of KPC was extraordinarily good with values between 96.1% (patients) and 97.4% (physicians) and assessment of AEs did not suggest any specific risks. Data of the sponsor’s pharmacovigilance system shows that, on average, 19 individual case safety reports (ICSRs) per year are recorded worldwide (i.e. an ICSRs is reported in about 0.006% of sold KPC units). The system organ class (SOC) gastrointestinal disorders is most frequently affected (ca. 25%), followed by SOC skin and subcutaneous tissue disorders (ca. 20%) and psychiatric disorders (ca. 15%). The underlying disease is very often involved in cases of the gastrointestinal as well as psychiatric disorders. On average only 1.6% of all ICSRs recorded were considered serious. In all serious cases, the relationship to KPC was assessed as unlikely due to serious underlying diseases and the intake of further medicinal products. The safety data of this RCT confirm the available very good safety profile from the NIS and the continuous market monitoring with regard to product safety via the sponsor’s pharmacovigilance system.
Conclusions
This double-blind RCT adds information to an anthroposophic medicinal product and completes the overall picture that KPC helps to relieve stress-related symptoms. In this RCT, no superiority of KPC over placebo was shown in the a priori analysis. In an exploratory post-hoc analysis, KPC was superior to placebo in the characteristic neurasthenia symptoms of irritability and nervousness. The safety of KPC can be assessed as markedly good. RCTs with a comparable design and a higher number of patients and a primary endpoint based on the difference from baseline rather than a mean at treatment end, may well clearly show that KPC is superior to placebo in the treatment of neurasthenia. Also, the current RCT suggests that typical symptoms such as irritability and nervousness are more suitable as primary endpoints. Future clinical studies could strengthen outcomes by including biomarkers for a stress-related dysregulation of the physiological system. Overall, KPC might be a good CAM approach in patients with neurasthenia.
Transparency
Declaration of funding
The trial was financed by the Weleda AG, Arlesheim, Switzerland.
Declaration of financial/other relationships
RH and LS are employees of Weleda AG, Germany. JH is the CEO of daacro GmbH & Co. KG, a clinical research organization in Germany and declares having received an honorarium for writing the publication. No further interests have to be declared. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
RH was responsible for the conception, design, and conduct of the trial. LS and JH drafted the manuscript and contributed substantially to the interpretation of the data. All authors reviewed the manuscript rigorously and critically and approved the final article.
Ethics approval and consent to participate
This clinical trial has been registered on the “German Clinical Trials Register” (ID: DRKS00003261) and on EudraCT as 2010-024189-23. The trial protocol, informed consent document(s), and any other appropriate trial-related documents were reviewed and approved by an independent Ethics Committee (Ethik-Kommission des Landes Berlin, Ref. No. 11/0131 – ZS EK 14) and the relevant regulatory authorities (German Federal Institute for Drugs and Medical Devices (BfArM)). There were no trial protocol amendments. All trial patients gave their written informed consent to participate in the clinical trial prior to any trial related procedures.
20231120_DR-CR-NEU01S01_Manuskript-cmro_3-0_Supplemental-material.pdf
Download PDF (399.7 KB)Acknowledgements
The authors would like to thank the clinical trial personnel of Emovis GmbH, a dedicated study site situated in Berlin, Germany, for the conduct of the RCT, the responsible persons of the CRO ACM Allied Clinical Management GmbH for their excellent trial management and monitoring, and the responsible persons from CSG Clinische Studien Gesellschaft (IGES Group) for their input regarding data management and statistics. The authors’ thanks also go to Cristina Semaca (former employee of Weleda AG) who took care of data analyses and their correctness, and Martin Schnelle for his contribution to the trial design and input for any medical matters. Also, we are grateful for the dedicated personnel of daacro GmbH & Co. KG for their support in writing the manuscript. Special thanks go to all the anonymous patients for their valuable contribution to the trial.
Data availability statement
The data that support the findings of this clinical trial are not publicly available due to containing information that could compromise the privacy of research participants. Disclosure of data would not be in accordance with the European General Data Protection Regulation.
Notes
i Neurodoron is a registered trademark of Weleda Trademark AG P-569108 Switzerland, National Trademark Registration, 006345516 European Union, Regional Trademark Registration.
References
- Beard G. Neurasthenia, or nervous exhaustion. Boston Med Surg J. 1869;80(13):217–221. doi: 10.1056/NEJM186904290801301.
- Van Deusen EH. Observations on a form of nervous prostration,(neurasthenia,) culminating in insanity. Am J Psychiatry. 1869;25(4):445–461. doi: 10.1176/ajp.25.4.445.
- Linde A. Chronic fatigue syndrome–a functional somatic syndrome. Ther Umsch. 2007;64(10):567–574. doi: 10.1024/0040-5930.64.10.567.
- Schäfer ML. On the history of the concept neurasthenia and its modern variants chronic-fatigue-syndrome, fibromyalgia and multiple chemical sensitivities. Fortschr Neurol Psychiatr. 2002; Nov70(11):570–582. doi: 10.1055/s-2002-35174.
- Wessely S. Neurasthenie. In: Helmchen HH, Lauter F, Sartorius N, editors. Psychiatrie der Gegenwart. Vol. 6. 4th ed. Berlin: Springer; 2000. p. 209–224.
- Üstün TB. WHO collaborative study: an epidemiological survey of psychological problems in general health care in 15 centers worldwide. Int Rev Psychiatry. 1994;6(4):357–363. doi: 10.3109/09540269409023273.
- Gamma A, Angst J, Ajdacic V, et al. The spectra of neurasthenia and depression: course, stability and transitions. Eur Arch Psychiatry Clin Neurosci. 2007;257(2):120–127. doi: 10.1007/s00406-006-0699-6.
- Sartorius N. Diagnosis and classification of neurasthenia. In: Judd LL, Saletu B, Filip, V, editors. Basic and clinical science of mental and addictive disorders. Bibliotheca psychiatrica. Basel: Karger; 1997. p. 1–5.
- Gaab J, Ehlert U. Chronische Erschöpfung und Chronisches Erschöpfungssyndrom. Vol. 26. Göttingen: Hogrefe Verlag; 2005.
- Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369(9565):946–955. doi: 10.1016/S0140-6736(07)60159-7.
- Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry. 2006;18(1):49–56. doi: 10.1080/10401230500464711.
- Abbot N, Ernst E. Patients′ opinions about complementary medicine. Complement Med Res. 1997;4(3):164–168. doi: 10.1159/000210318.
- Hubner R, van Haselen R, Klein P. Effectiveness of the homeopathic preparation neurexan compared with that of commonly used valerian-based preparations for the treatment of nervousness/restlessness - an observational study. Sci World J. 2009;9:733–745. doi: 10.1100/tsw.2009.95.
- Maisenbacher J. Baldrian und Melisse–milde Psychopharmaka. Therapiewoche. 1992;42:2140–2144.
- Müller SF, Klement S. A combination of valerian and lemon balm is effective in the treatment of restlessness and dyssomnia in children. Phytomedicine. 2006;13(6):383–387. doi: 10.1016/j.phymed.2006.01.013.
- Orth-Wagner S, Ressin W, Friederich I. Phytosedativum gegen Schlafstörungen. Klinische Wirksamkeit und Verträglichkeit eines Phytosedativums mit Auszügen von Baldrianwurzel, Hopfenzapfen und Melissenblättern. Z Phytother. 1995;16(3):147–156.
- Park SJ, Bae YC, Choi NR, et al. Clinical study on constitutional herbal tea for treating chronic fatigue. J Pharmacopuncture. 2014;17(4):55–60. doi: 10.3831/KPI.2014.17.037.
- Rother C, Oexle J. Einsatz von Neurodoron® bei Patienten mit nervöser Erschöpfung aufgrund von Stress [Use of neurodoron® for patients with nervous exhaustion due to stress]. Der Merkurstab. 2010;63:171–177.
- Hellhammer J, Spitznagel-Schminke L, Semaca C, et al. Neurodoron® in patients with neurasthenia – A randomized, double-blind, placebo-controlled clinical trial. medRxiv. 2022:2022.09.02.22279531.
- Hellhammer J, Schmidt K, Semaca C, et al. Neurodoron® for stress impairments: a prospective, multicenter non-Interventional trial. Evid Based Complement Alternat Med. 2022;2022:2626645. doi: 10.1155/2022/2626645.
- Glöckler M. Anthroposophische Arzneitherapie für Ärzte und Apotheker [Anthroposophic medication therapy for physicians and pharmacists]. Stuttgart: deutscher Apotheker Verlag; 2014. p. 1-1–1-2.
- Kienle GS, Albonico HU, Baars E, et al. Anthroposophic medicine: an integrative medical system originating in Europe. Glob Adv Health Med. 2013;2(6):20–31. doi: 10.7453/gahmj.2012.087.
- World Health Organization. WHO benchmarks for training in anthroposophic medicine. Geneva: World Health Organization; 2023.
- Arendt AK, Markus Kuck A, et al. Vademecum Anthroposophische Arzneimittel. Vol. 61, Supplement 1. Filderstadt: Gesellschaft Anthroposophischer Ärzte in Deutschland e.V., Medizinische Sektion der Freien Hochschule für Geisteswissenschaft, Dornach/Schweiz; 2008. (Merkurstab).
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Vol. 2. Geneva: World Health Organization; 1993.
- BfArM. Anthroposophische Arzneimittel: Aufbereitungsmonographien der Kommission C zu Kalium phosphoricum comp. Bundesanzeiger Nr. 43a vom 02.03.1991, incl. Korrektur: Bundesanzeiger Nr. 170 vom 10.09.1993.
- Levenstein S, Prantera C, Varvo V, et al. Development of the perceived stress questionnaire: a new tool for psychosomatic research. J Psychosom Res. 1993;37(1):19–32. doi: 10.1016/0022-3999(93)90120-5.
- Fliege H, Rose M, Arck P, et al. Validierung des “Perceived Stress Questionnaire” (PSQ) an einer deutschen Stichprobe [Validation of the “perceived stress questionnaire” (PSQ) in a german sample]. Diagnostica. 2001;47(3):142–152. doi: 10.1026//0012-1924.47.3.142.
- Fliege H, Rose M, Arck P, et al. The perceived stress questionnaire (PSQ) reconsidered: validation and reference values from different clinical and healthy adult samples. Psychosom Med. 2005;67(1):78–88. doi: 10.1097/01.psy.0000151491.80178.78.
- Morfeld M, Kirchberger I, Bullinger M. SF-36 Fragebogen zum Gesundheitszustand: Deutsche Version des Short Form-36 Health Survey. 2nd ed. Göttingen: Hogrefe; 2011.
- Pines A, Aronson E. Career burnout: causes and cures. New York (NY): Free Press; 1988.
- Pines AM, Aronson E, Ausgebrannt KD. Vom Überdruss zur Selbstentfaltung. 10th ed. Cranach AV, translator; Stuttgart: Klett-Cotta; 2006.
- Mallinckrod CH, Lane PW, Schnell D, et al. Recommendations for the primary analysis of continuous endpoints in longitudinal clinical trials. Drug Information J. 2008;42(4):303–319. doi: 10.1177/009286150804200402.
- Benedetti F, Mayberg HS, Wager TD, et al. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25(45):10390–10402. Nov 9 doi: 10.1523/JNEUROSCI.3458-05.2005.
- Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiol Rev. 2013;93(3):1207–1246. doi: 10.1152/physrev.00043.2012.
- Carlino E, Pollo A, Benedetti F. Placebo analgesia and beyond: a melting pot of concepts and ideas for neuroscience. Curr Opin Anaesthesiol. 2011;24(5):540–544. doi: 10.1097/ACO.0b013e328349d0c2.
- Sauro MD, Greenberg RP. Endogenous opiates and the placebo effect: a meta-analytic review. J Psychosom Res. 2005;58(2):115–120. doi: 10.1016/j.jpsychores.2004.07.001.
- Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16(11):1277–1283. doi: 10.1038/nm.2229.
- CAMDOC Alliance. The regulatory status of complementary and alternative medicine for medical doctors in Europe. Brussels; Vol. 13; 2010. p. 19.
- Gerdesmeyer L, Klueter T, Rahlfs VW, et al. Randomized placebo-controlled placebo trial to determine the placebo effect size. Pain Phys. 2017;20(5):387–396.
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–1892. doi: 10.1056/NEJM200006223422507.